Abstract
This study was implemented to investigate the effect of Ganoderma lucidum extract (0.0, 0.5, 1.0, 1.5 and 2.0%) on chemical properties, total phenolic content, antioxidant capacity, proteolysis, microstructure and sensory characteristics of the UF-white cheese during 90 days of ripening at 5 ± 0.1 °C. According to results, addition of Ganoderma lucidum extract into the UF-white cheese formulation caused significant decrease in pH and significant increase in moisture, titrable acidity, total phenolic content and antioxidant activity, during ripening (p < 0.05). Proteolysis indexes including water soluble nitrogen and trichloroacetic acid soluble nitrogen increased significantly (p < 0.05) with increasing the concentration of Ganoderma lucidum extract and reached to 31.16 and 21.42 (% of total nitrogen) at the end of the ripening, respectively. UF-white cheese containing high concentrations of Ganoderma lucidum extract (1.5 and 2%) had a porous structure of casein network with larger pores. The cheeses manufactured with 0.5 and 1.0 of Ganoderma lucidum extract had acceptable sensory properties compared to control cheese. In conclusion, UF-white cheese manufactured with 1.0% of Ganoderma lucidum had the best quality in terms of all quality characteristics.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the high nutritional value, different cheese varieties have remarkable role in the diet of many countries. During the ripening process of cheese, in addition to pH fluctuations and gradual accumulation of amino acids, the hydrolysis of lactose, fat and protein of milk is occurred by glycolysis, lipolysis and proteolysis, respectively. These changes cause a conversion from rubbery and bland curd to mature cheese with desirable texture and sensory properties [1,2,3].
Ultra-filtrated cheese is a group of soft cheeses with moisture content of 60–65% and salt content of 1–3%, which is produced using ultra-filtered pasteurized milk, mesophilic starter culture and commercial microbial rennet Soltani et al. [2]. In the process of cheese production by ultrafiltration, the stage of cutting the curd is not implemented. Therefore, significant amounts of nutrients are not wasted through the whey and preserved in the cheese matrix. On the other hand, and in addition to increasing the nutritional value of the final product, production efficiency is increased in ultrafiltered cheeses compare with cheese manufactured with conventional method due to the preservation of whey proteins in the curd [2, 4].
Food industry experts are trying to design and produce healthy foods in order to respond to consumer demands for the consumption of products with health benefits [5, 6]. In this regard, recently, food manufacturers have paid close attention to the use of natural preservatives including extracts and essential oils of medicinal plants, instead of chemical preservatives in their products. In addition to giving desirable flavor and color to foods, herbs and spices are used in food formulations for formation of antimicrobial and antioxidant properties in the final product [7]. Phenolic compounds present in the structure of plant extracts or essential oils carry bioactive properties like antioxidant capacity and antimicrobial activity. Therefore, food industry can use these compounds as natural preservatives in the formulation of healthy foods [8, 9].
Ganoderma lucidum (G. lucidum) belongs to the family Ganodermataceae that includes about 80 species and mainly grows in climate temperate and subtropical regions such as Europe, Asia, Americas, and Africa. G. lucidum has a kidney-shaped cap and its upper surface is russet with a cloud-like, ring pattern, glossy exterior and woody texture. Although there are many species of G. lucidum, but only six of them have been fully studied for their medicinal benefits, including red, yellow, black, white, blue, and purple Reishi. Among these six species, black, white, and red Reishi have the most important medicinal effects and are now widely used in global markets as health supplements. However, red Reishi has shown the most significant health-enhancing effects [10, 11]. The main contributors for biological efficiency of wild medicinal mushrooms are bioactive compounds. Recent studies have shown that G. lucidum contains over 400 bioactive compounds including triterpenoids, polysaccharides, fatty acids, steroids, proteins, amino acids, alkaloids and nucleosides [12, 13]. Triterpenoids, peptidoglycans and polysaccharides are the major bioactive compounds in G. lucidum, which have caused various biological activities such as antimicrobial, antioxidation, antiliver disorders, anti-inflammation, anti-tumor growth and metastasis [11, 14].
