Abstract
Kefir grain is a group of microorganisms in symbiosis, mainly yeasts and lactic acid bacteria (LAB), which stands out as a carrier of functional components and suitable option for enrichment. In present study, kefir as a functional beverage including purslane (Portulaca oleracea L.) seed oil at the different levels (0, 0.5, 1 and 1.5%) is produced. A reduction in pH and elevated acidity were detected significantly in all treatments during 1, 7, 14 and 21 days of shelf life. The higher concentration of seed oil induced more antioxidant capacity and total phenolic content. The elevated survival and activity of LAB were found by adding Portulaca oleracea L. seed oil; however, a reverse trend was observed on extended shelf life. The highest and lowest percentages of hydrophobicity are related to population for Lactobacillus spp. and Bifidiobacterium spp. isolated from treatment containing 1% Portulaca oleracea L. seed oil (72.4%) and control (35.1%) on the 1st and 21st day of shelf life, respectively. The sensory assessment outlined that panelists assigned the closest score to treatment with 0.5% of seed oil in contrast to control. Portulaca oleracea L. seed oil was extracted and fatty acid profiles were assessed by gas chromatography technique, which detected more concentration in treatment including 1.5% Portulaca oleracea L. seed oil compared to others. Also, fermentation procedure increased the conjugated linoleic acid in oil presence; as a conclusion, the potential application of enrichment kefir beverage has attracted intense attention for production in food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A functional product is consumed in a habitual diet, which supplies health benefits or avoidance of disease and physiological improvements beyond traditionally related to nutrients [1]. Nowadays, beneficial products with probiotic microorganisms and functional organic components have received an intense attention [2]. Lactic-alcoholic fermentation of milk with kefir grains or a starter culture produced fermented beverage such as kefir, which has abundant health promotions due to its anti‐diabetic, anti-inflammatory, antimicrobial attributes and can adjust the gut microbiota [3]. Lactic acid bacteria (LAB) as probiotic bacteria in kefir produce antimicrobial constituents such as organic acids, antimicrobial peptides, lantibiotics, hydrogen peroxide and bacteriocins. Also, LAB cause the control of pathogenic agents by reducing competition for nutrients, antagonistic activation and adhesion prevention of pathogens to intestinal wall [1, 4]. Hydrophobicity characteristic affects the adhesion of probiotics to intestinal membrane and probiotic efficiency improves by increasing this factor [2]. Kefir grains are considered as irregular granules with distinct size in diameter from 3 to 35 mm, which are applied to produce ancient beverage obtained from Western and Central Asia by fermented milk [5, 6].
There is an enormous attraction for functional product with beneficial improvements in different countries all over the world [4, 7]. Symbiotic correlations are observed among yeasts, acetic acid and LAB, which influence flavor perception and quality for kefir production [8]. The protein content (2.7%), lactic acid (0.6%) and fat level (< 10%) related to milk are observed in kefir [7, 8].
Regarding to previous researches, the nutritional status of kefir could be improved by applying distinct components such as Linum usitatissimum L. flaxseed (1% w/v), Rosmarinum officinalis L. essential oil (0.15% w/v) and hazelnut milk (25–50–75% w/v), which positively affected on hypoglycemic, anti-inflammatory, antioxidant activities and microbial survival [9, 10].
Purslane (Portulaca oleracea L.) is applied as a beneficial oil for cardiovascular diseases, because is rich in α-linolenic acid and ß-carotene as well as in some clinical researches for its hepatoprotective and hypolipidemic effects has higher volatile terpenoids and phenol components [11]. Portulaca oleracea L. seed oil has saturated fatty acids (SFA), monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA) such as palmitic acid, stearic acid, linoleic acid, oleic acid specifically alpha-linolenicacid and less omega-6/omega-3 ratio < 2 [11, 12]. It is a prominent factor for acceptable equilibrium of mentioned fatty acids (FAs) in human body systems and has vitamin [11].
The tocopherols as natural antioxidants can neutralize free radicals and assist to the avoidance of correlated disorder [13]. Not many researches have been performed on Portulaca oleracea L. application in the food industry such as its frozen powder to replace with soy protein in bread [12] and extract in yogurt during 21 days [14]. Conjugated linoleic acids (CLAs-conjugated linoleic acids) are produced by the fermentation of linoleic acid with probiotic bacteria and also, these FAs are found naturally in milk and meat [15].
The fermented milk with adding pro-healthy components is attracted much attention due to fat including suitable n-3 and n-6 FAs; as a result, vegetable oils are employed for enriching products with essential FAs [12]. The present research aims to explore the impact of Portulaca oleracea L. seed oil in kefir beverages with 0, 0.5, 1 and 1.5% concentrations on some physicochemical attributes (pH, titratable acidity and color), biochemical characteristic (total phenolic contents (TPC) and antioxidant activity), survival microorganisms, hydrophobicity of kefir microorganisms, sensory stimulation (flavor, color, odor, texture and overall acceptance) and FA profiles of fortified kefir.
Materials and methods
Cow milk (whole milk) with 2.5% fat was purchased from Kalleh Dairy Co. (Iran, Amol) as well as Kefir grains as a starter culture was attained from household in Tehran, Iran and glass bottles were applied to maintain the produced functional beverage of kefir.
Sodium hydroxide was obtained from Fluka Co. (United States of America) and other chemical materials including sulfuric acid, isoamyl alcohol, phenolphthalein, sodium sulfate, methanol, hexane and MRS media (De Man, Rogosa and Sharpe agar) were obtained from Merck Co (Germany). Fatty acid methyl ester (FAME) standards were achieved from Sigma (Supelco™ 37 component FAME Mix, Oakville, ON, Canada).
