Abstract
The aim of this study was to investigate the effect of different sweeteners on the physiochemical, minerals, bioactive, and microstructure of sand pear cubes was investigated under different blanching time (0, 2.5, and 5 min). Compared with the unblanched sample, blanching led to a significant increase in the water activity, total soluble solids (TSS), and total sugars of sand pear cubes. The results regarding minerals and bioactive compounds indicated that maximum retention was observed in 2.5 min blanched samples. Meanwhile, ranking and cluster analysis showed that sweeteners had most significant effect on all the quality attributes as compared to blanching time. The low-calorie sweeteners result in low TSS and total sugars with high retention of bioactive compounds. Among these sweeteners aspartame, stevia, and sucralose indicated the same effect on physico-chemical parameters, whereas stevia-based cubes had high bioactive compounds which is also confirmed by FTIR characterization. The structural characterization revealed that the stevia-based cubes had a porous and less crystalline surface, which was responsible for the soft texture of cubes. Hence, it was observed that stevia can be a good alternative to reduce sugar in fruit cubes along with, high retention of bioactive compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Confectionary products are generally prepared with white sugar using artificial food additives (including coloring and flavoring agent).The high rate of sugar consumption has a negative impact on health such as obesity, diabetes, oral plague, cardiovascular diseases etc. [1, 2]. According to the World Health Organization, sugar consumption should be less than 10% of the daily caloric intake. Furthermore, increased awareness about the maintenance of good health and tackling health problems through dietary habits has also led to the expansion of natural food products having specific health interests such as low sugar products with high nutritional value [1, 3]. As a consequence, the food industries are looking for new solutions to develop healthy products. In recent years, low-calorie sweeteners become important part of food industry. They are characterized by different degree of sweetness, having much lower energy value and glycemic index than sucrose [4]. However, these sweeteners affect the retention of phytocompounds and the quality of the final product [5].
In addition consumer satisfaction is also the major concern [6]. Recently, many researchers have worked on the development of fruit-based confectionary products using low calorie sweeteners such as apple jam, gelatin candies [7], velvet candy [8], mango jam [9], fruit beverage, bakery products [10] etc. Among these products, fruit based candies have high demand in market owing to their high acceptability in terms of taste and nutrition,
Sand pear (Pyrus pyrifolia) is a sub-tropical fruit that belongs to the Rosaceae family and is mainly cultivated in Asia [11]. It is abundant in sugars, phenolic acids, flavonoids, minerals, vitamins, and possesses high antioxidant activity. Due to high phytochemicals, sand pear exhibits various health-promoting properties such as anti-inflammatory, anti-diabetic, anti-obesity, and prevention against several cardiovascular and carcinogenic diseases. Sand pear flavonoids have been proven to play a key function in the prevention of degenerative and infectious diseases [12, 13]. However, being highly perishable with limited availability an enormous amount of fruit is wasted hence, needs to be preserved [14]. Few studies have been available on the valorization of sand pear in the development of different food products such as wine, cubes, juice [12, 15, 16] etc. Blanching is an important step prior to the processing of fruits and vegetables. In addition to the blanching temperature, exposure time is also a crucial parameter, which directly affects the nutritional quality of the resulted product [17].
However, no detailed study is available on effect of processing on phytocompounds of sand pear cubes. It was aimed to determine the effect of blanching time (0, 2.5, and 5 min) and different sweeteners (sucrose, sucralose, aspartame, stevia, and sorbitol) on the physico-chemical, mineral profile, bioactive and structural attributes of sand pear cubes were studied. Therefore, this comprehensive study provides a deeper understanding of the effect of blanching time and different sweeteners on the quality of sand pear cubes.
Materials and methods
Materials
Sand pears were procured from the Department of Fruit Science, Punjab Agricultural University, Ludhiana, India. Sweeteners such as sucrose, aspartame, stevia, sucralose, and sorbitol were procured from the certified store of Ludhiana.
Preparation of syrup
Five types of syrup such as sucrose, aspartame, stevia, sucralose, and sorbitol were prepared. The sucrose syrup (70° Brix) was taken as control, whereas the base syrup for other sweeteners was prepared using sucrose (40° Brix) and sweeteners (aspartame, stevia, sucralose, and sorbitol) were added based on sweetness accordingly [8].
Preparation of sand pear cubes
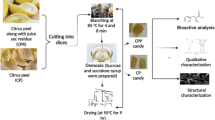
The sand pear was cut into cubes with the cube cutter having length 4 ± 0.02 cm and width 3 ± 0.3 cm. Peeled cubes were blanched at 85 ± 3 °C for 0 (control), 2.5 and 5 min and cubes were allowed to cool immediately in cold water then blanched samples are dipped in prepared sugar syrups. This process was carried out at room temperature 27 °C ± 2. until the equilibrium for the TSS (total soluble solids) of the solution was reached. After completing osmotic dehydration, cubes were dried by a hot air cabinet tray dryer at 55 °C until constant moisture content [12].