The proteins in this mushroom contains all the essential amino acids, especially lysine and leucine. The low content of total fat and the high ratio of unsaturated fatty acids to total fatty acids show the significant medicinal value of this mushroom. Ganodric acid in G. lucidum has various important medicinal activities such as anticancer properties, histamine secretion, inhibition of cholesterol synthesis, anti-inflammatory, strengthening the immune system, antibacterial and antiviral properties. G. lucidum is also a rich source of minerals and vitamins including potassium (438 mg/100 g), phosphorus (167 mg/100 g), calcium (83 mg/100 g), manganese, magnesium (566 mg/100 g), iron (5 mg/100 g). 10), copper, zinc (1.64 mg/100 g) and vitamin D (438 mg/100 g) [15, 16].
Numerous G. lucidum products are manufactured industrially in the form of dietary supplements in many countries around the world, because of various benefits in maintaining human health. G. lucidum is also mentioned in the list of the Dietary Supplements and Herbal Medicines of U. S. Pharmacopeia [17]. Dairy products, such as cheese, are able to carry functional compounds and release them into the human body because of their ability to form gels. Adding these compounds to cheese can improve its antioxidant activity, functional properties, color and nutritional value [18]. In this study, the effect of using different concentrations of G. lucidum extract as a functional food supplement on chemical properties, antioxidant activity, proteolysis, microstructure and sensory characteristics of UF-white cheese were investigated during 90 days of ripening at 5 ± 0.1 °C.
Materials and methods
Materials
G. lucidum was purchased from Sepid Khosh Company (Qazvin, Iran) and then its scientific name was confirmed by the Herbarium of the Department of Medicinal Plants, Tehran University (Tehran, Iran). Raw milk was prepared from Sharifabad Agro-Industry Co. (Qazvin, Iran). The starter (Lctococcus Lactis subsp. lactis and Lactococcus Lactis subsp. cremoris) and microbial rennet (Rhizomucor miehei) were purchased from Chr. Hansen (Denmark) and Caglificio Clerici (Italy) Companies, respectively. Chemicals used during analysis were all analytical grade and obtained from Sigma Aldrich (St. Louis, USA) and Merck (Darmstadt, Germany) Companies.
Preparation of G. lucidum extract
The selection of G. lucidum mushroom in the cheeses was based on a completely random design. G. lucidum mushroom was washed with distilled water and chopped into small pieces. Then, it was dried in a vacuum oven (UF55/UN, Germany) at 45 °C for 24 h to reach a constant weight. The dried mushrooms were grinded and passed through a 16 µm sieve. The prepared powder was stored at 25 °C in a dry container for further use. G. lucidum powder and methanol 1:5 (w/v) were placed into an Erlenmeyer flask and treated with ultrasound in an ultrasonic bath (Tecno-Gas.S.P.A; Italy) at 300 W for 15 min (based on the best conditions achieved in pre-experimental trails) Then, the mixture of powder and methanol was filtered by Whatman No. 1 filter paper and the solvent was evaporated by rotary evaporator under vacuum (EKA overy 10; Germany) at 40 °C.
Manufacture of UF-white cheese
Cheese samples were manufactured in Rabta Dairy Company (Qom, Iran) using pasteurized bovine milk (pH 6.6, 3.2% fat, 3.12% protein, 11.78% total solid content and 1.029 gml−1 specific gravity) according to method described by Soltani et al. [1] (Fig. 1). First, the raw milk preheated at 45–48 °C and fat content was standardized to 3.0%. Then, two-stages of bactofugation and pasteurization (72 °C for 15 s) were applied to the milk. After ultrafiltration (52 ± 1 °C and pressure of 140 kPa), 1 kg of retentate was obtained from 5.1 kg of milk. The retentate was then pasteurized (78 °C for 60 s), homogenized (5 MPa), cooled to 35 °C and inoculated with starter culture at the rate of 2 g/100 kg of retentate. G. lucidum extract (GLE) was added in different concentrations (0.0. 0.5, 1.0, 1.5 and 2.0% w/w) to the retentate according to Table 1. At the next stage, appropriate amount of microbial rennet (3 g/100 kg of retentate) was added to the milk and the mixture filled into containers (300 g) and sent to coagulation tunnel for coagulation at 35 °C for 20 min. After formation of a uniform coagulum, parchment paper was placed on the top of the coagulum and dry salt (3%) was sprayed on the parchment paper. Finally, cheese containers were sealed with aluminum foil and after holding for 24 h at 26 ± 1 °C, kept in a cold room (5 ± 0.5 °C) for 90 days. Chemical properties, total phenolic contents, free radical scavenging activity (IC50), proteolysis, microstructure and sensory characteristics of cheese samples were evaluated during 90 days of ripening at 5 ± 0.1 °C.
