Abstract
Selective extraction is an improved method applied to fenugreek seeds to isolate specific bioactive compounds from different seed fractions. This process leads to the recovery of two fixed oils (FO) from husk (HK) and cotyledon (CT) as by-products of the defatting process. The main objective of this study is to investigate and compare the properties obtained from these fractions FO. A comprehensive analysis of the extracted FO was performed, including lipid class distribution, fatty acid analysis, molecular species of triacylglycerols, nutraceutical profile (sterol and tocopherol content), antioxidant activity (polyphenol content) and physicochemical properties. The percentage oil yield of the husk and cotyledon fractions was 2.625 ± 0.2% and 11.6 ± 0.69%, respectively. In addition, nutrient profiling identified β-sitosterol, 1340.31 ± 1.01 and 1670.28 ± 1.22 and α-tocopherol, 0.17 and 0.13 mg/100 g in HK and CT, respectively. Fatty acid analysis revealed a higher proportion of linoleic acid (50.17 µmol %) in HK FO and α-linolenic acid (15.28 µmol %) in CT FO as major fatty acids. The Husk FO exhibited significant antioxidant activity due to its high polyphenol content. Other parameters related to the physical and chemical properties determine the quality, stability and characteristics of the fixed oils from both fractions. The results provide valuable insights into the specific properties of FO obtained from these fractions and shed light on differences in the composition of bioactive compounds such as sterols, tocopherols and fatty acids. This research contributes to the understanding of the constituents of fenugreek seeds and their potential applications in the food and pharmaceutical industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fenugreek (Trigonella foenum-graecum L.) seeds belonging to the Fabaceae family are frequently used in both cooking and medicine. They are rich in nutrients and contain a variety of bioactive compounds that has proven potential in preventing and treating a range of health issues such as diabetes, cancer, cardiovascular problems and obesity [1]. As a counterpart of legumes, these seeds are also rich in protein, fiber and oil. Among the oils, fixed oil (FO) is the major oil component in fenugreek seeds [2].
FO are non-volatile extracts obtained with organic solvents such as hexane or petroleum ether that maintain a liquid state at room temperature. Oil content in fenugreek seeds ranges from 3.25 to 8.4%, and the fixed oils account for the major share due to the significantly lower essential oil content [3]. While a number of studies have explored the health benefits of fenugreek seed oil, the focus of research till date has been on the whole seeds [4,5,6]. The present study aims to address this gap by investigating the properties of fenugreek fraction FO and evaluating their difference in composition and attributes. Fraction FO is obtained through selective extraction, a technique exclusively used for fenugreek seeds to improve efficiency in extraction to meet the increasing industrial demand. Selective extraction enables the extraction of specific compounds from distinct seed parts rather than the entire seed. These seed fractions facilitate the focused extraction of major bioactive compounds present in each of them [7]. A study conducted by Naidu et al. (2011) also demonstrated significant antioxidant activity among these seed fractions [8].
Concurrently, there is a growing interest in the use of by-products and the optimization of resource utilization through efficient extraction conditions. Selective extraction starts with fractionation of the seeds and prior to the start of extraction process, these fractions are subjected to a defatting procedure, resulting in retrieval of a considerable amount of FO which is often underutilized or discarded. However, the oils extracted from the husk and cotyledons contain numerous compounds with pharmaceutical potential. This highlights the importance of exploring and maximizing the use of these by-products for their inherent bioactive compounds.
The main objective of this study is to conduct a thorough evaluation of fenugreek seed fraction FO, which includes determination and evaluation of lipid class composition, fatty acid analysis, lipidomic analysis, thermal stability and chemical parameters. In addition, the study aims to estimate the content of sterols, tocopherols and polyphenols in the FO and their anti-oxidant activity. A comparative analysis between two fractions will be performed, providing valuable insights into the differences between them. This study is the first comprehensive analysis of fenugreek seed fraction FO.
Materials and methods
Materials
Fenugreek (Trigonella foenum-graecum L.) seeds were purchased from the local market at Mysuru, India. ABTS, Heptadecanoic acid (C17:0), boron trifluoride-methanol (BF3 methanol), DPPH, Folin-Ciocalteu (FC) reagent, Gallic acid, HPLC standards for tocopherols and phytosterols, 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), Trolox were purchased from Sigma Aldrich, St. Louis, MO, USA. Other chemicals used, such as aluminium chloride (AlCl3), potassium hydroxide (KOH), sodium thiosulphate, sodium hydroxide, and solvents such as petroleum ether, were of analytical grade from Hi-media, India.
Fractionation, extraction of fraction FO and yield estimation
The first step of selective extraction is the fractionation of the fenugreek seeds [7]. The fenugreek seeds were conditioned for two hours at room temperature with moisture (16%) to slightly soften the seed coat. This makes the seeds suitable for fractionation. Plate milling was performed to obtain different fractions, husk and cotyledon. These fractions were then separated using a Schule-type paddy separator (FH Schule GmbH, Germany). The separated fenugreek seed fractions were ground and then used to extract the FO using a Soxhlet apparatus. Petroleum ether was chosen as the solvent and the extraction process involved ten cycles per hour with duration of 10 h. After extraction, the solvent was removed by evaporation under reduced pressure using a flash evaporator, specifically the Heidolph Hei-Vap R-300 from Germany. This process made it possible to recover the fenugreek oil. The weight of the recovered oil was measured and the yield calculated using a special formula.
The fixed oil and other experimental samples were stored at -20 °C. All the experiments were conducted within 30 days of the extraction.
Lipid-class composition of FO
Lipid classes provide comprehensive insights into various parameters and properties of oil. The lipid class composition of the extracted fixed oil (FO) samples from fenugreek seeds was evaluated according to CD 11 C-93-AOCS2003. The lipid classes analyzed include glycolipids, phospholipids and neutral lipids. Column chromatography was used to separate the different lipid classes. A Supelclean LC-Si SPE (Solid Phase Extraction) tube (Merck, Germany) with a capacity of 3 mL and a silica gel bed of 500 mg was used for the separation. The column was first equilibrated with chloroform, adding 2 mL of chloroform to ensure proper equilibration. Next, 5 mg of the FO sample dissolved in 2 mL of chloroform was added to the column. The column was then washed with 2 mL of chloroform to remove all impurities. Subsequently, 4 mL of chloroform was used to elute the neutral lipids. A mixture of acetone and isopropanol at a ratio of 9:1 (v/v) was used as an eluent to extract the glycolipids, with 4 mL of the mixture added to the column. Finally, 4 mL of methanol was used to elute the phospholipids from the column. After elution, the individual fractions corresponding to GL, PL and NL were collected and the solvent was removed. The weight of the individual fractions was determined by weighing the collected material [9].