Methods
Physico-chemical analysis
Water activity was measured by water activity meter (PREAQUA LAB, Water activity analyzer, SN: PRE000197). The TSS of sand pear cubes was measured by a digital refractometerand data were expressed as °Brix. The NEB index of sand pear cubes was determined by taking 2 g of sample, which was mixed for 2 h with 25 ml of 2% acetic acid [18]. The material was then centrifuged for 20 min at 10,000 rpm. A UV-VIZ spectrophotometer was used to measure the absorbance of the supernatant at 420 nm. For the determination of total sugars, 1 g of the sample was refluxed with 80% isopropyl alcohol for 4 h. Extracts were kept for evaporation in a boiling water bath and the filtrate was diluted to 100 ml using distilled water. The 1 ml from the obtained extract was diluted 10 times for further analysis. The aliquot was placed in a test tube and 1 ml of 5% phenol and 5 ml of concentrated sulphuric acid were added. Samples were chilled in ice water and thoroughly mixed with a vortex. The color intensity was measured on UV-VIZ spectrophotometer at 490 nm and the results were expressed in percentage [19]. Color measurement was performed using a Colorimeter (Hunter Lab) by determining the color value; L* (100 = white; 0 = black), a* (positive = red; negative = green), and b* (positive = yellow; negative = blue). For the texture analysis (TA-XT2i Texture analyzer) with a cylinder probe (50 mm) was used to examine the texture of cubes. Pre-test speed was 1 mm/s, test speed was 0.5 mm/s, post-test speed was 0.5 mm/s, distance between probe and sample was 10 mm, trigger force was 10 g, and the time between two compressions was 4 s.
Mineral profile
The mineral content of prepared cubes was determined using thermo-electron inductively coupled plasma atomic emission spectrometry (ICP-AES) [20]. In a conical flask, 0.5 g of sample was taken and 10 ml of diacid mixture (nitric acid and perchloric acid in a 3:1 ratio) was added and left overnight. After that, the flask was placed on a heated plate for digestion, and the samples were digested until the white fumes ceased evaporating. The digested samples were diluted in double distilled water. The filtrate volume was then increased to 25 ml with distilled water and used for mineral analysis.
Bioactive analysis
The cube samples were extracted at 45 ± 1 °C with 50 ml of 80% methanol for 4 h. After shaking, the extract was centrifuged for 10 min at 6000 rpm. For further analysis, the supernatant was collected and kept in amber colored flasks at 4 ± 1 °C for further analysis [21].
Total phenolic content
The 1 ml of extract was added in a test tube, along with 2.5 ml of F–C reagent. After mixing, 2 ml of 20% saturated sodium carbonate was added and the mixture was left in the dark for 15 min. The absorbance of this solution was measured with UV–VIS spectrophotometer at 765 nm [21]. The results were expressed as gallic acid equivalent (GAE) mg/100 g.
Flavonoid content
The 1 ml of extract was mixed with 1 ml pure methanol, 0.1 ml of 10% aluminium chloride, 0.1 ml potassium acetate solution and 1.8 ml distilled water [22]. The absorbance of the solution was measured at 415 nm. The flavonoids were expressed in mg quercetin equivalent (QE) /100 g.
DPPH (2,2-diphenyl-1-picryl hydrazyl) assay
The mixture's potential to scavenge DPPH free radicals was examined. 1 ml extract was blended with 1 ml tris buffer in a test tube. DPPH (2 ml) was added to this mixture and incubated for 30 min at room temperature in the dark. Methanol was used as a control to evaluate absorbance at 517 nm [21]. The results were expressed in trolox equivalent (TE) µmol/100 g.
Reducing power assay (RPA)
The mixture of 1 ml extract and 2.5 ml phosphate buffer, 2.5 ml of 1% potassium ferricyanide was added before incubating at 50 °C for 20 min. The mixture was then mixed with 2.5 ml of 10% trichloroacetic acid and centrifuged at 3000 rpm. Finally, 2.5 ml of supernatant solution was collected and mixed with distilled water (2.5 ml) and 1 ml ferric chloride (0.1%) before measuring absorbance at 700 nm [23]. The findings were reported in mg of ascorbic acid equivalent (AAE)/100 g.