Chemical composition
The UF-white cheese samples were analyzed in duplicate from three batches for moisture by the oven drying method at 102 °C, total nitrogen by the micro-kjeldahl method and titratable acidity by method as described in AOAC [19]. The pH of cheese samples (10 g sample which diluted in 10 ml distilled water) was measured using a digital pH meter (testo® 230, Testo, GmbH & Co, Germany).
Total phenolic content (TPC) and antioxidant activity
Cheese samples were mixed with methanol for 10 min in ratio of 1:10 w/v and centrifuged (3000 rpm, Merck, Germany) for 15 min. Supernatant was used to determine TPC and antioxidant activity. The TPC was measured in cheeses by Folin–Ciocalteau method as described by Ribas et al. [20]. 125 µL of supernatant were mixed with 125 µL Folin-Ciocalteu solution (1:1 deionized water) and 80 µL sodium carbonate (75 g/L). Samples kept in a dark place for 60 min and the absorbance was recorded at 765 nm in a spectrophotometer (Aqualytic AL800, Germany). Gallic acid was used for the standard curve and results were reported in mg of Gallic acid equivalent (GAE)/g of sample.
The antioxidant activity of the samples was determined by the method of Tan et al. [21] based on the DPPH free radical scavenging capacity. A certain amount of the supernatant (2 ml) was mixed with 2 mL of 0.2 mol/L methanolic solutions of DPPH. The mixtures were well shaken for 1 min and then kept in a dark place. The decrease in absorbance at 517 nm was determined with UV–Visible spectrophotometer (Aqualytic AL800, Germany) after 30 min for all samples. The percentage of DPPH free radical quenching activity was determined using the following equation (Eq. 1).
Proteolysis
Water-soluble nitrogen (WSN) and 12% trichloroacetic acid soluble nitrogen (TCA-SN) fractions as % of total nitrogen of the cheeses were determined by the methods described by Hayaloglu et al. [22]. The WSN and TCA–SN were expressed as % of TN. The hydrolysis degree of the cheese casein system during the ripening was performed using urea-polyacrylamide gels (Urea-PAGE) electrophoresis instrument [23]. For this purpose, 10 mg of the pH 4.6-insoluble fraction from each sample in freeze-dried form was dissolved in 1 mL of buffer (containing 40% urea), vortexed for 1 min and stored at − 20 °C until analyzed. Then 10 µL of it was loaded onto the gel. Electrophoresis was performed through stacking and separating gels at 280 and then 300 V, respectively. Staining process was performed using Coomassie Blue G-250 according to the method described by Blakesley and Boezi [24].
Microstructure
Scanning electron microscopy (SEM) with ×3000 magnification (MIRA-TESCAN, Czech Republic) was used to study the microstructure of the manufactured cheese. The samples were coated with a layer of gold by a coating device and then the surface of the prepared samples was imaged at a voltage of 10 kV 1 day after production [25].
Sensory properties
The cheese samples were analyzed after 1, 45 and 90 days of ripening by ten expert panelists from the staff members of dairy products Rabta Company (Qom, Iran). Cheeses were evaluated for appearance and color, body and texture, odor and flavor and overall acceptance of the cheese samples by using an evaluation form. Panelists scored the sample on the basis of five-point hedonic scale, ranging from excellent (score = 5) to dislike extremely (score = 1) as described by Afzaal et al. [26].
Statistical analysis
The data obtained from three trials was analyzed by the analysis of variance (ANOVA) using SPSS software (SPSS package program, version 22.0, SPSS Inc., USA). Significant differences between means were determined by Duncan’s multiple range test. Analysis was implemented at 1, 45 and 90 days of ripening and the obtained results were considered significant at α = 0.05.