Thin-layer chromatography (TLC)
TLC was used to separate and analyze different lipid classes, including TAGs, DAGs, SEs and FFAs. TLC silica gel 60 sheets (Merck, Germany) were used as the stationary phase. The mobile phase used for the separation was a mixture of petroleum ether, diethyl ether and acetic acid in a ratio of 80:20:1 (v/v/v). This specific composition of the mobile phase contributes to an optimal separation of the lipid classes. To perform the TLC analysis, the FO samples were spotted onto the TLC plates together with a standard reference such as rice bran oil. The plates were then placed in a glass chamber into which iodine vapors were introduced. The iodine vapors act as a visualizing agent and react with the lipid bands on the TLC plates, making them visible as distinct spots or bands. By comparing the migration distances and patterns of the lipid bands on the TLC plates with the standard reference, the different lipid classes, including TAG, DAG, SE and FFA, can be identified and quantified [9].
Fatty acid profile analysis
Fatty acid analysis of oil is important in determining the physical, nutritional and chemical properties of oils. The fatty acid composition of the fixed oils extracted from the FO was determined by GC-MS analysis [10]. Fatty acid methyl esters (FAME) were prepared by esterification of the fixed oils with a BF3-methanol complex solution. Specifically, 1 mL of the BF3-methanol complex solution was added to the FOs and the mixture was heated to 60 °C for 30 min to facilitate the esterification process. A mixture of water and hexane in a 1:1 ratio (v/v) was used to extract the FAMEs. The mixture was added to the esterified FOs and the upper organic hexane layer containing the FAMEs was separated and transferred to GC vials. Prior to the methylation step, 50 µg of heptadecanoic acid (C17:0) was added as an internal standard. This helps to accurately quantify the fatty acids during the analysis. The FAMEs obtained from the extraction were analyzed using an Agilent DB-23 column, a (50% cyanopropyl) methylpolysiloxane column with specific dimensions (60 m length, 0.25 mm ID and 0.25 μm film thickness). GC-MS analysis was performed using a 7890B-GC-5977 A MSD system from Agilent Technologies. The identified fatty acids were confirmed using a mass spectrum search, specifically NIST version 2.0 g. This allows accurate identification of the fatty acids based on their mass spectra.
TAG molecular species fingerprinting by high-resolution mass spectroscopy electrospray ionization (HR-MS ESI)
The molecular species of TAGs in oil are the specific combinations of fatty acids attached to the glycerol backbone. The evaluation of these molecular species was critical to understand the physical and nutritional properties of oils. The molecular species of the TAGs was analyzed using the MS/MSALL technique. The FO was dissolved in a mixture of chloroform and methanol (1:2, v/v) with 7.5 mM ammonium acetate. The resulting solution was prepared with a volume of 250 µl. The prepared samples were injected directly into an AB Sciex Triple TOF 5600 system at a flow rate of 7 µL/min. The analysis was performed using MS/MSALL in positive mode, which allows the identification and quantification of the TAG molecular species. The MS/MS detector conditions were set according to the parameters reported in a previous study [11]. These conditions include specific instrument settings such as ionization mode, collision energy, and other parameters relevant to MS/MSALL analysis. Analyst TF 1.6 software was used to control the AB Sciex Triple TOF 5600 system during the analysis. The acquired data was processed using Peak View software, which enables the identification and quantification of TAG molecular species based on their total fatty acyl carbons and total fatty acyl double bonds.
Determination of phytosterols and tocopherols
Phytosterols
The phytosterol analysis was performed to identify different phytosterols as well as their concentration in the fenugreek fraction FO. The phytosterols from the FO sample were separated by a method involving alkaline saponification and high performance liquid chromatography (HPLC). First, the FO was subjected to alkaline saponification by boiling with ethanolic potassium hydroxide under reflux for 2 h. This process hydrolyzes the ester bonds and converts the phytosterols into their free form. The unsaponified material containing the phytosterols was then dissolved in acetone for further analysis. HPLC separation was performed using an LC-10 A system equipped with a photodiode array detector (PDA) from Shimadzu, Tokyo, Japan. A reversed-phase C18 column (Chromasil, 250 × 4.6 mm, 5 μm) was used for the separation of phytosterols. The column was kept at a constant temperature of 30 °C. Isocratic elution was performed with a mobile phase consisting of a mixture of methanol and acetonitrile at a ratio of 98:2 (v/v). The eluent was pumped through the column at a flow rate of 1.2 mL/min.
During HPLC analysis, the separated phytosterols were detected using the PDA detector, which measures their absorbance at a wavelength of 205 nm. This detection wavelength is commonly used for phytosterol analysis. The absorbance values provide information about the presence and quantity of phytosterols in the sample. The specific spectral properties of phytosterols enable their identification and quantification [12].
Tocopherols
Similarly, evaluation of tocopherols was vital in identification of various tocopherols and their concentration in the fenugreek fraction FO. The tocopherols in the FO samples were determined by HPLC. The FO samples were dissolved in HPLC-hexane, and the solution was filtered through syringe filters with a pore size of 0.2 μm to remove all particles before injection. HPLC analysis was performed using a Shimadzu HPLC system with methanol:water (95:5, v/v) as the solvent system for isocratic elution. A C18 column (Chromasil, 250 × 4.6 mm, 5 μm) was used to separate the tocopherols. The column was kept at a constant temperature of 30 ºC and the eluent was pumped through the column at a flow rate of 1.5 mL/min. Tocopherols was detected using an RF 20 A detector with fluorescence detection at an excitation wavelength of 290 nm and an emission wavelength of 330 nm. This fluorescence detection method enables the sensitive and specific measurement of tocopherols present in the FO samples. The results of the analysis were expressed as milligrams (mg) of tocopherols per 100 g (g) of oil. This expression standardizes the tocopherol content and facilitates comparison between different samples. The described HPLC method provides a reliable and accurate means of determining the tocopherol composition in the FO samples [13].