Ferric reducing antioxidant power (FRAP) assay
The antioxidant capacity of each sample was estimated by FRAP assay, briefly, 2.7 ml of freshly prepared FRAP reagent (TPTZ, FeCl3 and acetate buffer) at 37 °C was mixed with 1 ml of extract. The absorbance at 595 nm was determined after 30 min [21]. Aqueous solutions of known Fe(II) concentrations were used for calibration and results were expressed as FeSO4 µmol/100 g.
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assay
To make the ABTS radical cation, 5 ml of an ABTS solution (7 M) was mixed with 88 ml of potassium persulfate 140 M (final concentration: 2.45 mM). This solution was diluted with ethanol after 16 h in the dark to get an absorbance of 0.7 (approx.) at 734 nm. The absorbance was measured at 743 nm in UV-VIZ spectrophotometer after 1 ml of the sample extract was combined with 3 ml of the ABTS radical [24]. The results were expressed as Trolox equivalents (TE) µmol/100 g.
Hydroxyl radical scavenging activity (HRSA)
The hydroxyl radicals (OH) were taking 10 μl of FeCl3 (0.1 mM), 10 μl of ascorbic acid (0.1 mM), 10 μl of H2O2 (1 mM), and 10 μl of EDTA (0.1 mM). At 37 °C for 1 h, 20 μl of the sample was treated with 10 μl of desoxyribose (1 mM). The mixture was incubated at 100 °C for 15 min. It was then allowed to cool to room temperature. At 532 nm reading were noted for detection of malondialdehyde released from the breakdown of desoxyribose. The outcome was expressed as a percentage inhibition (%) in comparison to a control test [25].
Anti-diabetic activity
The 1 ml of the extract in 0.02 M sodium phosphate buffer of pH 6.9 were added to alpha amylase (0.5 mg/ml) and thereafter incubation was done for 10 min at 25 °C. Then, 1 ml starch solution (one percent) in sodium phosphate buffer was added. The reaction mixture was incubated for 10 min at 25 °C and stopped with 1 ml of dinitrosalicylic acid (DNSA) reagent. The mixture was incubated in a boiling bath for 5 min and cooled to room temperature. The reaction mixture was further diluted with distilled water 10 ml and the absorbance was measured at 540 nm. The inhibitory impact of the extracts was determined and given as % inhibition [25]
FTIR
The prepared cubes were tested for FTIR spectra using an Agilent Cary 630 FTIR spectrometer The FT-IR spectra of the sand pear cubes were recorded in the range of 689 to 3559 cm−1 and the peaks were compared with the standard table provided by [26].
SEM analysis
The prepared cubes were fixed graded ethanol series (20–100%) followed by mounting on different aluminum stubs. These samples were coated with gold under vacuum and then scanned under different magnifications to obtain a clear micrograph using scanning electron micrograph (JEOL, JSM-6510 LV, Tokyo, Japan) [12].
Statistical analysis
The data obtained was analyzed using the SPSS software. The statistical analyses were carried out using one-way and two-way ANOVA procedures. Statistical differences were investigated at p ≤ 0.05.
Results and discussion
Effect of blanching time on the physico-chemical, mineral profile, and bioactive attributes of sand pear cubes. In order to develop the sand pear cubes, blanching time must be optimized to retain the texture and bioactive compounds
Effect on physico-chemical attributes
Blanching time had a very pronounced and significant (p ≤ 0.05) effect on all the physico-chemical attributes of sand pear cubes, as presented in Table 1. Water activity is an important factor that affects the food product’s quality and microbial activity, hence product stability. The water activity of sand pear cubes varied from 0.51 to 0.68. Higher water activity was observed in 5 min blanched samples, irrespective of the type of sweetener. Blanching time has an impact on the cell structure of fruit such as disruption of the cell membrane [27] resulting in more gain of the water during blanching hence led to increase in the water activity of the final product [28,29,30]. The TSS and total sugars of sand pear cubes were ranging from 36.33 to 65.50 °Brix and 36.17 to 52.74%, respectively. As expected the higher TSS and total sugars value was observed in 5 min blanched samples, which might be due to pre-treatment (blanching) as it enhances the osmosis rate by increasing the cell membrane permeability therefore, more absorption of the sugar during osmosis [30, 31]. Browning index, enzymatic browning is critical because it not only deteriorates colour and reduces shelf life, but it also has a negative impact on the quality of the cut fruits [32]. So it is also considered an important parameter during the processing of food products. The oxidation reaction of phenolic compounds by polyphenol oxidase (PPO) results in the formation of o-quinone, which is polymerized to form the dark pigment melanin. Browning can thus be avoided by inhibiting enzyme activity through processes such as thermal processing [32], In the sand pear cubes, the highest browning was found in control samples due to high enzymatic reactions during the drying of the sand pear cubes. On the contrary, in the pre-treated samples blanching reduced the oxygen content and inactiveate the enzymes, thereby preventing the browning reaction [33, 34].