Portulaca oleracea L. seed oil extraction
The seeds were obtained by the Iranian Agriculture Research Center in Karaj, Iran. Since germination enhances unsaturated fatty acids (USFA) and reduces SFA of seed, this procedure was done before oiling [16]. After germination, seeds were mixed by 5% NaClO (about 5 min), after that washed and maintained in sterile water for 4 h. Afterwards, they were dried at 50 °C during 12 h in an oven and then crushed into powder by a grinder with a size range of 0.55 to 1.0 mm. Crude oil was achieved from dried seeds using solvent hexane (1:10, w/v) at 70 °C and for 6 h by a Soxhlet apparatus [11].
Preparation of kefir beverage treatments
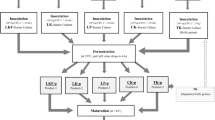
The pasteurized milk was cooled to 22 °C, inoculated with kefir grains at 3% (w/v) and mixed for 15 min. Then, Portulaca oleracea L. seed oil was added at distinct concentrations (0, 0.5, 1 and 1.5%) to inoculate milk. After the oil addition, milk was homogenized 3 times in Armfield Ltd. Ringwood (England) homogenizer under a pressure of 7 MPa. The obtained treatments were packaged in glass bottles and incubated at 25 °C until reaching the desired acidity and then transferred to 4 °C. The treatments were expressed as CK, K1, K2 and K3 for 0, 0.5, 1 and 1.5% of Portulaca oleracea L. seed oil, respectively and the treatments were assayed at 1, 7, 14 and 21 days of shelf life.
Physicochemical attributes
The pH was potentiometrically measured according to AOAC (2000) using a pH meter (Seven Compact pH/Ion S220, Schwerzenbach, Switzerland), acidity was expressed as g/100 mL in anhydrous lactic acid, which were determined [18]. Color values were evaluated using a Konica Minolta CR1 10 colorimeter (Konica Minolta Solutions Ltd., Basildon, UK). The determinations were carried out according to non-natural light to minimize daylight impacts and also color factors L* (lightness), a* (red/greenness) and b* (yellow/blueness) of kefir beverages were investigated regarding to the International Commission on Illumination [19].
DPPH radical scavenging potential
The antioxidant attribute was described by measuring the scavenging stability of free radical DPPH (2,2-diphenyl-1-picryl hydrazyl). In present method, 100 μL treatment was blended with 3.9 mL DPPH (0.0227 g/L methanol) and maintained in darkness at 25 ºC during 2 h. Methanol was applied as a blank and after centrifugation (10 min at 1400×g), the solution absorbance was detected at 517 nm using the spectrophotometer. The scavenging of DPPH free radical (%) was determined through Eq. 1:
Absorption values for blank and treated samples were expressed as A0 and A1, respectively [12].
TPC assessment
The TPC of kefir beverages were measured using Folin-Ciocalteu reagent as a standard. Briefly, 1 mL for each treatment was added to 6 mL deionized water and 0.5 mL of 1 N Folin-Ciocalteu phenol reagent; then, the mixture was allowed to stand for 5 min and 1 mL sodium carbonate was added. After 1 h in darkness, the absorbance was calculated at 725 nm by spectrophotometry (Thermo Scientific, Madison, WI, USA) and the equation of total phenolic standard curve (as Gallic acid equivalents, mg/g) was plotted [4].
Viable count measurement
An active isolate culture was first prepared and then 200 µL was added to 4 mL of MRS culture medium. After maximum serial dilution, Lactobacillus spp. (MRS culture at 37 °C for 72 h), Lactococcus spp. (MR17 medium at 20 °C during 120 h) and Bifidiobacterium spp. (MRS-NNLP (nalidixic acid, neomycin sulfate, lithium chloride and paromomycin sulfate) at 37 °C about 72 h) were counted and log of colony forming units (CFU) was determined in kefir treatments. Viable yeast counts were calculated as log CFU count on agar medium, which included 0.5% yeast, 1% glucose, 1.5% agar and 0.5% polypeptone, before counting, agar plates were incubated at 30 °C during 48 h [20].
Hydrophobicity assay of kefir strains
Probiotic microorganisms must be adhere to the intestinal wall in order to function properly in the gastrointestinal tract and the assessment of hydrophobicity was performed. Initially, the dilution of kefir sample was performed and incubated using a pour plate technique. Then, Lactobacillus spp. (MRS culture at 37 °C about 72 h), Lactococcus spp. (MR17 medium at 20 °C during 120 h) and Bifidiobacterium spp. (MRS-NNLP at 37 °C for 72 h) were cultured until the optical density (OD0) reached 0.6 to 0.7. The 3 mL microbial suspension was added to 1 mL n-hexadecane and incubation was done at 15 min. The tubes containing the treatments were vortexed for 3 min, incubated about 30 min at room temperature and finally OD was measured. The hydrophobicity assay was calculated by Eq. 2:
where OD0 and OD are related to initial and final absorption rate of sample, respectively [21].
Sensory assessment
Sensorial evaluation of kefir beverages was conducted by 22 trained panelists (equal male and female) and treatments were randomly served in transparent glass cups for assessors, after that they reported their feelings by a score scale. A hedonic scales were ranged from disliked extremely (1) to liked extremely (5), which applied to investigate appearance, flavor, consistency, odor and overall acceptability [22].