Polyphenol content and antioxidant activity
Polyphenols are a diverse group of naturally occurring compounds found in plants. They are known for their antioxidant properties, which help neutralize reactive molecules that can cause oxidative stress and cell damage widely known as free radicals. The ABTS, DPPH, and FRAP assays are crucial to evaluate antioxidant capacity of the oil. ABTS and DPPH assays both measured the ability of antioxidants to scavenge free radicals, using color changes in the ABTS•+ and DPPH radicals, respectively that helps in quantifying antioxidant capacity whereas the FRAP assay assessed the reducing power of antioxidants by monitoring their ability to convert a ferric tripyridyltriazine (Fe (III)-TPTZ) complex to its ferrous form, which was indicated by a color change [14].
Extract preparation
To extract the polyphenols, the FO sample (1 g) was mixed with 0.1% HCl in 4 mL of 70% methanol on a multi-tube vortexer (Benchmixer XL, Benchmark Scientific, USA) at 2500 rpm for 1 h. The reaction mixture was centrifuged at 10 °C at 12,000 rpm for 15 min. The procedure was repeated three times for complete extraction. The supernatants were collected and filtered and kept in the dark due to photosensitivity and stored at -20 °C to be used for determination of phenolic content and antioxidant activity of the fixed oil [15].
The polyphenol content was determined by the Folin-Ciocalteu (FC) method and the antioxidant activity of the fixed oils was estimated by three different methods, namely 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP) and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS).
Total phenolic content
The FC method is standard in determining the total phenolic content of the oil [16]. A linear standard curve was developed to adjust the concentrations of the sample extracts. 10% FC reagent (1 mL) and 7.5% sodium carbonate (Na2CO3) (0.8 mL) were mixed and kept at RT for 90 min. The TPC content in the fixed oils was expressed as mg gallic acid equivalent (GAE) per 100 g of oil by using the regression equation obtained from the calibration curve for gallic acid [15].
2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
The sample extract (8–40 µL) was mixed with the DPPH reagent prepared with 0.1 mM methanol to obtain a total volume of 2 mL. The reaction mixture was kept at RT for 20 min in the dark. The absorbance was measured at 517 nm against methanol as a blank. The radical scavenging activities of the fixed oils were expressed as inhibition percentage (IC50), which was referred to ascorbic acid as a standard [5].
Ferric reducing antioxidant power (FRAP) assay
The method described by Benzie & Strain was used to evaluate the ferric reducing antioxidant power of fixed oils [17]. The FRAP reagent was freshly prepared by mixing acetate buffer, 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) solution and ferric chloride solution in a 10:1:1 ratio. The reagent was then incubated for 30 min at 37 °C in a water bath. The sample extracts (200 µL) and FRAP reagent (3 mL) were added and incubated for 6 min at RT. The optical density was read at 593 nm as a measure of Trolox equivalent expressed as mM Trolox equivalent (TE) per 100 g of oil.
2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay
Different concentrations of the FO extracts (8–40 µL) were added to 2 mL ABTS solution prepared by adding 26.4 mg potassium persulfate in 40 mL ABTS (7.4 mM) stored in the dark for 16 h for 6 min and the optical density was measured at 734 nm. The percent inhibition (IC50) of the samples was expressed as Trolox equivalents [18].
Physico-chemical characterization of fraction FO
Color
The color of oils can vary widely depending on the source, processing methods, and presence of certain compounds. Color is important in evaluating quality, purity, and processing state along with suitability for various application. The color of the fractionated FO was evaluated using the Konica Minolta CM-5 color measuring system, specifically from Konica Minolta Sensing, Inc., USA. The FO was filled into a cuvette, and a reading was taken using the color measuring system [6].
Optical rotation
The assessment of optical rotation was crucial for gauging purity, authenticity, and chemical composition of oils, serving as a key parameter in quality control for industries. The optical rotation ([α] D) of a 1% (1 mg mL− 1) solution of the FO was determined using the Anton Paar Polarimeter MCP200. To obtain the reading, the solution was filled into the sample cell of the polarimeter [7].
Refractive index
The evaluation of refractive index served as an indicator of solute concentration of the oil that also provide insights regarding optical properties and transparency. The refractive index was measured using a Butyro refractometer. The reading was taken by comparing the FO against reference oil.
Differential scanning calorimetry (DSC)
The DSC analysis was important in determining the thermal properties of the extracted oil. The thermal stability of the FO obtained from fenugreek seeds were evaluated using a Double furnace DSC system (Perkin Elmer DSC 8000 USA). The FO sample, after drying, was placed in a sealed aluminum pan. To understand the melting point and the enthalpy change of the FO, an empty pan was used as a reference. The DSC analysis was conducted using a single ramp program, with a temperature range of 30–100 °C and a ramp rate of 10 °C per min. During the analysis, nitrogen was used as the purge gas to maintain an inert atmosphere and minimize any potential oxidative reactions or sample degradation. By performing DSC analysis, the thermal behavior and stability of the fixed oil were assessed, providing insights into its melting properties, enthalpy changes, and overall thermal characteristics. Additionally crystallization of oils were measured as temperature decreased at 10 °C/min upto 100 °C with a holding time of 10 min [7].
Fourier transform infrared (FTIR) spectroscopy
FTIR analysis assisted in understanding of the chemical composition and molecular structure of the oil samples. The FTIR measurements were carried out in the wavenumber range of 400 to 4000 cm− 1 and a spectral resolution of 2 cm− 1, allowing for the collection of the infrared spectra of the fixed oil. The instrument was equipped with a Deuterated Lanthanum α Alanine doped TriGlycine sulphate (DLATGS) detector (Bruker in Germany). By obtaining the spectra through FTIR transmission measurements, the functional groups and structural information of the fixed oil could be analyzed and identified [7].
Free fatty acid (FFA)
FFA analysis facilitated as an indicator of oil quality, freshness, and potential degradation, impacting both sensory attributes and shelf life. Sodium hydroxide (0.1 N) dissolved in neutralized alcohol was titrated against FO sample (1 g), where phenolphthalein was used as an indicator to determine the FFA (CA 5 A-40) [19].