Color is a prime factor in determining the consumer acceptability of any food product. The L*, a* and b* value of the sand pear cubes varied between 28.07 to 44.27, 0.16 to 0.92, and 4.76 to 8.75, respectively (Table 1). In general, the higher value of L* (brightness) and b* (yellowness) coordinates, the better quality of the sand pear cubes. A remarkable decrease was observed in L* and b* values of the control samples, indicate the more darkening of the cubes compared to blanched samples. These results can be explained by the fact that enzymes are very active in control samples, compared to their inactivation in blanched samples as a result of heat treatment [35, 36]. Desirable qualities of food products are generally associated with good textural properties as texture influences the overall acceptability of food product. The results of the texture profile analyzer showed (Table 1) that the textural properties of cubes are strongly dependent on the blanching time. In this study, the hardness (N) varied from 11.58 ± 0.69 to 37.80 ± 2.28, springiness (mm) from 3.86 ± 0.19 to 10.00 ± 0.31, cohesiveness from 0.20 ± 0.02 to 1.04 ± 0.01, gumminess (N) from 8.64 ± 0.51 to 14.74 ± 0.72, chewiness (J) from 33.46 ± 2.00 to 123.89 ± 4.95. Results for the hardness of cubes showed that it decreased with an increase in blanching time. The springiness is related to the elasticity of the cubes, which increased from 3.86 to 10.00 mm with an increase in blanching time. A similar trend was observed for other parameters such as cohesiveness, gumminess, and chewiness of the sandy pear cubes. Several studies have reported the adverse effects (such as loss of cell turgidity and degradation of cell wall components) of thermal blanching for a long time (> 10 min) in different plant tissue [36]. Generally, tissue softening with low temperature and time blanching treatment is mainly due to pectin conversion during blanching [36, 37] resulting in the soft texture of the cubes.
Effect on mineral profile
Sand pear is also a good source of minerals as reported in the literature [13, 32]. Micronutrient analysis of sand pear cubes showed the presence of significant (p ≤ 0.05) amount of minerals such as boron, calcium, iron, potassium, magnesium, manganese, sodium, phosphorus, sulphur, and zinc (Table 2). The five major minerals in the sand pear cubes were in the following order: calcium > sulphur > potassium > sodium > magnesium followed by phosphorus, iron, boron, zinc, copper and manganese. In general, the blanching time had a significant effect (p ≤ 0.05) on mineral profile of sand pear cubes. As the blanching time increases, a decrease in the mineral profile was observed regardless of the type of mineral. These results were in line with the study of Jabbar et al. (2014), who reported that, a decrease in the mineral content of carrot juice with the blanching treatment [20]. This can be related to the leaching of minerals during blanching and osmosis of sand pear cubes.
Effect on bioactive attributes
The blanching caused a noticeable change in the bioactive attributes of the sand pear cubes as shown in Table 3. The highest retention of total phenols and flavonoids was observed in 2.5 min blanched samples. However, further increase in blanching time (upto 5 min) results in the loss of phenolics and flavonoids this may be due to applying a high temperature over an extended period of time which may damages functional compounds. In the case of 5 min blanching time, there was more softening of tissue (also confirmed by texture results) as compared to 2.5 min blanching time, which leads to a high rate of mass flow and may cause a leaching effect on polyphenols [38,39,40]. A similar effect of blanching time on total phenols in bottle gourd was observed [41]. Samples blanched for 2.5 min had 19.50 and 32.20% retention in phenolic acids and flavonoids, respectively as compared to the control samples. Giovanille et al. 2012 also reported that blanching as pre treatment before osmosis cause more retention of phenolic compounds as well as antioxidant capacities in berries [42, 43]. In carrots blanching treatment gives highest retention of polyphenols but reduction was noticed after 2 to 4 min of blanching time [36]. The application of blanching releases the bound phenols from the cell wall due to the breakage of the ester bond between phenolic and cell wall [35, 36]. On the contrary, higher degradation of phenols and flavonoids in the control samples indicated more enzymatic and oxidation reactions during the drying of sand pear cubes; blanching at 80 °C to 100 °C significantly reduce the polyphenol oxidase and peroxidase activities reported by Magangana et al. (2021) [44]. This was due to inactivation of enzymes peroxidise and polyphenoloxidase which prevents the polyphenols by oxidation [40, 45].