FA evaluation using gas chromatography flame ionization detector (GC-FID)
The FA components were investigated using GC-FID (CP-3800 GC device, Varian, Walton-on Thames, UK) on the 1st and 21th days of storage. At first, kefir fat was separated, then 30 mg of isolated oil were blended with 5% (v/v) sulphuric acid (2 mL) and methanol in a glass tube. Triheptadecanoin (C17:0) and toluene (300 μL) were applied as a standard; afterwards, mixture was heated and cooled at 80 °C during 95 min as well as 0.9% NaCl (3 mL) and hexane (2 mL) were added to FAME. After centrifugation, the organic component was placed into a GC and FAMEs were assessed on a Varian GC-450 system with auto samplers, FID detector and a capillary RTX column (25 m × 0.25 mm ID, 0.25 μm thickness) and also Excalibur software (Fisons) was applied. Treatments were run at a flow rate (1.5 mL/min) with helium (with 99.99% purity) as a carrier gas as well as injector and detector temperatures (260 °C). The temperature of oven was changed from 150 to 240 °C about 3 and 10 min, respectively; after that, treatments were maintained at this situation and FAME standards were injected individually for peak detection [23].
Statistical analysis
Test was performed by three repetitions with mean and standard deviations; therefore, 0, 0.5, 1 and 1.5% Portulaca oleracea L. seed oil were evaluated on 1, 7, 14 and 21 days of storage, respectively. Duncan's multiple test was done at 0.05 level and statistical analysis was conducted using Minitab software Version 15.
Results and discussion
Physicochemical aspects
The pH value of kefir beverages decreased and titratable acidity increased during incubation time (p < 0.05). These results implied that changes in pH and titratable acidity were correlated with each other and pH of kefir beverages is reduced during 21 days of storage (Fig. 1a). The highest and lowest pH were obtained for K3 (4.31) and CK (4.02) on 1st and 21st days, respectively. It should be noted that no significant difference was observed between treatments on 14th and 21st days (p > 0.05). However, the pH of samples enriched by oil is longer than control without additive during whole shelf life, so this factor was enhanced using adding oil. Titratable acidity of kefir beverages produced from milk fortified with Portulaca oleracea L. seed oil is given in Fig. 1b. The highest acidity for CK (87.96°D) and lowest value in K3 (74.89°D) were recorded on 21st and production days, respectively. Also acidity increased significantly (p < 0.05) during storage and CK and K1 indicated the highest level (p < 0.05).
pH (1a) and acidity values (1b) of kefir beverages supplemented with different purslane seed oils (CK: 0%, K1: 0.5%, K2: 1% and K3: 1.5%) on 1, 7, 14 and 21 days of storage (a to b indicates a significant difference between different samples (p < 0.05). Small letters have been used for comparison of times in each treatment (row) and capital letters have been used for comparison of treatments in each times (column) (p < 0.05).
These findings illustrated that fermentation procedure was occurred using the production of acids. The pH values for kefir beverages were slightly higher with seed oil because the microbial inhibition effect [24]. The changes in pH and acidity are mainly associated with the production of certain organic acids, ethanol, CO2 and other volatile components, which caused by microbial population in kefir beverages [3].
In line with results of present study, a pH range was diminished from 4.31 to 4.24 for kefir beverage by adding 10% black mulberry, pomegranate and strawberry during shelf life [25] as well as 4.34 to 4.25 in fortified kefir with walnut and Camelina sativa oil [20]. However, the pH was increased from 4.31 to 4.36 using 10 to 25% black carrot in kefir beverage during 0 to 12 weeks of storage [25] which was in contrast to present research. The acidity was lowered by adding and 3% (w/w) honey [10] as well as increased by using 2% (w/v) brown lentils [26] and 15% (w/w) pomegranate juice [27] in kefir beverage, which were similar to present study.
The L*, a* and b* parameters for color are investigated on 1, 7, 14 and 21 days of shelf life, which portrayed in Table 1. All color factors were affected by supplementation of Portulaca oleracea L. seed oil and also highest and lowest values for L* were about 74.16 and 52.63 in CK (1st day) and K3 (21st day), respectively; on the contrary, a* and b* values were elevated significantly by adding seed oil (p < 0.05). The highest a* (− 0.78) and b* (11.03) were achieved for K3 at the 21st day and these factors were significantly influenced due to anthocyanins of seed oil (p < 0.05). The addition of Portulaca oleracea L. seed oil has significantly darkened the kefir color and reduced its brightness. Storage time until the 14th day had no significant effect on L* factor, but it changed significantly at the 21st day. The control had higher L* compared to the other treatments, which was associated with casein micelles and its role in light reflection. In general, there is a significant difference between the color factors of distinct treatments and the reduction in color quality is caused by Portulaca oleracea L. color, which is dark yellow or light brown and its interior is light yellow [28]. The reduction in L* and enhanced a* were observed by adding 15% (w/w) pomegranate juice [27] and 30% (w/v) honey [10] in treatments of kefir beverages.
Biochemical evaluation of kefir beverages
The biological attributes of treatments are outlined in Fig. 2 and antioxidant activity was elevated by adding seed oil such as K2 and K3, which was in contrast to extended fermentation. The highest capacity of antioxidant was found the highest levels (72.12%) for K3 on 1st day and the lowest (29.78%) in control on 21st day.
An antioxidant activity and TPC of kefir beverages supplemented with purslane seed oils (CK: 0%, K1: 0.5%, K2: 1% and K3: 1.5%) on 1, 7, 14 and 21 days of storage. Small letters have been used for comparison of times in each treatment (row) and capital letters have been used for comparison of treatments in each times (column) (p < 0.05).