Iodine value (IV)
IV evaluation was important in determining the degree of unsaturation, aiding in the assessment of oil stability, susceptibility to oxidation, and suitability for various applications. The samples (1 g) were dissolved in carbon tetrachloride, and 25 mL of Wij’s solution was added. During the reaction, the formation of iodine monochloride was treated with potassium iodide, which led to the liberation of iodine and titrated against 0.1 N of sodium thiosulphate solution using starch as an indicator (CD 1D-92) [19].
Saponification value (SV)
SV assessment was vital in measuring the average molecular weight of fatty acids, providing insights into the oil’s potential for saponification and its overall fatty acid composition. Saponification of FO sample (5 g) was determined by taking 50 mL of 5% ethanolic KOH solution connected to an air condenser heated until complete saponification of the oil. The samples were cooled and titrated against 0.5 N hydrochloric acid (HCl) using phenolphthalein as an indicator (CD 3–25) [19].
Unsaponifiable matter (USM)
The determination of unsaponifiable matter in oils was critical to identify and quantify non-saponifiable components, offering insights into the oil’s unique bioactive compounds, nutritional value, and potential therapeutic properties. Reflux of the sample (5 g) was performed using 50% KOH solution (5 mL) in the presence of ethanol (30 mL) until complete saponification of the oil. Petroleum ether was used to extract unsaponifiable matter, and 70% ethanol was used to wash the extract. The extract was dried and weighed (CA 6 A-40) [19].
Peroxide value (PV)
PV analysis was necessary in measuring the extent of peroxidation in oils that indicated the initial stage of oxidative rancidity. The samples (5 g) were titrated with 0.1 N sodium thiosulphate and saturated potassium iodide solution using starch as an indicator (CA 8–53) [19].
p-anisidine value (p-A value)
p-A V assessment was essential to understand the level of secondary oxidation products in oil, providing information about the degree of oxidation and the development of off-flavors. Schiff base formed due to the reaction of aldehydes carbonyl bond of the p-anisidine amine group was measured against isooctane containing p-anisidine at 350 nm to find p-A value [19].
Total oxidation (TOTOX) value
TOTOX value serves as a composite index considering both the peroxide value (PV) and the p-anisidine value (p-A value), providing a more comprehensive measure of the oxidative status of oil. The following equation by Wai et al., 2009, was used to find TOTOX value [19].
where, PV (peroxide value) and p-AV (p-anisidine value).
Statistical analysis
All analyses were performed in biological triplicates (n = 3). Data are presented as mean ± SD of independently prepared samples performed with MS-Excel. Significance between groups was determined by an independent t-test using Graphpad Prism. Significant differences between samples were labeled as follows: *p < 0.05 and ns as non significant.
Results and discussion
Fixed oil yield of fenugreek seed fractions
The fixed oil content of two fractions obtained by Soxhlet extraction and flash evaporator drying was determined as a percentage based on dry weight. The cotyledon fraction had a higher fixed oil content of 11.6 ± 0.69%, while the husk fraction had a lower fixed oil content of 2.625 ± 0.2% (Fig. 1). The combination of both fractions resulted in an overall yield of approximately 7%, which is in the range observed for the whole seed fixed oil. This indicates a higher yield of fixed oil in the cotyledon fraction compared to the husk fraction.
The husk, which makes up about 45% of the total seed, consists mainly of carbohydrates and fiber and contributes only minimally to the overall lipid content. In contrast, the cotyledon fraction, including the germ part, makes up about 55% of the seed and contains a considerable amount of lipids [8]. The fixed oil content of whole fenugreek seeds varies from region to region and from variety to variety. Reported values range from 5.8 to 15.2%, 8.4%, 7.5% and 3.25–6.88% [20,21,22,23].
Significant differences in the yield of fixed oil between the cotyledon and husk fractions highlight the husk as a poor supplier of fixed oil. The observed differences in fixed oil content can be attributed to factors such as genetic differences, varietal differences, growing conditions and extraction methods used in the various studies. This study emphasizes the importance of fractionation in the study of lipid composition and properties of different seeds and provides a comprehensive understanding of various factors influencing fraction-specific properties. These results contribute to the existing knowledge on the variability of fixed oil content in fenugreek seed fractions and emphasize the need for fractionation for detailed studies.
Lipid-class composition
The fenugreek seed fraction FO was further analyzed to determine its lipid class compositions. The composition of the extracted oils differed in terms of quantity and concentration between the different lipid classes (Fig. 2a). Of the three lipid classes analyzed (glycolipids, phospholipids and neutral lipids), neutral lipids were present in significantly higher concentrations in both the husk and cotyledon oils. This was followed by glycolipids in moderate amounts, while phospholipids were found in minimal amounts in the fractionated oils. In the case of husk fixed oil, the concentration of neutral lipids was the highest, while the phospholipids were negligible. On the other hand, the oil from the cotyledons had the highest concentration of glycolipids among the lipid classes studied. Higher neutral lipids, including triglycerides, in fenugreek fixed oils serve as energy stores and contribute to the nutritional profile of the oil, as revealed by the lipid class composition analysis. Fenugreek glycolipids and phospholipids contribute to the structural integrity of cell membranes and are involved in various cellular functions [24].
a Lipid composition of neutral lipids (NL), glycolipids (GL) and phospholipids (PL) among different fraction FO. Values are represented as mean ± SD of 3 independent experiments. b TLC profile of FO. TLC was run using solvent system petroleum ether: diethyl ether: acetic acid 80:20:1 (v/v/v) the bands were visualized in iodine vapours. TAG: triacylglycerol, DAG: diacylglycerol, SE: steryl ester, FFA: free fatty acids. c Fatty acid composition of fixed oil from different fractions (C14:0- myristic acid; C16:0- palmitic acid; C18:0- stearic acid; C18:1- oleic acid; C18:2- linoleic acid; C18:3- α-linolenic acid). Values are represented as mean ± SD of 3 independent experiments
In addition, the lipid classes present in the fenugreek seed oil samples were separated and identified using the TLC separation method. The analysis revealed the presence of several lipid classes, including TAG, DAG, SE and FFA. TLC analysis (Fig. 2b) revealed that both the cotyledon and husk FO contained all four lipid classes. However, the CT FO had a higher abundance of TAGs than the HK FO. The proportion of FFA was slightly higher in HK FO. Other important classes were SE, 1, 3 DAG and 1, 2 TAG. The differences in lipid class composition between fractions may have implications for their nutritional and functional properties, as different lipid classes may contribute differently to the overall composition and properties of the oils. The presence of DAG, SE and FFA in both fractions indicates the occurrence of partial hydrolysis and rearrangement reactions during the extraction and processing of the oils [6, 25].