Results and discussion
Chemical properties
The results of changes in pH and acidity of UF-white cheese samples during 90 days of ripening at 5 °C are given in Table 2. As can be seen, GLE had a significant effect on changes in pH and titratable acidity of UF-cheese samples (p < 0.05). The values of these parameters also changed significantly during ripening (p < 0.05). The phenolic compounds in the extract may be decomposed into phenolic acids with different acid strengths by oxidative hydrolysis, which increases the acidity of the cheese and decreases the pH [20]. GLE also contains various monosaccharides including glucose, galactose, mannose and xylose, which can affect the fermentation of sugars by lactic acid bacteria and more production of lactic acid during cheese ripening [12]. In a similar study, Solhi et al. [27] in the evolution the effect of asparagus powder on the qualitative characteristics of processed cheese found that the addition of different concentrations of asparagus powder led to a decrease in pH of the samples compared to the control and this decrease continued during 90 days of ripening in the refrigerator. The relationship between pH and titratable acidity of cheese during ripening does not depend only on the amount of lactic acid produced by the microbial flora, but also the buffering capacity of the curd, which itself is due to the amount of casein, citrate and phosphate, are also effective in these changes [28, 29].
On the other hand, ripening period had significant effect on pH and titratable acidity of cheese samples (p < 0.05). As, significant decrease and increase were observed with progressing the ripening in the pH and titratable acidity, respectively, probably due to conversion of lactose to lactic acid by starter cultures and formation of ionic groups during proteolysis [4, 30, 31].
The moisture content of UF-cheese manufactured with different concentrations of GLE during 90 days of ripening are shown in Table 2. According to the results, the addition of GLE in different concentrations has led to a significant (p < 0.05) increase in the moisture content of the samples from 66.94% in the control cheese to 74.21% in the cheese containing 2% of GLE. Moisture content of all cheese samples decreased significantly (p < 0.05) over ripening period. Temperature, pH, incubation time, proteolysis and fat content are important and influential factors on syneresis of curd. Reducing the amount of casein or any other effective factor that leads to weakening of the tissue, also increases the amount of syneresis, so it can affect the moisture content of the cheese [32]. The increase in moisture content of cheese samples by adding different concentrations of the GLE is probably due to the increase in protein degradation because of higher acidic conditions in the cheese matrix. A decrease in protein content can lead to the formation of weaker and fewer bonds between protein and water molecules and increasing in the moisture content of the cheese. Therefore, by decreasing the ability of proteins to absorb and retain water molecules, the amount of dry matter in cheese decreases [27]. The increase in the dry matter of the cheese during ripening is due to the withdrawal of water from the cheese (syneresis) with the development of acidity and shrinkage of the curd [5]. When the pH decreases, the protein matrix is compressed and shortened, which reduces the water holding capacity of the proteins. Therefore, with progressing the ripening and increasing the activity of lactic acid-producing bacteria, the pH and as a result, the moisture content of the cheese decreased [32]. It was reported that low fat UF-soft cheese containing 1–5% of rosemary extract had higher moisture content than control cheese which is consistent with the results of this study [5].
Total phenolic contents and antioxidant activity
Phenolic compounds are secondary metabolites derivate from plants and produced in response to environmental stresses. These compounds have hydroxyl groups and can deactivate free radicals as the result of donating electron or hydrogen donors. These bioactive compounds have antioxidant activity in the result of their ability in chelating heavy metals, neutralizing free radicals and reactive oxygen spices [33, 34]. The amounts of total phenolic contents (TPC) and IC50 of UF-white cheese containing different concentrations of GLE during the ripening period are shown in Table 2. The results showed that different concentrations of GLE and ripening period had significant effect on TPC and IC50 index in UF-white cheese. As expected, with increasing different concentrations of GLE, proportional increase was seen in TPC and antioxidant activity of UF-white cheese. However, TPC and antioxidant activity decreased significantly in all cheese samples, during ripening. Phenolic compounds are unstable and exposed too many enzymatic and chemical reactions during ripening [5]. This is one of the important reasons for the decrease in phenolic compounds during the ripening period. Furthermore, absorption of phenolic compounds of GLE by special peptides produced in cheese could be the reason for the decrease of total phenolic contents in fortified UF-white cheeses during ripening. These peptides can be produced by the residual proteolytic agents during ripening, which deactivate the phenolic compounds present in cheese [27]. Researches have shown that the main reason for the anti-inflammatory and antimicrobial properties of G. lucidum is due to the presence of phenolic compounds such as tannins, flavonoids and terpenes that are able to inhibit metal elements and interfere with the metabolic mechanism of bacteria and fungi [11, 35]. On the other hand, it has been reported that phenolic compounds interact with proteins through hydrogen, hydrophobic, ionic, covalent and other reactions and these interactions can alter the activity of polyphenol compounds in cheese [36]. It has been also stated that polyphenols in their free form have stronger antioxidant effects than when they are bound to proteins [37]. In consistency with the result of the present study, the results of a study conducted by Ribas et al. [20] on fresh cheeses showed that by adding basil extract to the cheese formulation, the amount of phenolic compounds in cheese increased due to the presence of different phenolic compounds in basil. In a relevant study, in addition to determining the antioxidant and antibacterial activity, the anti-Listeria effect of broccoli sprout extract was confirmed in Ricotta cheese [9].