Milk and its derivative products have antioxidants such as lactoferrin, beta lactoglobulinand bovin serum albumin [24]. This study demonstrated that kefir supplemented with Portulaca oleracea L. had high antioxidant activity which was consistent with previous findings [24, 29]. The major active compositions of Portulaca oleracea L. are beta-carotene, alpha-tocopherol, phenolics, ascorbic acid and glutathione [30]. The produced polystyrene (predominantly kefiran) with LAB had distinct functional groups (–COOH, –OH and –CO), which could react with free radicals and finally quench [3]. This benefit of kefiran and expanded polystyrene showed important applications in food industry and used as natural antioxidants in human diet [31, 32]. The previous results illustrated that 30% w/v honey [10], 0.15% w/v Rosmarinum officinalis L. essential oil [10], 8% v/v cinnamon [24], 5% w/v soy whey [20] and 5% w/v mango peel [31] indicated superior antioxidant activity in kefir, which were in line with present study.
Also Fig. 2 demonstrated changes in TPC for distinct fermented treatments during 21 days of fermentation (p < 0.05). K3 had the highest 109.85 (mg GAE/g) TPC and the lowest level was presented 48.28 (mg GAE/g) in CK. In a constant time, K2 and K3 exhibited more TPC in comparison with CK and K1 as well as TPC was lowered by extending fermentation. Portulaca oleracea L. seed oil had antioxidant feature and IC50 values of 0.388 ± 0.2033 mg/mL for hydroxyl free radical scavenging activity [33]. Furthermore, the antioxidant function and TPC for this oil were in the rage of 53.90 to 63.01% and 66.51 to 155.65 mg GAE/kg oil and also vegetable oils are considered as an enriched source of volatile terpenoids and TPC [34]. Portulaca oleracea L. includes more USFAs (especially high omega-3 FAs) and polyphenols, which are great absorbers of reactive oxygen and causes a significant antioxidant in this seed [11, 33]. Free radicals of oil was removed by antioxidant function and inhibit the chain reaction, thus prevent the lipid oxidation and effect on stability behavior [34]. Polyphenols are recognized to interact with proteins of milk and constitute insoluble composition that reduced free total polyphenol [19]. The TPC for kefir were improved by adding 20% (w/w) aqueous extract of dried berry and 2% (w/v) brown lentils [26], which were similar to present research.
Survival of LAB, mold and yeast
The results of LAB count are represented in Table 2 and the highest populations for Lactobacillus spp., Lactococcus spp. and Bifidiobacterium spp. were 8.25, 8.89 and 28.3 log CFU/mL for K2 on the 7th day and the lowest number were 7.12, 6. 39 and 7.4 log CFU/mL in K3 at the 21st day, respectively. The effect of shelf life was investigated on bacteria count and it could be concluded that microorganism numbers did not change significantly until the 14th day, but after that a reduction trend was considerably observed on the 21st day, which was corresponded to a lower substrate (lactose) and also inhibition influence of seed oil [35]. During inoculation of starter culture, microorganisms fermented lactose to LAB, alcohol and carbon dioxide as secondary metabolites, which could limit their growth due to reduction of lactose/lactic acid ratio [3]. The population of Lactobacillus spp., Lactococcus spp. and Bifidiobacterium spp. in K3 is significantly lower than other treatments, which is probably owing to the inhibition effect of oily substrate. The enriched plant forms (extract, essential oil and etc.) with polyphenols and other antimicrobial components such as saponins acted as inhibitor factors and diminished microbial growth [19]. Furthermore, kefiran exopolysaccharide influenced on microbial growth and redox potential by LAB with microbial cells through coating a thin film that lowered oxygen transfer [3].
The results of mold and yeast counts are presented in Table 2 and the highest rate was about 13.2 CFU/mL for K2 on 1st day and the lowest was 1.8 CFU/mL for CK on 21st day of incubation. The mold and yeast counts are generally higher in fermented dairy products especially kefir than natural bacterial flora, which is related to rapid growth [36]. In general, the effect of storage was significant on mold and yeast count; so, the highest population was obtained on 1st day in all treatments as well as the lowest was achieved at 21st day of fermentation. Accordingly, the most level for mold and yeast was observed in kefir beverage at beginning of storage due to more lactose presence, but their populations were reduced over a time [35]. Yeast and mold are considered as key factors in preparation of fermented dairy products, which supplied essential nutrients such as vitamins, amino acids, pH, ethanol and CO2 [37]. Yeasts in kefir clearly provided a favorable environment for bacteria growth producing metabolites that contributed to flavor and mouth feel [3].
The amount of 2% w/v brown lentils [26], 1% w/v Linum usitatissimum L. as flaxseed [9], 5% w/v soy whey [38] and 3% w/v Viciafaba L. bean [39] indicated higher LAB population, which were similar to present study. Ginger and cinnamon extracts were supplemented into goat milk kefir, which reduced LAB from 1 × 1010 for kefir without extract to 9 × 109 with 8% cinnamon and 3.2 × 109 with 12% ginger [24].