Fatty acid composition
The oil extracted from the cotyledon and husk fractions was derivatized to form fatty acid methyl esters (FAMEs), which were subsequently analyzed by gas chromatography-mass spectrometry (GC-MS). GC-MS analysis revealed the presence of fatty acids with a length of 14 to 18 carbon lengths in both the cotyledons and husk fixed oils (Fig. 2c). The major fatty acids identified in both fractions included myristic acid (C14:0), palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2) and α-linolenic acid (C18:3), which are mainly known for their important role in human health and are considered essential fatty acids [26].
The analysis showed that husk fixed oil had a higher content of myristic acid (1.29 µmol %), palmitic acid (28.6 µmol %), stearic acid (5.55 µmol %) and linoleic acid (50.17 µmol %) compared to the oil from the cotyledon. Conversely, cotyledon fixed oil had higher levels of oleic acid (16.78 µmol %) and α-linolenic acid (15.28 µmol %). It is noteworthy that myristic acid was exclusively present in husk fixed oil, while the cotyledon fixed oil contained significantly higher amounts of essential fatty acids such as α-linolenic acid and oleic acid. Husk fixed oil had significantly more palmitic acid. In addition, the difference in concentration between stearic acid and linoleic acid was higher in husk fixed oil, although not statistically significant. These findings emphasize that fenugreek fixed oils are rich sources of essential fatty acids, especially linoleic acid and α-linolenic acid, which are associated with various health benefits and are used in the treatment of various diseases [27].
TAG molecular species fingerprinting by HRMS-ESI analysis
The TLC profile showed that TAGs are the major fraction in both fenugreek FOs. Plant oils exhibit a wide variety of molecular TAG species characterized by variations in fatty acid composition, including chain length and degree of saturation. To investigate the TAG composition of fenugreek FOs, a neutral loss (NL) scan was performed targeting specific neutral loss values corresponding to different fatty acid chains, such as NL245 (C14:0), NL273 (C16:0), NL301 (C18:0), NL299 (C18:1), NL297 (C18:2), and NL295 (C18:3) [28]. The analysis revealed important TAG molecules with carbon chain lengths between 52 and 54 (Table 1). These TAG species exhibited varying degrees of unsaturation, with a predominance of unsaturated molecules. Of note, one saturated TAG species, C54:0, was detected in CTFO. The major molecular TAG species identified in both CT and HK FO were C54:6 and C54:5. The prevalence of unsaturated fatty acids in the TAG molecules of fenugreek FO is significant because as unsaturated fatty acids are associated with various health benefits [29]. This analytical approach provides insights into the specific TAG composition of fenugreek FOs, highlighting the presence of diverse molecular species with varying chain lengths and degrees of saturation, contributing to the nutritional profile and potential health-promoting effects of these oils.
Determination of phytosterols and tocopherols
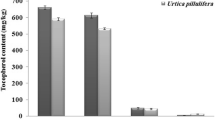
Phytosterols and tocopherols, integral components of the unsaponifiable portion of oils, are recognized for their functional properties and health benefits [30, 31]. β-sitosterol, a phytosterol found in fenugreek fixed oil (FO), is acknowledged for its cholesterol-lowering capabilities, while α-tocopherols, a form of vitamin E, play a crucial role in various bodily processes [32, 33]. The fenugreek FO analysis from husk and cotyledon fractions revealed significant amounts of phytosterols and tocopherols. HPLC was employed to separate and analyze these compounds, and the chromatograms depicting the separation of sterols and tocopherols from husk and cotyledon fixed oils are presented (Fig. S1 and S2). Among phytosterols, cotyledon FO exhibited significantly higher levels of ergosterol and β-sitosterol than husk FO, while other sterols were higher in husk FO. Squalene, though not a significant phytosterol, was also detected, showcasing fenugreek FO as a plant source of this compound [34]. Concerning tocopherols, cotyledon FO contained significantly higher levels of α-tocopherol, while other tocopherols were higher in husk FO. Detailed concentrations of these compounds can be found in Table 2.
Phytosterols like β-sitosterol and tocopherols like α-tocopherol in fenugreek FO highlight their functional properties and potential health benefits. Phytosterols, known for their cholesterol-lowering effects, contribute to cardiovascular health. Tocopherols, recognized for their antioxidant properties, are vital in safeguarding cells from oxidative damage [35].
Total phenolic content (TPC) and anti-oxidant activity
Total phenolic content
The quantification of TPC in plant seed oil is a crucial determinant of oil quality, with phenolic compounds playing a pivotal role in free radical scavenging and lipid peroxidation [36]. In this study, the Folin-Ciocalteu assay, utilizing Gallic acid as a standard, was employed to ascertain the TPC in fenugreek seed fixed oil (FO). The phenolic content was 19.12 ± 0.23 mg/g in the husk and 16.53 ± 0.61 mg/g in the cotyledon, expressed as Gallic acid equivalents. This substantial phenolic content underscores the significant antioxidant activity of the oil attributed to the presence of phenolic components. Comparatively, both fractions of the fixed oil exhibited nearly similar amounts of polyphenols, with the husk fraction displaying a slightly higher concentration. This correlation aligns with the reported antioxidant activity of the husk and cotyledon, as documented previously [8]. The observed variations in phenolic content between the husk and cotyledon fractions contribute to understanding the antioxidant potential and overall quality of fenugreek seed fixed oil. The study highlights the importance of phenolic compounds as critical contributors to the antioxidant capacity of the oil, emphasizing their role in promoting health benefits associated with free radical scavenging [36].