Sand pear (Pyrus pyrifolia) is rich in antioxidant compounds [13]. The antioxidant activity of sand pear cubes was assessed in terms of DPPH (2.15–3.18 µmol/100 g), RPA (74.28–123.72 mg/100 g), FRAP (1.72–2.28 µmol/100 g), ABTS (1.61–2.30 µmol/100 g), HRSA (43.90–66.63%). In this study, the total antioxidant capacity values for all the methods were also higher in 2.5 min blanched samples (Table 3). Better retention of antioxidant activity could be related to the retention of phenolic acids and flavonoids in these samples. Kaseke et al. (2020) found similar results in blanched samples compared with controls [46]. In okra pods blanching with hot humid air gave better retention for phenolics, flavonoids and antioxidant activities [47]. The authors hypothesised that higher antioxidant activity of pomegranate seeds could be related to an increase in extractability of antioxidant compounds with blanching. The anti-diabetic activity of sand pear cubes was ranged from 14.77 to 38.53%, as expected the anti-diabetic activity of 2.5 min blanched samples were higher than control samples. The effect of polyphenol extract of different fruits on the inhibition of α-amylases [48]. Similarly, various studies have also reported the inhibition of α-amylase by the polyphenolic extracted of different fruits [49,50,51,52]. These results demonstrate that with the effective blanching time (2.5 min), sand pear cubes retains high bioactive compounds with the maintenance of texture.
Effect of sweeteners on the physico-chemical, mineral profile, and bioactive attributes of sand pear cubes
Effect on physico-chemical attributes
The effect of different sweeteners on the physico-chemical attributes of cubes are presented in Table 1. In this study, it was observed that the water activity was determined lower in sucrose followed by aspartame, stevia, sucralose, and sorbitol samples. This may be explained by the fact that due to the high concentration of solute (initial concentration of sweetener); the osmosis rate of sucrose was the highest regardless of the blanching time. The increase in the concentration of osmotic solution increased the osmotic pressure of the surrounding solution which, in turn, results in higher free water loss hence lowering the water activity of the resultant sample [53] Among the sweeteners, sorbitol samples owing to the humectant effect of sorbitol as it doesn’t easily absorb and loss moisture [54]; whereas lower the concentration of sucralose in the osmotic solution hence higher the water activity of sucralose samples. The trend of TSS in the sand pear cubes was sucrose > sorbitol > aspartame > stevia > sucralose which was based on the concentration of sweeteners was used in osmotic solution. Results regarding the effect of different sweeteners on the browning index are presented in Table 1. However, incorporation of low calorie sweeteners lowers the water activity and TSS of the resultant product as shown in Tables 1 and 2 also diminished the browning reactions during processing, probably due to the stability of polyphenols. Color and texture is the important parameter of cubes and significantly affected by the rate of osmosis of the solution [55]. Among all the sweetener sucrose, stevia, sucralose and aspartame there is non-significant effect on color except sorbitol. Sorbitol possessed more lightness as compared to others. Similar effect of sorbitol on L*, a*, b* values was scene in osmo-dried apple slices [56]. Among the sweeteners, sucrose cubes had the highest level of hardness, whereas sorbitol cubes has the lowest hardness. It could be owing to sorbitol's humectant tendency, which keeps the cubes moist. The other textural parameter such as springiness, cohesiveness, gumminess and chewiness was significantly different. Sorbitol cubes possess maximum springiness, cohesiveness, gumminess and chewiness as compared to other samples. This could be due to the more stickiness of sorbitol based cubes [56].
Effect on mineral profile
The mineral content was increased in the stevia sweeteners-based cubes as compared to other sweeteners (Table 2). Non-significant change was observed among the different sweeteners. This may be attributed to the high mineral content in stevia [57].
The effect of incorporation of different sweeteners was clearly observed the mineral content of sand pear cubes. The highest mineral content was observed in the stevia based samples as shown in Table 3. This could be attributed to the high mineral content of stevia [57]. The more loss of minerals were observed in sucrose based samples may due to the higher concentration of sucrose in osmotic solution results in high osmotic rate [58].
Effect on bioactive attributes
Regarding the effect of sweeteners, the sand pear cubes prepared using stevia had highest polyphenols would be owing to its own bioactive compounds [57]. The sucralose and aspartame has shown almost same effect on polyphenols retention. Whereas least retention was observed in sorbitol samples. It was found that, the concentration of osmotic solution also affect the phenolic and flavonoids retention, high concentration of osmotic solution cause more leaching of phenols as compare to low concentration [59]. Moreover, the availability of free phenols provided by blanching treatment followed by osmotic solution of high concentration causing more osmotic stress to leach them out. Resulting in low retention of polyphenols in cubes sample made with 5 min blanching time with syrup of 70°Brix, whereas cubes without blanching cause degradation of polyphenolic compounds due to enzymatic reactions [44]. As expected, this was observed that cubes prepared from stevia posses high antioxidant activity and high inhibition activity followed by aspartame, sucralose and sucrose, sorbitol cubes posses least antioxidant activity. It was stated that antioxidant activity depends upon heating temperature, sugar concentration and polyphenol concentration [60]. Hence, after evaluating the effect of sweeteners on the bioactive attributes, it was observed that, stevia based sample possess more bioactive compounds among all the sweeteners.