Hydrophobicity
As can be seen in Table 3, the most and least percentages of hydrophobicity are related to Lactobacillus spp. isolated from K2 on the 1st day (72.4%) and Bifidiobacterium spp. isolated from CK on 21st day (35.1%). The hydrophobicity rate of Lactobacillus spp. was higher in all treatments compared to others and this level for kefir containing oil was more than without oil and also a significant effect was not observed on hydrophobicity until the 14th day, but a noticeable reduction was found on the 21st day. Also, K3 had a significantly higher hydrophobicity percentage throughout the shelf life among different treatments. Several factors affect the hydrophobicity characteristic such as chemical and structural properties including protein, amino acid, polysaccharide and lipid compositions in bacterial cells, environmental agents and growth [21]. The hydrophobicity of cell surface was reported to be 50.32 to 77.8% by previous researchers [40]. Various agents are involved in the adhesion of different probiotics to intestinal cells, which can mention the hydrophobic and hydrophilic properties for cell membrane of microorganisms [2]. Distinct culture mediums have an influence on FAs in cell membrane and its effects change the hydrophobicity and hydrophilicity of cell membranes [21]. Therefore, the ability to adhere surfaces for epithelial cells is one of the most important conditions in probiotic selection [41]. The constituents of microorganism culture have a great effect on changing cell wall and its function [42]. The effect of the culture components has been observed on microorganisms in various researches [21]. Membrane composition, morphology and technical functions of Lactobacillus johnsonii NCC 533 were significantly changed by enrichment with oleic, linoleic and linolenic acids [43]. When 10 μg/mL non-USFAs were added to culture medium, the ratio of SFAs to USFAs in the bacterial membrane decreased by almost two times [43]. The impacts of elaidic acid on the growth and surface hydrophobicity of Lactobacillus bacteria could change the physicochemical attributes [41].
Sensorial quality
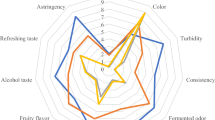
The sensory attributes of beverages supplemented with different Portulaca oleracea L. seed oil concentrations are depicted in Fig. 3. All scores were received by panelists, which significantly lessened (p < 0.05) using seed oil addition in treatments. Accordingly, the highest general acceptance was assigned to K1 that was closest to CK; so, panelists preferred kefir with a slightly acidic or alcoholic flavor and odor instead of higher acidity.
According to sensory aspects, the addition of 3% w/w honey [10] and 15% w/w pomegranate juice [27] to kefir production reduced red color intensity, acidity and increased viscosity with sweetness at the same time. All kefir treatments indicated acceptable sensory scores by adding 5% w/v soy whey [38] and 2 to 4% w/v black and green tea [14] in contrast to 8% v/v cinnamon [24], during 21 days of storage.
FA components of fortified kefir
The SFAs and USFAs of CK, K1, K2 and K3 (1st and 21st day) are detected by GC (Fig. 4). According to obtained results, FA of K3 is higher than others and also the lowest was observed in CK. The addition of oil and this fermentation had increased FA in treatments as well as PUFA was a prominent composition of Portulaca oleracea L. seed oil and present result was in agreement with previous articles [28, 30].
Chromagoram GC of kefir beverages supplemented with different Purslane seed oil (CK: 0%, K1: 0.5%, K2: 1% and K3: 1.5%) on 1 and 21 days of storage. Small letters have been used for comparison of times in each treatment (column) and capital letters have been used for comparison of treatments in each times (row) (p < 0.05).
In general, total SFA increased at the end of 21st days, but MUFA and PUFA reduced in all kefirs. Based on pervious result, the main FAs are detected stearic and oleic acids in LAB population [20]. The number of microorganisms and their diversity in kefir produced with starter culture can change during the shelf life and increase oleic acid [42]. The effect of fermentation and adding 2 g/100 g camellia oil on arachidic acid can be related to long chain FAs with a length of C:20 to C:24 by Lactobacilli; as a result, these factors play an important role in increasing SFA [37]. The reduction of MUFA especially in oleic acid (C18:1, n-9) has been obtained in present study, which turns this phenomenon into products and also increases the stiffness of cell membrane and its permeability as well as a decrease in stress conditions caused by storage [44]. According to results of this study, CLA lowered in kefir medium containing vegetable oil during the shelf life [20]. These FAs were probably reduced as a result of oxidation (more sensitivity for MUFA and PUFA to oxidation), metabolism and Lactobacilli dominance in the environment during storage [20]. CLAs are spatial and positional isomers of octadecadienoate (18:2), which are found in foods prepared from ruminants (sheep, cattle and dairy products manufactured) [37]. Carcinogenesis is reduced when a mixture of FA isomers is given to animals that have suffered various cancers such as breast, intestine and skin with chemicals [1]. FA isomers also play a role in treatment of diabetes, osteoporosis (improving calcium absorption), weight loss (more fat lipolysis), heart diseases and have high antioxidant activity [45]. Nowadays, FA is available in the market as a supplement and CLA is synthesized by LAB especially probiotic bacterial strains [46]. The substrate for the synthesis of CLAs is required through linoleic acid fermentation by microorganism, which has been reported by Lactobacillus and Bifidobacterium genera and vegetable oils are a rich source of FA [11]. Reactions of hydration, dehydration and change in the position of double bond by short chain dehydrogenase, oxidoreductase and acetoacetate decarboxylase are involved in synthesis of CLA from linoleic acid [47].
Isomerization and conjugation of USFAs are probably caused by linoleic isomerase, which is produced by various bacterial strains, mainly probiotics [16]. Bifidobacterium animalis subsp. lactis Bb-12 as one of the most popular probiotic strains can convert 27% of free linoleic acid to cis-9 and trans-11-CLA, when grows in MRS medium [48]. The differences in CLA synthesis are due to distinct isomerase activities among species or strains of microorganisms [15]. Three isomers for CLA, butyric, palmitic and palmitoleic, oleic acids were higher in kefir than yogurt or milk [42]. Since stearic and vaccinic acids are the final and intermediate products of CLA metabolism, respectively [5]. As a result, FAs were increased by adding oil and fermentation of kefir in parallel with linoleic acid.