Proteolysis
Proteolysis as the main biochemical event of cheese ripening causes development in cheese texture via hydrolysis of protein matrix and formation of desirable flavor compounds through the release of short peptides which contribute to umami taste in final product [38]. While the action of coagulant enzyme and indigenous proteinases is responsible for primary proteolysis of the caseins in the cheese, secondary proteolysis is mainly due to role of starter and non-starter lactic acid bacteria in degradation of caseins [39, 40]. The contents of WSN (% of total nitrogen) as primary proteolysis index and TCA-SN (% of total nitrogen) as secondary proteolysis index in UF-white cheese samples manufactured with different concentrations of GLE during ripening, are shown in Table 3. Cheese samples containing GLE had more WSN than the control cheese and with increasing the concentration of the extract, the WSN content was increased significantly (p < 0.05) probably due to the positive effect of GLE compounds on expansion of proteolysis. In a related research, it was stated that the using asparagus powder led to a significant increase in the amount of WSN during ripening of processed cheese [27]. Moreover, it was reported that chili pepper extract did not inhibit proteolysis and caused significant increase in free amino acids of Gouda cheese, during ripening [41]. On the other hand, the results showed that in all cheese samples, the amount of WSN increased significantly during ripening due to the proteolytic activity of lactic acid bacteria and expansion of proteolytic activities in cheese during ripening [1, 42].
Measuring test of non-protein nitrogen compounds is a useful technique for assessing secondary proteolysis products including small peptides, urea and amino acids [27, 43]. According to the results presented in Table 3, different GLE concentrations used for manufacturing of UF-white cheese caused significant changes in the amount of TCA-SN. As, increasing the GLE concentration led to significant increase in TCA-SN during ripening (p < 0.05), probably due to the higher availability of primary proteolysis products as a substrate for continued proteolysis [40, 41]. The values of TCA-SN were also increased significantly (p < 0.05) in all cheese samples during ripening due to proteolytic activity of lactic acid bacteria and liberation of intermediate and lower molecular weight peptides [40, 42].
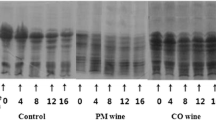
The protein network that forms the curd structure in fresh cheeses, loses some parts of its original structure during cheese ripening. Electrophoresis can be useful for measuring protein status at different stages of ripening period [44]. This method detects degraded proteins and to some extent the existing proteases. The intensity of cheese aroma seems to be related to soluble nitrogen compounds, especially small peptides and amino acids [45]. Urea-polyacrylamide gel electrophoresis (Urea-PAGE) was used to evaluate the proteolysis process. For this purpose, casein of UF-white cheeses containing different concentrations of GLE were tested at the first and 90th days of the ripening period. The results of Urea-PAGE showed that with increasing ripening time, the intensity of αs1-casein bands decreased in all cheese samples, and these degradation of αs1-casein was higher in cheeses containing the GLE than control sample (Fig. 2), which indicated an increase in the intensity of proteolysis during the ripening period, especially in samples containing GLE extracts.
In ripened cheeses, αs1-casein and β-casein are hydrolyses to medium and small peptides by chymosin and cell wall proteases. These peptides are also broken down by starter lactic acid bacteria into smaller peptides and amino acids [38]. It was also observed that the hydrolysis rate of β- casein is lower during ripening due to the higher resistance of this proteins to salt and acidic conditions [45]. Also, plasmin which plays a major role in the breakdown of β-casein in UF-white cheeses is inhibited by existing inhibitors, especially β- lactoglobulin and this factor is responsible for reducing the breakdown of β- casein in this type of cheese. Hydrolysis of β-casein by plasmin leads to the formation of γ1, γ2 and γ3 caseins and peptone protease [44, 46]. As the proteolysis process progresses, the casein proteins are decomposed to soluble compounds (Table 4), resulting in a decrease in the staining of the bands in the gel electrophoresis, which is consistent with the results of data obtained from soluble nitrogen, and indicated the significance effect of ripening time and GLE on proteolysis intensity [27]. The basis of isolation in electrophoresis is the difference in molecular weight, electric charge and structure of different proteins [44]. During the proteolysis process, as the pH decreases, more calcium phosphate in the casein structure comes out of the colloidal state and the casein micelle becomes more unstable and in consequence, is exposed to more proteolytic enzymes [46].