Molecular characterization
The FTIR was done for the qualitative detection of functional groups present in the sand pear cubes as shown in Fig. 1. The FTIR spectra of the cubes samples were recorded in the range of 689 to 3559 cm−1 and the peaks were compared with the standard Table provided by [42]. The wavelength at 3000–3560 cm−1, have shown the presence of intermolecular bonded O–H stretching which show the presence of hydroxyl groups such as carboxylic acids and water. Sand pear cubes prepared without blanching did not exhibit any visible peaks at 3560–3500 cm−1, which indicates a lack of hydroxyl O–H stretching (indicates presence of bound phenols or unbounding of phenols not yet started) whereas; bands at 3560–3500 cm−1are more visible with high intensity when the blanching time is increased to 2.5 min.
Among sweeteners, the cubes containing sorbitol had a high intensity peak at 3600–3200 cm−1 is due to increased hydrogen bonding of water with sorbitol molecules [56]. The bands around 2929- 2937 cm−1 indicates the presence of C–H stretching, the intensity of these bands decrease with the increase in blanching time.
From wavelength 1237.41 to 1259.84 cm−1, the cubes without blanching showed the highest absorption at this peak, which indicates aliphatic C–O stretching of esters. This stretching decreased with increase in blanching time.d-Glucose is indicated by the absorption between 905.74 and 920.65 cm−1. With longer blanching times, it was seen that the peak for d-glucose increased in cubes samples. When it came to sweeteners, sucrose showed a high peak for d-glucose. Stevia cube sample was the second-lowest peak, behind sorbitol cubes, while sucralose cubes recorded the second-highest peak. Increased peak for d-sucrose and d-fructose were seen with increasing blanching times, and the sucrose concentration in the cubes sample had the greatest peak of all the samples. Peak values for d-sucrose and d-fructose ranged from 864.76 to 868.46 cm−1. The trend for remaining was, sorbitol the second highest and the sucralose was the third and stevia was the least for this wave number.
Scanning electron microscopy (SEM)
Morphology of control and selected sand pear cubes was evaluated with SEM with the magnification from × 100, × 300, and × 500. Alteration in the structural and arrangement of macromolecules due to blanching is clearly visible in Fig. 2. Both control and blanched the sample had uneven and rough outer surface. It can be clearly seen that due to blanching cell walls deformed and structure collapsed resulting information of larger vacuoles (Fig. 2). Blanched sample had porous, denser surface and had exposed open solid matrix. Blanching leads to internal heating in product and creates a temperature difference in inner core and surface thereby leading to formation of micro-cracks and pores [61]. This microstructure is the possible explanation for liberation of bound phenols and flavonoids thereby increasing their bioavailability and concentration in blanched sample [36]. The number of pores and the pores size affects the texture of cubes results in soft texture of blanched cubes as compared to control.
Cluster analysis
Cluster analysis was performed to get the overview of effect of treatments on the different variables and observed that major clustering based on the sweeteners effect. Two major cluster were found where the sorbitol possesses different and separate attributes. A further clustering of sucrose was observed irrespective to blanching time. However, stevia, aspartame and sucralose could not be able to form separate cluster indicated that these sweeteners almost possesses similar effect in terms of physico-chemical and bioactive attributes.
Conclusion
The present study indicated that 2.5 min blanched sample had lowest water activity with high retention of bioactive compounds in sand pear cubes as compared control. However, 5 min blanching time caused the destruction of bioactive compounds with significant changes in the antioxidant activity. Among sweeteners, stevia had a positive impact on the retention of bioactive compounds, which was also confirmed by FTIR analysis. The SEM results indicated that blanching caused the change in cell wall structure of cubes which results in low hardness of the resultant sample. Thus, this sweetener could be used to prepare fruit based cubes.