Anti-oxidant activity
The assessment of potential antioxidant activity in this study employed three crucial assays. The observed antioxidant effects of plant extracts on DPPH radical scavenging are likely attributed to their hydrogen-donating abilities, which reduce the stable violet DPPH radical to the yellow DPPH-H. The degree of discoloration in the sample or extract indicates the potential free radical scavenging capacity [36]. In this investigation, the inhibition concentration (IC50) of fenugreek seed fixed oil (FO) was determined through the DPPH assay, revealing values of 7.2 ± 3.1 mg/mL in the husk and 9.86 ± 0.59 mg/mL in the cotyledon. The FO derived from husk exhibits notable antioxidant activity, possibly attributed to palmitic acid and tocopherols. The antioxidant capacity of the fraction oil was further evaluated using the ABTS assay, yielding IC50 values of 35 ± 2.21 mg/mL in the husk and 41.42 ± 1.51 mg/mL in the cotyledon. Both DPPH and ABTS assays significantly reduced proton free radicals when exposed to proton radical scavengers. Total antioxidant capacity was calculated and expressed as Fe(II) equivalents. Both fractions’ ferric-reducing antioxidant power was determined as 2.36 ± 0.01 M and 1.88 ± 0.01 M of Trolox equivalent per 100 g in the husk and cotyledon, respectively. The findings from the three assays collectively indicate promising antioxidant activity in both fractions of the fixed oil. This activity is associated with the potential to promote radical scavenging and the reduction of ferric ions. The elevated antioxidant activity aligns with the inherent phenolic compounds in fractions, as demonstrated in this study. The overall phenolic content and antioxidant activity were comparable in both fractions, with the husk samples exhibiting a higher edge over the cotyledon samples. The results indicated a positive relevance between the antioxidant activity of the oil and its total phenolic contents. This observation is consistent with the previously studied higher antioxidant activity in husk fractions compared to cotyledon samples [8]. The results underscore the potential health-promoting benefits of the antioxidant-rich components in fenugreek seed fractions.
Color, optical rotation and refractive index
The color characteristics of the extracted fixed oils (FO) from husk and cotyledon were evaluated using color measurement spectroscopy, and the results are presented in Table. 3. The color parameters include L* (lightness/darkness), a* (red-green color), b* (blue-yellow color), and dEab (overall color difference between a* and b* values). Both husk and cotyledon FOs exhibited high L* values, indicating lightness in color. Positive a* values suggest a reddish color, while positive b* values indicate a yellowish color in both samples. The dEab value reflects the total color difference between the red-green and blue-yellow components. Husk FO appeared darker with a brownish tinge, while cotyledon FO appeared reddish-yellow.
Optical rotation, crucial for detecting adulteration and added compounds, and refractive index, indicative of possible rancidity due to oxidation, were measured. The refractive index values for both fraction oils fell within the range of 1.4 to 1.5, signifying acceptable values for these properties [37]. Detailed values for these parameters can be found in Table 3.
Thermal stability analysis
DSC is a rapid and highly sensitive technique employed to measure enthalpy changes associated with alterations in the physical and chemical properties of oils as a function of temperature or time. The thermograms obtained for husk and cotyledon fixed oils (FO) using DSC (Fig. 3) provide valuable insights into their thermal stability. Thermograms are instrumental in evaluating the stability of various materials, particularly in industrial processes. The husk and cotyledon FO samples exhibit distinct patterns in terms of peak characteristics. The husk FO demonstrates a higher melting point at 203.11 °C, contrasting with the 185.96 °C observed for cotyledon FO, indicating a difference of ± 15 °C. These melting points suggest the presence of saturated fatty acids and high-melting glycerolipids in both FO samples. The observed transition in the thermogram is endothermic, signifying heat absorption during the decomposition process. Furthermore, the crystallization of husk FO occurs at a higher cooling point of -28.63 °C compared to -10.84 °C for cotyledon FO. Additional details of crystallization include onset temperatures of -16.13 °C and − 5.88 °C, offset temperatures of -36 °C and − 20 °C, enthalpy changes (ΔH) of -13.47 and − 4.7, peak heights of -0.33 and − 0.12, and peak areas of -29.64 and − 10.58 for husk and cotyledon FO, respectively. These parameters collectively reflect the crystallization behavior during cooling. Both FO samples are rich in unsaturated fatty acids, contributing to their high crystallization points during cooling [3, 6]. Variations in thermal properties are due to differences in their fatty acid composition, degree of saturation, and molecular structure. Variations in the proportion of saturated and unsaturated fats, presence of specific fatty acids, and the arrangement of triglycerides influence the melting and crystallization behaviors of oils, resulting in distinct thermal profiles. The DSC analysis provides a comprehensive understanding of the thermal characteristics of husk and cotyledon FO, crucial for applications in various industrial processes. The details of these peaks, such as their temperatures and specific transitions, are described in the figure.
FTIR spectroscopy analysis
The functional groups present in fenugreek seed FO were analyzed using FTIR spectroscopy. The symmetric and asymmetric stretching of methylene (-CH2) and methyl (-CH3) groups, associated with carboxyl groups (COOH) in fatty acids and methyl esters, were observed in the spectral range of 2854–3010 cm− 1. The presence of saturated fat aldehyde was identified at approximately 1743–1744 cm− 1. This characteristic peak indicates the presence of aldehydes derived from saturated fats. The peak at 1461 cm− 1 corresponds to the presence of alcoholic (C-OH) groups in the FO. This peak represents the stretching vibration of the carbon-oxygen bond in alcohols. The amide groups, phenolic compounds (C-OH), and aromatic acid esters (C-O-C) were observed in the spectral range of 1101 − 1099 cm− 1 to 1238–1242 cm− 1. These peaks indicate the presence of amides, phenolic compounds, and esters containing aromatic acid groups in the FO. The presence of a benzene ring was identified at 722–723 cm− 1. This peak indicates the presence of aromatic compounds, possibly derived from phenolic compounds or other aromatic structure [5]. The analysis of the fixed oil (FO) revealed the presence of various functional groups, including amides, alcohols, hydroxyl groups, carboxyl groups, and phenolic compounds, as illustrated (Fig. S3). The spectra of the two FO fractions exhibit high alignment, with variations in wavenumber of approximately ± 5 among all functional groups. This spectroscopic analysis provides valuable insights into the chemical composition of the FO, highlighting distinctive functional groups present in the husk and cotyledon fractions.