Conclusion
The supplementation of Portulaca oleracea L. seed oil has affected FA, physicochemical, antioxidant, microbiological and sensorial attributes of kefir beverages. According to obtained results, FAs in treatment containing 1.5% oil are more than others and the lowest was observed in control. Also, fermentation improved CLAs in oil presence; in general, SFA enhanced during the storage period, but mono and PUFAs declined as well as higher concentration of seed oil induced more antioxidant capacity and TPC level. A negative correlation was observed between TPC, antioxidant potential and survival of LAB in kefir beverages supplemented with Portulaca oleracea L. seed oil. Hydrophobicity of Lactobacillus spp., Lactococcus spp. and Bifidiobacterium spp. isolated from oil-enriched kefir treatments has elevated, which indicates the improvement of their efficiency in the human digestive system. Otherwise, the addition of seed oil into kefir beverages lessened sensory quality compared to control and also sensorial aspects of K1 were quite similar to CK. Portulaca oleracea L. Therefore, reasonable levels of seed oil could be applied as natural preservatives and antioxidants especially in probiotic dairy products, which could have several beneficial effects on human health.
Data Availability
The data that support the findings of this study are available on request.
References
C. Leyva-Porras, Z. Saavedra-Leos, M. Román-Aguirre, C. Arzate-Quintana, A.R. Castillo-González, A.I. González-Jácquez, F. Gómez-Loya, An equilibrium state diagram for storage stability and conservation of active ingredients in a functional food based on polysaccharides blends. Polymers 15(2), 367–383 (2023). https://doi.org/10.3390/polym15020367
M. Saboktakin-Rizi, B. Alizadeh Behbahani, M. Hojjati, M. Noshad, Identification of Lactobacillus plantarum TW29–1 isolated from Iranian fermented cereal-dairy product (Yellow Zabol Kashk): probiotic characteristics, antimicrobial activity and safety evaluation. J. Food Meas. Charact. 15(3), 2615–2624 (2021). https://doi.org/10.1007/s11694-021-00846-5
R. Korzhov, A. Ponomarev, E. Melnikova, E. Bogdanova, Preclinical studies of kefir product with reduced allergenicity of β-lactoglobulin. Foods Raw Mater. 3(2), 115–121 (2015). https://doi.org/10.12737/13128
F. Falah, Z. Zareie, A. Vasiee, F. Tabatabaee Yazdi, S.A. Mortazavi, B. Alizadeh Behbahani, Production of synbiotic ice-creams with Lactobacillus brevis PML1 and inulin: functional characteristics, probiotic viability, and sensory properties. J. Food Meas. Charact. 15(6), 5537–5546 (2021). https://doi.org/10.1007/s11694-021-01119-x
P. Gómez-Cortés, M. Juárez, M.A. De la Fuente, Milk fatty acids and potential health benefits: an updated vision. Trends Food Sci Technol. 81, 1–9 (2018). https://doi.org/10.1016/j.tifs.2018.08.014
L. Ould Saadi, F. Zaidi, T. Sanz, C.M. Haros, Effect of faba bean and chickpea mucilage incorporation in the structure and functionality of kefir. Int. J. Food Sci. 26(6), 503–511 (2020). https://doi.org/10.1177/1082013220908089
C. Puerari, K.T. Magalhães, R.F. Schwan, New cocoa pulp-based kefir beverages: Microbiological, chemical composition and sensory analysis. Food Res. Int. 48(2), 634–640 (2012). https://doi.org/10.1016/j.foodres.2012.06.005
M. Azizkhani, P.E.J. Saris, M. Baniasadi, An in-vitro assessment of antifungal and antibacterial activity of cow, camel, ewe, and goat milk kefir and probiotic yogurt. J. Food Meas. Charact. 15, 406–415 (2021). https://doi.org/10.1007/s11694-020-00645-4
D.H. Kim, D. Jeong, Y.T. Oh, K.Y. Song, H.S. Kim, J.W. Chon, H. Kim, K.H. Seo, Stimulating the growth of Kefir-isolated lactic acid bacteria using addition of crude flaxseed (Linum usitatissimum L.) extract. J. Dairy Sci. Biotechnol. 35(2), 93–97 (2017). https://doi.org/10.22424/jmsb.2017.35.2.93
A. Perna, A. Simonetti, E. Gambacorta, Phenolic content and antioxidant activity of donkey milk kefir fortified with sulla honey and rosemary essential oil during refrigerated storage. Int. J. Dairy Technol. 72(1), 74–81 (2019). https://doi.org/10.1111/1471-0307.12561
S.M. Osman, M.A. Hussein, Purslane seeds fixed oil as a functional food in treatment of obesity induced by high fat diet in obese diabetic mice. J. Nutr. Sci. 5(1), 1–7 (2015). https://doi.org/10.4172/2155-9600.1000332
M. Salehi, M. Ghorbani, A. Sadeghi Mahoonk, M. Khomeiri, Physicochemical, antioxidant and sensory properties of yogurt fortified with common purslane (Portulaca oleracea) extract. J. Food Meas. Charact. 15(5), 4288–4296 (2021). https://doi.org/10.1007/s11694-021-00949-z