Data Availability
Data will be available on request from the authors. The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
S. Bernardini, A. Tiezzi, V. LaghezzaMasci, E. Ovidi, Nat. Prod. Res. 32, 1926 (2017). https://doi.org/10.1080/14786419.2017.1356838
N. Konar, R. Gunes, I. Palabiyik, O.S. Toker, Trends Food Sci. Technol. 123, 57 (2022)
P. Selvasekaran, R. Chidambaram, Trends Food Sci. Technol. 113, 315 (2021)
A.C. Sylvetsky, K.I. Rother, Physiol. Behav. 164, 446 (2016)
P. Nowicka, A. Wojdyło, K. Lech, A. Figiel, Food Bioprocess. Technol. 8, 824 (2015)
J.M. Cha, C.P. Borchgrevink, J. Qual. Assurance Hosp. Tour 20, 143 (2018). https://doi.org/10.1080/1528008X.2018.1512934
R. Rivero, D. Archaina, N. Sosa, C. Schebor, LWT 141, 110894 (2021)
R. SukeawSamakradhamrongthai, T. Jannu, Food Chem. 352, 129353 (2021)
S. Basu, U.S. Shivhare, Food Bioprocess. Technol. 6, 1401 (2013)
S. Ghosh, M.L. Sudha, Int. J. Food Sci. Nutr. 63, 372 (2012). https://doi.org/10.3109/09637486.2011.627846
Y. Zhi Wang, M. Song Dai, D. Ying Cai, S. Zhang, Z. Bin Shi, Sci. Hortic. (Amsterdam). 210, 138 (2016)
P. Baniwal, B.S. Hathan, J. Food Process. Preserv. 39, 3098 (2015)
R. Chandel, V. Kumar, R. Kaur, S. Kumar, A. Kumar, D. Kumar, S. Kapoor, Nutr. Food Sci. (2023). https://doi.org/10.1108/NFS-09-2022-0306
A. Sridhar, M. Ponnuchamy, P.S. Kumar, A. Kapoor, Environ. Chem. Lett. 19, 1715 (2021)
P.S. Panesar, V.K. Joshi, V. Bali, R. Panesar, Technology for Production of Fortified and Sparkling Fruit Wines (Elsevier, Amsterdam, 2017)
D. Zhang, B. Yu, J. Bai, M. Qian, Q. Shu, J. Su, Y. Teng, Sci. Hortic. (Amsterdam). 134, 53 (2012)
N. Nan An, W. QiaoLv, D. Li, L. Jun Wang, Y. Wang, Food Chem. 398, 133925 (2023)
A.K. Baloch, K.A. Buckle, R.A. Edwards, J. Sci. Food Agric. 24, 389 (1973)
M. Dubois, K.A. Gilles, J.K. Hamilton, P.A. Rebers, F. Smith, Anal. Chem. 28, 350 (1956)
S. Jabbar, M. Abid, B. Hu, T. Wu, M.M. Hashim, S. Lei, X. Zhu, X. Zeng, LWT - Food Sci. Technol. 55, 16 (2014)
H.R. De Moraes Barros, T.A.P. De Castro Ferreira, M.I. Genovese, Food Chem. 134, 1892 (2012)
A. Pękal, K. Pyrzynska, Food Anal. Methods 7, 1776 (2014)
N. Singh, P.S. Rajini, Food Chem. 85, 611 (2004)
N. Pellegrini, R. Re, M. Yang, C. Rice-Evans, Methods Enzymol. 299, 379 (1999)
A. Gupta, R. Kumar, A.K. Pandey, S. Afr. J. Bot. 130, 308 (2020)
B.H. Stuart, Infrared Spectroscopy (Wiley, Hoboken, 2005)
M.S. Tapía, S.M. Alzamora, J. Chirife, Water Activity in Foods: Fundamentals and Applications (Wiley, Hoboken, 2020)
A. Ciurzyńska, J. Falacińska, H. Kowalska, J. Kowalska, S. Galus, A. Marzec, E. Domian, Foods 2021(10), 132 (2021)
J.L.G. Corrêa, D.B. Ernesto, J.G.L.F. Alves, R.S. Andrade, Int. J. Food Sci. Technol. 49, 2008 (2014)