Chemical characterization
The chemical properties of the fractionated FO were analyzed to assess the quality and characteristics of the oil. These properties play a crucial role in determining the overall quality and stability of the oil during processing, storage, and preservation. The free fatty acid (FFA) value indicates the quality of the raw source material and the purity of the oil during processing and storage. A higher FFA value may indicate lower quality or degradation of the oil [38]. Acid value among fraction FO did not vary significantly. The peroxide value (PV) is a measure of oxidation in the oil and can indicate the quality and shelf life of the oil. Higher PV values may suggest increased oxidation and potential deterioration of the oil [39, 40]. PV of both fraction FO did not vary much and were considerably low, indicating resistance to oxidation and hence have a longer shelf life. The p-anisidine value (p-AV) measures secondary oxidation products that can contribute to off-flavors and odors in the oil. It reflects the stability of the oil at higher temperatures and its susceptibility to oxidative degradation. The values obtained should be lower than 30 for better quality [41]. The values obtained for fraction FO of both are within this range, depicting resistance to off-flavors and degradation. The TOTOX value was calculated using both the p-AV and PV values and provided an overall measure of the total oxidation in the oil. A higher TOTOX value indicates a higher degree of oxidation. Overall, HK FO has significantly better oxidation resistance than CT FO [37]. The iodine value (IV) measures unsaturation in the oil. Oils with higher IV values contain more unsaturated fatty acids, which can impact their stability and susceptibility to oxidation [42]. The samples from HK and CT FO had significant differences in the IV values, and higher values indicate highly unsaturated fatty acids, which are also prone to fast degradation reactions such as auto-oxidation and polymerization.
The saponification value (SV) reflects the average molecular weight and length of the fatty acid chains present in the oil. It provides information about the composition and characteristics of the oil [39]. HT FO had significantly higher SV than CT FO, indicating higher medium-chain fatty acids and low impurities in the FOs. The unsaponifiable matter (USM) in oils consists of non-saponifiable compounds, including antioxidant compounds, oil-soluble vitamins and phytosterols [43]. USM content can contribute to the oil’s overall quality and health benefits. Fenugreek FO has higher USM values than other oils, indicating health-promoting effects. Table 4 provides an overview of the different chemical properties of the fenugreek fraction fixed oils. The cotyledon oil exhibited higher values for IV, USM, p-AV, and TOTOX, indicating higher unsaturation, antioxidant content, and lower oxidation stability. On the other hand, the husk oil had higher values for FFA, SV, and PV, suggesting higher acidity, molecular weight, and oxidative stability. These parameters provide valuable insights into the quality, stability, and characteristics of the fenugreek fraction fixed oils and can guide their utilization in various applications.
Conclusion
In conclusion, our study comprehensively analyzed the fixed oil (FO) extracted from husk and cotyledon fractions of fenugreek seeds obtained through selective extraction. Comparative assessments revealed that the cotyledon fraction (CT) exhibited a higher yield of FO than the husk fraction (HK). Fatty acid profiling demonstrated elevated levels of linoleic acid in the HK fraction, while linolenic acid predominated in the CT fraction. Additionally, both fractions demonstrated enriched sterols and tocopherols, particularly CT FO, in concentrations of β-sitosterol and α-tocopherol, respectively, emphasizing their potential health-promoting properties. Analysis of lipid classes indicated a prevalence of neutral lipids, followed by glycolipids, whereas only CT FO contained higher phospholipid content. TLC analysis showed a higher concentration of TAGs, which was further analyzed to contain higher unsaturated species. Other properties, such as the thermal stability of HK and CT FO, were attributed to an increased concentration of fatty acids, mainly oleic acid. FTIR analysis revealed minimal differences among samples. Polyphenol analysis and antioxidant activity assessments indicated superior antioxidant capabilities in HK FO. Our findings underscore the potential of the fixed oil obtained as a by-product during the selective extraction of fenugreek seeds for diverse industrial applications. Utilizing this by-product facilitates effective resource management, minimizing waste, and maximizes the utilization of available resources. In summary, this study highlights the intrinsic value and versatility of fraction fixed oils from fenugreek seeds, advocating for their incorporation as functional ingredients in various industries and promoting sustainable utilization of by-products.
Abbreviations
- ABTS- 2:
-
2′-azino-bis (3-ethyl benzo thiazoline-6-sulphonic acid
- AOCS:
-
American oil chemists society
- BF3 :
-
Boron trifluoride
- DAG:
-
Diacylglycerol
- DLATGS:
-
Deuterated L-alanine doped triglycene sulphate
- DPPH:
-
1,1-diphenyl-2-picryl hydrazyl
- DSC:
-
Differential Scanning Calorimeter
- FAME:
-
Fatty acid methyl esters
- FC:
-
Folin-Ciocalteu
- FFA:
-
Free fatty acid
- FRAP:
-
Ferric reducing antioxidant power
- FO:
-