S. Farnejad, M. Nouri, S. Safari Dolatabad, Obtaining of chickpea protein isolate and its application as coating enriched with essential oils from Satureja hortensis and Satureja mutica in egg at room temperature. Int. J. Food Sci. Technol. 57(1), 400–407 (2022). https://doi.org/10.1111/ijfs.15413
C. Karagözlü, Ü. Gülfem, A.S. Akalin, A. Ecem, Ö. Kinik, The supplementary effect of black and green tea infusion on antimicrobial activities of kefir. Food Health. 4(2), 124–131 (2018). https://doi.org/10.3153/FH18012
L. Gorissen, F. Leroy, L. De Vuyst, S. De Smet, K. Raes, Bacterial production of conjugated linoleic and linolenic acid in foods: a technological challenge. Crit. Rev. Food Sci. Nutr. 55(11), 1561–1574 (2015). https://doi.org/10.1080/10408398.2012.706243
M.A.E.B. Salama, M. Owon, M. Osman, A. Ibrahim, B. Matthäus, Effect of germination and roasting on oil profile of Moringa oleifera and Moringa peregrina seeds. J. Food Meas. Charact. 14, 2220–2229 (2020). https://doi.org/10.1007/s11694-020-00469-2
Association of Officiating Analytical Chemists (AOAC), Official Method of Analysis of the AOAC (AOAC, New York, 2005)
M.M. Cavia, M.A. Fernández-Muiño, E. Gömez-Alonso, M.J. Montes-Pérez, J.F.M.T. Huidobro, Sancho, evolution of fructose and glucose in honey over one year: influence of induced granulation. Food Chem. 78(2), 157–161 (2002). https://doi.org/10.1016/S0308-8146(01)00393-4
E.S. Entezarisareshkeh, M. Nouri, Improving stability of bioactive components and folate and survival of Bifidobacterium bifidum and Bifidobacterium lactis in probiotic ice creams containing Japanese Loquat pulps. Nutr. Food Sci. 9(1), 49–56 (2022)
K. Turek, M. Wszołek, Effect of walnut oil on the fatty acid content of probiotic kefir produced either with kefir grains or kefir starter cultures. Int. Dairy J. 127, 105290 (2022)
H.B. Barzegar, B. Alizadeh Behbahani, F. Falah, Safety, probiotic properties, antimicrobial activity, and technological performance of Lactobacillus strains isolated from Iranian raw milk cheeses. Food Sci. Nutr. 9(8), 4094–4107 (2021). https://doi.org/10.1002/fsn3.2365
H. Mooliani, M. Nouri, Optimization of oxidative, physical stability and microbial characteristics of salad dressing emulsions based on avocado and whey protein combined with mint (Mentha spicata L.) extract. J. Food Meas. Charact. 15(6), 5713–5724 (2021). https://doi.org/10.1007/s11694-021-01131-1
M. Ledoux, J.M. Chardigny, M. Darbois, Y. Soustre, J.L. Sébédio, L. Laloux, Fatty acid composition of French butters, with special emphasis on conjugated linoleic acid (CLA) isomers. J. Food Compost. Anal. 18(5), 409–425 (2005). https://doi.org/10.1016/j.jfca.2004.01.001
F. Setiyoningrum, G. Priadi, F. Afiati, Supplementation of ginger and cinnamon extract into goat milk kefir. AIP Conf. Proc. 2175, 20069 (2019). https://doi.org/10.1063/1.5134633
S.A. Kabakcı, M. Türkyılmaz, M. Özkan, Changes in the quality of kefir fortified with anthocyanin-rich juices during storage. Food Chem. 326, 126977 (2020). https://doi.org/10.1016/j.foodchem.2020.126977
A. Gunenc, M.H. Yeung, C. Lavergne, J. Bertinato, F. Hosseinian, Enhancements of antioxidant activity and mineral solubility of germinated wrinkled lentils during fermentation in kefir. J. Funct. Foods. 32, 72–79 (2017). https://doi.org/10.1016/j.jff.2017.02.016
G. Dimitreli, D. Petridis, N. Kapageridis, M. Mixiou, Effect of pomegranate juice and fir honey addition on the rheological and sensory properties of kefir-type products differing in their fat content. LWT. 111, 799–808 (2019). https://doi.org/10.1016/J.LWT.2019.05.071
S.A. Ahmed, S.E. Shaker, H. Shawky, Solvent polarity dictates the anti-inflammatory potency and mechanism of two purslane (Portulaca oleracea) seed extracts. J. Food Biochem. 46(10), 14281 (2022). https://doi.org/10.1111/jfbc.14281
A. Aussanasuwannakul, K. Puntaburt, W. Treesuwan, Rheological, tribological, and sensory analysis of coconut-oil-modified coconut milk Kefir. Curr. Res. Nutr. Food Sci. 8(2), 496–503 (2020). https://doi.org/10.12944/CRNFSJ.8.2.15
A. Gunenc, O. Rowland, H. Xu, A. Marangoni, F. Hosseinian, Portulaca oleracea seeds as a novel source of alkylresorcinols and its phenolic profiles during germination. LWT. 101, 246–250 (2019). https://doi.org/10.1016/j.lwt.2018.10.075
G.M. Vicenssuto, R.J.S. De Castro, Development of a novel probiotic milk product with enhanced antioxidant properties using mango peel as a fermentation substrate. Biocatal. Agric. Biotechnol. 24, 101564 (2020). https://doi.org/10.1016/j.bcab.2020.101564