Microstructure
The SEM images of the UF-white cheeses at the first day of ripening are shown in Fig. 3. The results revealed that the addition of GLE to formulation had an important effect on the porous structure of casein network in the UF-white cheeses. As can be seen, the control cheese presented more homogeneous and denser microstructure due to regular and smaller accumulations of casein and fat globules. While samples containing GLE especially at higher concentrations (1.5 and 2%), did not have a uniform structure and had a weaker and more open protein network. According to Fig. 3, using higher levels of GLE for manufacturing UF-white cheese led to the creation of the large and deeper porous structure in the protein network, which can be seen in the images as darker areas.
The addition of GLE to UF-white cheese increased the acidity and accelerated the process of proteolysis, and these factors are also effective in breakdown the casein network and creating porous structure in the cheese. There are also various enzymes in the structure of GLE, including glutamic protease, α-1,2-mannosidase, β-N-Acetylhexosaminidase and β-1,3-glucanase, which can be effective in hydrolyzing the casein network. Hydrolyze of protein lead to production of water-soluble breakdown products and these products can not contribute to framework provided by the protein matrix [47, 48]. In terms of cheese structure, more protein breakdown may cause the formation of less solid-like structure and more presence of spaces in cheese [2].
Sensory properties
The sensory analysis is very important in the elaboration of food products. The average score points of UF- cheese fortified by GLE are described in Table 4. Evaluation of the appearance and color score of cheese samples showed that the addition of GLE at concentrations of 0.5 and 1% had no significant effect on the appearance and color scores of the UF-white cheese samples compared to the control cheese. However, with increasing GLE concentration to 1.5 and 2%, appearance and color score of the cheeses samples decreased significantly compared to other samples (p < 0.05) and at the end of the ripening, the lowest color score belonged to the sample containing 2% GLE (Table 5). Appearance and color are one of the most important visual features in dairy products. The yellowish color of ripe cheese is due to the scattering of light by casein and fat network, which occurs during the process of proteolysis and lipolysis in cheese [20]. The results of this study was in accordance with those found by Giroux et al. and Ribas et al. [20, 49], which determined more yellow intensity for cheddar cheese and fresh cheese with addition of green tea and basil extract, respectively.
The results also showed that the use of the GLE in concentrations less than 1% improved the body and texture and odor and flavor scores of cheese compared to the control, while the cheese samples manufactured with 1.5 and 2% GLE had lower scores of these parameters and these changes were noticeable during ripening. The free fatty acids produced during the process of lipolysis and the metabolism of carbohydrates and amino acids by lactic acid bacteria during the proteolysis process, are directly involved in the development of cheese flavor, especially when there is a proper balance between proteolysis and lipolysis products and other reactions in cheese. Lactic acid bacteria, because of their ability to release proteases, lipases and β-galactosidase, can be used as a starter in the cheese fermentation process to create a unique taste, aroma and texture [50]. As previously mentioned, the GLE due to its protease activity and the presence of glucose and galactose monosaccharides is effective in promoting the process of proteolysis and more activity of lactic acid bacteria, and thus play a role in improving the proper flavor of cheese. The decrease in flavor score at higher concentrations of the GLE is probably due to the production of bitter peptides in the result of higher proteolysis and the strong aroma of the extract, which limits the use of its higher doses. These findings were similar to the results obtained by Adesokan et al. and Abd El-Aziz et al. [51, 52] which reported that addition of ginger extract into Ogi cheese and soft cheese at lower concentrations significantly improved cheeses sensory properties.