Fixed oil
- FTIR:
-
Fourier transform infrared
- GC-MS:
-
Gas Chromatography-Mass Spectrometry
- GL:
-
Glycolipid
- HPLC:
-
High-performance liquid chromatography
- HR-MS ESI:
-
High-resolution mass spectroscopy electrospray ionization
- IC50 :
-
Inhibition concentration
- IV:
-
Iodine value
- NL:
-
Neutral lipid
- OD:
-
Optical density
- PDA:
-
Photodiode array
- PL:
-
Phospholipid
- p-A:
-
Value- p-anisidine Value
- PV:
-
Peroxide value
- RT:
-
Room temperature
- SV:
-
Saponification value
- SE:
-
Steryl ester
- TLC:
-
Thin-layer chromatography
- TPC:
-
Total phenolic content
- TAG:
-
Triacylglycerol
- TPTZ:
-
2,4,6-tripyridyl-triazine
- USM:
-
Unsaponifiable matter
References
U.M. Srinivasa, M.M. Naidu, Studies in Natural Product Chemistry, Vol. 71 (Eds. Atta-ur-Rahman) Elsevier Ch.6 (2021), p. 141-184K
J. Żuk-Gołaszewska, Wierzbowska, J. Elementology. 22, 3 (2017)
K. Hamden, H. Keskes, S. Belhaj, K. Mnafgui, N. Allouche, Lipids Health Dis. 10, 1 (2011)
M.A. Al-Sebaeai, M. Alfawaz, A.K. Chauhan, A. Al-Farga, S. Fatma, Int. J. Food Sci. Nutr. 6, 52 (2017)
V. Manasa, S.R. Chaudhari, A.W. Tumaney, RSC Adv. 10, 43975 (2020)
V. Manasa, S.R. Vaishnav, A.W. Tumaney, J. Food Sci. Technol. 12 (2020)
U.M. Srinivasa, M.M. Naidu, J. Sci. Food Agric. 11, 4751 (2021)
M.M. Naidu, B.N. Shyamala, J.P. Naik, G. Sulochanamma, P. Srinivas, LWT 44, 451 (2011)
V. Venkateshwari, A. Vijayakumar, A.K. Vijayakumar, L.P.A. Reddy, M. Srinivasan, R. Rajasekharan, Planta. 248, 347 (2018)
P. Daga, S.R. Vaishnav, A. Dalmia, A.W. Tumaney, J. Food Sci. Tech. 1 (2021)
P.K. Yadav, R. Rajasekharan, Mol. Cell. Biochem. 434, 89 (2017)
D.I. Sanchez-Machado, J. Lopez-Herna´ndez, P. Paseiro-Losada, J. Lopez Cervantes Biomed. Chromatogr. 18, 183 (2004)
B.T. Diwakar, P.K. Dutta, B.R. Lokesh, K.A. Naidu, J. Am. Oil Chem. Soc. 87, 539 (2010)
P. Shah, H.A. Modi, Int. J. Res. Appl. Sci. Eng. Technol. 3(6), 636–641 (2015)
D. Sreeramulu, M. Raghunath, Food Nutr. Sci. 02, 422 (2011)
S. Dande, R. Manchala, FNS,. (2011)
I.F.F. Benzie, J.J. Strain, Methods Enzymol. 299, 15 (1999)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Free Radic Biol. Med. 26, 1231 (1999)
AOCS, (2003)
A.M.E. Sulieman, A.O. Ali, J. Hemavathy, Int. Food Sci. Technol. 43, 380 (2008)
J. Hemavathy, J.V. Prabhakar, Food Chem. 31, 1 (1989)
M. Arivalagan, K.K. Gangopadhyay, G. Kumar, Indian J. Pharm. Sci. 75, 110 (2013)
O.N. Ciftci, R. Przybylski, M. Rudzinska, S. Acharya, J. Am. Oil Chem. Soc. 88, 1603 (2011)
S. Asokapandian, S. Sreelakshmi, G. Rajamanickam, Food biopolymers: Structural, functional and nutraceutical properties. 389–411 (2021)
S.K. Lo, C.P. Tan, K. Long, M.S.A. Yusoff, O.M. Lai, Food Bioprocess. Tech. 1, 223–233 (2008)
V.S. Shramko, Y.V. Polonskaya, E.V. Kashtanova, E.M. Stakhneva, Y.I. Ragino, Biomolecules. 10, 1127 (2020)
A.P. Simopoulos, Am. J. Clin. Nutri. 70, 560s–569s (1999)
L.B. Gu, X.N. Liu, H.M. Liu, H.L. Pang, G.Y. Qin, Molecules. 22, 228 (2017)
J. Lunn, H.E. Theobald, Nutr. Bull. 31, 178–224 (2006)
E.A. Trautwein, I. Demonty, Oléagineux, Corps Gras Lipides. 14, 259–266 (2007)
F. Shahidi, A.C. De Camargo, Int. J. Mol. Sci. 17, 1745 (2016)
D.W. Peterson, Exp. Biol. Med. 78, 143 (1951)
L. Packer, Am. J. Clin. Nutr. 53, 1050S (1991)
V.K.C. Nagulapalli, A. Swaroop, D. Bagchi, A. Bishayee, Mol. Nut Food Res. 61, 1600950 (2017)
Y. Wu, W. Yuan, X. Han, J. Hu, L. Yin, Z. Lv, Ind. Crops Prod. 154, 112655 (2020)
S. Akbari, N.H. Abdurahman, R.M. Yunus, O.R. Alara, O.O. Abayomi, Extraction, characterization and antioxidant activity of fenugreek (Trigonella-Foenum Graecum) seed oil. Mat. Sci. Energy Tech. 2, 349–355 (2019)
A. Bayrak, A. Akgül, J. Sci. Food Agri. 64, 441–448 (1994)
V.M. Paradiso, T. Gomes, R. Nasti, F. Caponio, C. Summo, Food Res. Int. 43, 1389–1394 (2010)
A.I. Alajtal, F.E. Sherami, M.A. Elbagermi, J. Mater. 4, 43–47 (2018)
J.W. Irwin, N. Hedges, Understanding and measuring the shelf-life of food. 289–316 (2004) Vol. 1 (Eds. R Steele) Woodhead publishing Ch.13 (2004), p. 289–311
J. Cao, L. Deng, X.M. Zhu, Y. Fan, J.N. Hu, J. Li, Z.Y. Deng, J. Agri Food Chem. 62, 12545–12552 (2014)
D. Firestone, J. AOAC Int. 77, 674–676 (1994)
S. Punia, M. Kumar, A.K. Siroha, S.S. Purewal, Rice Sci. 28, 217–232 (2021)
Acknowledgements
We acknowledge Director, CSIR-CFTRI Mysuru, India, for her support and encouragement. Ms. Uma Maheshwari S is thankful to Department of Biotechnology, New Delhi for her Junior Research Fellowship.
Funding
This work was supported and funded by the CSIR-CFTRI, Mysuru and Department of Biotechnology, Government of India, under GAP-0462 project.
Author information
Authors and Affiliations
Contributions
UMS and AD have done the investigation of the experiments; AWT and MMN have supervised and funded the work; Manuscript was written by UMS.
Corresponding author
Ethics declarations
Consent for publication
All the authors approved the consent for publishing the manuscript in Journal of food measurement and characterization and if accepted for publication, it will not be published elsewhere in the same form or in any other language.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srinivasa, U.M., Dalmia, A., Tumaney, A.W. et al. Exploring the lipidome, nutraceutical profile, anti-oxidant activity and physico-chemical properties of fixed oil derived from fenugreek (Trigonella foenum-graecum L.) seed fractions: a comparative analysis. Food Measure 18, 4388–4401 (2024). https://doi.org/10.1007/s11694-024-02501-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02501-1