M. Nouri, F. Khodaiyan, Green synthesis of chitosan magnetic nanoparticles and their application with poly-aldehyde kefiran cross-linker to immobilize pectinase enzyme. Biocatal. Agric. Biotechnol. 29, 101681 (2020). https://doi.org/10.1016/j.bcab.2020.101681
G. Guo, L. Yue, S. Fan, S. Jing, L.J. Yan, Antioxidant and antiproliferative activities of purslane seed oil. J. Hypertens. 5(2), 1–6 (2016). https://doi.org/10.4172/2167-1095.1000218
S. Delfan-Hosseini, K. Nayebzadeh, L. Mirmoghtadaie, M. Kavosi, S.M. Hosseini, Effect of extraction process on composition, oxidative stability and rheological properties of purslane seed oil. Food Chem. 222, 61–66 (2017). https://doi.org/10.1016/j.foodchem.2016.11.150
B.N.P. Sah, T. Vasiljevic, S. McKechnie, O.N. Donkor, Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT. 65, 978–986 (2016). https://doi.org/10.1016/j.lwt.2015.09.027
S.J. Eom, J.E. Hwang, K.T. Kim, H.D. Paik, Antibacterial effects against various foodborne pathogens and sensory properties of yogurt supplemented with Panax ginseng Marc extract. Korean J. Food Sci. Anim. Resour. 37(5), 787–793 (2017). https://doi.org/10.5851/kosfa.2017.37.5.787
C.P. Vieira, C.C. Cabral, B.R. Da Costa Lima, V.M.F. Paschoalin, K.C. Leandro, C.A. Conte-Junior, Lactococcus lactis ssp. cremoris MRS47, a potential probiotic strain isolated from kefir grains, increases cis-9, trans-11-CLA and PUFA contents in fermented milk. J. Funct. Foods. 31, 172–178 (2017). https://doi.org/10.1016/j.jff.2017.01.047
C. Tu, F. Azi, J. Huang, X. Xu, G. Xing, M. Dong, Quality and metagenomic evaluation of a novel functional beverage produced from soy whey using water kefir grains. LWT. 113, 108258 (2019). https://doi.org/10.1016/j.lwt.2019.108258
L. Ould Saadi, F. Zaidi, T. Sanz, C.M. Haros, Effect of faba bean and chickpea mucilage incorporation in the structure and functionality of kefir. Food Sci. Technol. 26(6), 503–511 (2020). https://doi.org/10.1177/1082013220908089
H. Nagaraja, G. Chennappa, S. Rakesh, M.K. Naik, Y.S. Amaresh, M.Y. Sreenivasa, Probiotic properties of lactic acid bacteria isolated from neera: a naturally fermenting coconut palm nectar. Front Microbiol. 10, 1382–1395 (2019). https://doi.org/10.3389/fmicb.2019.01382
Q. Wu, N.P. Shah, Gas release-based prescreening combined with reversed-phase HPLC quantitation for efficient selection of high-γ-aminobutyric acid (GABA)-producing lactic acid bacteria. J. Dairy Sci. 98(2), 790–797 (2015). https://doi.org/10.3168/jds.2014-8808
Z. Guzel-seydim, J.T. Wyffels, A.C. Seydim, A.K. Greene, Turkish kefir and kefir grains: microbial enumeration and electron microscobic observation. Int. J. Dairy Technol. 58(1), 25–29 (2005). https://doi.org/10.1111/j.1471-0307.2005.00177.x
J.A. Muller, R.P. Ross, W.F.H. Sybesma, G.F. Fitzgerald, C. Stanton, Modification of the technical properties of Lactobacillus johnsonii NCC 533 by supplementing the growth medium with unsaturated fatty acids. Appl. Environ. Microbiol. 77(19), 6889–6898 (2011). https://doi.org/10.1128/AEM.05213-11
M.E. Guerzoni, R. Lanciotti, P.S. Cocconcelli, Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiology 147(8), 2255–2264 (2001). https://doi.org/10.1099/00221287-147-8-2255
D. Kritchevsky, Antimutagenic and some other effects of conjugated linoleic acid. Br. J. Nutr. 83(5), 459–465 (2000). https://doi.org/10.1017/S0007114500000581
M.H. Abd El-Salam, K. El-Shafei, O.M. Sharaf, B.A. Effat, F.M. Asem, A.M. El-Aasar, Screening of some potentially probiotic lactic acid bacteria for their ability to synthesis conjugated linoleic acid. Int. J. Dairy Technol. 63(1), 62–69 (2010). https://doi.org/10.1111/j.1471-0307.2009.00541.x
L. Gorissen, L. De Vuyst, K. Raes, S. De Smet, F. Leroy, Conjugated linoleic and linolenic acid production kinetics by bifidobacteria differ among strains. Int. J. Food Microbiol. 155(3), 234–240 (2012). https://doi.org/10.1016/j.ijfoodmicro.2012.02.012
M. Coakley, R.P. Ross, M. Nordgren, G. Fitzgerald, R. Devery, C. Stanton, Conjugated linoleic acid biosynthesis by human-derived Bifidobacterium species. J. Appl. Microbiol. 94(1), 138–145 (2003). https://doi.org/10.1046/j.1365-2672.2003.01814.x
Author information
Authors and Affiliations
Contributions
The authors are equally corresponded to the manuscript writing and responsible for plagiarism.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moradi, S., Nouri, M. Production of functional kefir supplemented by Portulaca oleracea L. seed oil. Food Measure 17, 5000–5011 (2023). https://doi.org/10.1007/s11694-023-01993-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-01993-7