M. Nowacka, L. Laghi, K. Rybak, M. Dalla Rosa, D. Witrowa-Rajchert, U. Tylewicz, Food Chem. 299, 125122 (2019)
G. Giovanelli, A. Brambilla, A. Rizzolo, N. S.-F. R. International, and U. 2012, Food Res. Int. (n.d.).
F. Alipoorfard, M. Jouki, H. Tavakolipour, J. Food Sci. Technol. 57, 3165 (2020)
C. Zhang, C. Hu, Y. Sun, X. Zhang, Y. Wang, H. Fu, X. Chen, Y. Wang, Food Chem. 345, 128756 (2021)
L. Shrestha, B. Kulig, R. Moscetti, R. Massantini, E. Pawelzik, O. Hensel, B. Sturm, Foods 2020(9), 76 (2020)
R. FeumbaDibanda, E. PanyooAkdowa, A. Rani, P.Q. MetsatedemTongwa, C.M. Mbofung, Food Chem. 302, 125308 (2020)
H. Wang, X.M. Fang, P.P. Sutar, J.S. Meng, J. Wang, X.L. Yu, H.W. Xiao, Food Chem. 338, 127799 (2021)
L. Zhao, N. Kristi, Z. Ye, Food Res. Int. 140, 110043 (2021)
P. Baniwal, B. H.-A. J. of Chemistry, and undefined 2017, Researchgate.Net (n.d.).
S.Y. Hong, E. Lansky, S.S. Kang, M. Yang, B.M.C. Complement, Med. Ther. 21, 1 (2021)
L.Z. Deng, A.S. Mujumdar, Q. Zhang, X.H. Yang, J. Wang, Z.A. Zheng, Z.J. Gao, H. W. Xiao (2017). https://doi.org/10.1080/10408398.2017.140919259,1408
S. Bhat, C.S. Saini, H.K. Sharma, Food Sci. Biotechnol. 26, 29 (2017)
S.S. Sablani, P.K. Andrews, N.M. Davies, T. Walters, H. Saez, R.M. Syamaladevi, P.R. Mohekar, J. Sci. Food Agric. 90, 769 (2010)
G. Giovanelli, A. Brambilla, A. Rizzolo, N. Sinelli, Food Res. Int. 49, 263 (2012)
T.P. Magangana, N.P. Makunga, O.A. Fawole, U.L. Opara, Process. 2021(9), 1012 (2021)
A. Sulaiman, M.J. Soo, M. Farid, F.V.M. Silva, J. Food Eng. 165, 133 (2015)
T. Kaseke, U. Opara, O. F.- Molecules, and undefined 2020, Mdpi.Com (n.d.).
S. Zielinska, I. Staniszewska, J. Cybulska, A. Zdunek, M. Szymanska-Chargot, D. Zielinska, Z. L. Liu, H. W. Xiao, Z. Pan, and M. Zielinska, J. Sci. Food Agric. (2022).
M.N. Beidokhti, A.K. Jäger, J. Ethnopharmacol. 201, 26 (2017)
Z. Sun, X. Wang, Bioorg. Chem. 94, 103485 (2020)
T. Wang, X. Li, B. Zhou, H. Li, J. Zeng, W. Gao, J. Funct. Foods 13, 276 (2015)
A. Wojdyło, P. Nowicka, I.P. Turkiewicz, K. Tkacz, F. Hernandez, Sci. Rep. 11, 1 (2021)
C. Quesada, B. Bartolomé, … O. N.-J. of F., and undefined 1996, Meridian.Allenpress.Com (n.d.).
M.A. Mauro, N. Dellarosa, U. Tylewicz, S. Tappi, L. Laghi, P. Rocculi, M.D. Rosa, Food Chem. 195, 19 (2016)
W. Zhang, J. Chen, Q. Chen, H. Wu, W. Mu, Appl. Microbiol. Biotechnol. 104, 9487 (2020)
K. Falade, J. Igbeka, F. A.-J. of F. Engineering, and undefined 2007, Elsevier (n.d.).
O. Chauhan, A. Singh, A. Singh, P. R.-I. J. of, and undefined 2011, Taylor Fr. 14, 1037 (2011).
R. Lemus-Mondaca, A. Vega-Gálvez, L. Zura-Bravo, A.H. Kong, Food Chem. 132, 1121 (2012)
S.P.M. Germer, M.A. Morgano, M.G. da Silva, N.F.A. Silveira, Dry Technol. (2016). https://doi.org/10.1080/07373937.2015.109044634,997
E. Devic, S. Guyot, J.D. Daudin, C. Bonazzi, J. Agric. Food Chem. 58, 606 (2009)
R.P.F. Guiné, M.J. Barroca, F.J. Gonçalves, M. Alves, S. Oliveira, P.M.R. Correia, Int. J. Fruit Sci. 15, 173 (2015)
L. Neri, I. Hernando, I. Pérez-Munuera, G. S.-F. chemistry, and undefined 2014, Elsevier (n.d.).
Acknowledgements
Authors express their sincere gratitude to Punjab Agricultural university for providing the necessary facilities to carry out this work.
Author information
Authors and Affiliations
Contributions
RC: conceptualization, methodology, preparation, analysis, writing–original draft, editing: VK: supervision, project administration, formal analysis; RK: formal analysis, writing—original draft, review and editing; SK: writing—review and editing; MS: raw material provided RVW: review DK: visualization, RS: editing.
Corresponding authors
Ethics declarations
Competing interests
No conflict of interest is to declare as such.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandel, R., Kumar, V., Kaur, R. et al. Functionality enhancement of osmo-dried sand pear cubes using different sweeteners: quality, bioactive, textural, molecular, and structural characterization. Food Measure 17, 3709–3721 (2023). https://doi.org/10.1007/s11694-023-01894-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-01894-9