Finally, the results of overall acceptance of the cheeses demonstrated that in both fresh and ripe state, the cheese samples manufactured using 0.5 and 1% GLE received an acceptable score from the panelists (up to 4.79) and the cheese samples manufactured with 1.5 and 2% GLE were not satisfied with the consumers. According to the results of this study, it can be stated that the addition of bioactive compounds (such as extracts) in foods at the appropriate dose leads to improved sensory properties and antioxidant activity of them. However, using higher doses of mentioned compounds can lead to a bitterness and negatively affect color, texture and flavor of foods and decreases consumer acceptability.
Conclusions
The quality characteristics of UF-white cheese manufactured using different concentrations of GLE were evaluated during 90 days of ripening. The cheeses fortified with GLE had significantly higher acidity, moisture, total phenolic content and antioxidant activity than the control one, during ripening. Proteolysis in UF-white cheese was affected by contribution of GLE, where significant increase in WSN and TCA-SN observed by increasing GLE concentration. On the other hand, protein content of all cheese samples decreased significantly, during ripening due to partial decomposition of casein network by proteolysis. Regarding sensory properties, UF-white cheeses manufactured with 0.5 and 1% GLE received higher scores among the cheeses fortified with GLE. In conclusion, the incorporation of 1% GLE caused the best results in UF-white cheese in terms of overall quality properties.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
M. Soltani, N. Guzeler, A.A. Hayaloglu, J. Dairy Res. 82, 365–374 (2015)
M. Soltani, D. Sahingil, Y. Gokce, A.A. Hayaloglu, J. Dairy Sci. 99, 7744–7754 (2016)
A. Najafi, M. Ziabakhsh Deylami, H. Karimian, A. Abedinia, M. Hosseini Nejad, Food Technol. Nutr. 8, 85–91 (2011)
M. Soltani, S. Saremnezhad, A.R. Faraji, A.A. Hayaloglu, Int. Dairy J. 125, 105232 (2022)
M.F. Hala, E.D. Ebtisam, I. Sanaa, M.A. Badran, A.S. Marwa, Am. J. Sci. 6, 570–579 (2010)
E. Kondyli, E.C. Pappa, D. Arapoglou, M. Metafa, Ch. Eliopoulos, C. Israilidis, Foods. 11, 417 (2022)
C. Martinez-Gracia, C.A. Gonzalez-Bermudez, A.M. Cabellero-Valcarcel, M. Santaella-Pascual, C. Frontela-Saseta, Curr Opin. Food Sci. 6, 38–43 (2015)
A. Abedinia, F. Alimohammadi, F. Teymori, N. Razagardani, M.R. Saeidi Asl, F. Ariffin, A. Mohammadi Nafchi, N. Huda, J. Roslan, Foods 10, 1761 (2021)
Z. Azarashkan, S. Farahani, A. Abedinia, M. Akbarmivehie, A. Motemedzadegan, J. Heidarbeigi, A.A. Hayaloglu, Int. J. Food Microbiol. 376, 109761 (2022)
D. Cör, Ž Knez, H.M. Knez, Mol. 23, 649–670 (2018)
Y. Yang, H. Zhang, J. Zuo, Biomed Dermatol. 3, 1–7 (2019)
A. Kubota, M. Kobayashi, S. Sarashina, R. Takeno, K. Okamoto, K. Narumi, J. Ethnopharmacol. 214, 240–243 (2018)
J. Dimitrova-Shumkovska, F. Kosharkoska-Spasovska, L. Krstanoski, M. Karadelev, J. Food Biochem. 5, e14364 (2022)
R. Sarnthima, S. Khammaung, P. Sa-Ard, J. Food Sci. Technol. 54, 3724–3730 (2017)
M.F. Moradali, G.H.A. Hedjaroude, H. Mostafavi, M. Abbasi, Sh. Ghods, A. Sharifi-Tehrani, Mycotaxon 99, 251–269 (2007)
M. Obodai, D.L. Narh Mensah, A. Fernandes, N.K. Kortei, M. Dzomeku, M. Teegarden, S.J. Schwartz, L. Barros, J. Prempeh, R.K. Takli, I.C. Ferreira, Mol. 22, 196 (2017)
D.T. Wu, D.Y. Deng, L.X. Chen, J. Zhao, A. Bzhelyansky, S.P. Li, Sci Rep. 7, 1–10 (2017)
A.F. Farrag, M.M. El-Sheikh, M.T. Fouad, A.F. Sayed, M.A. El-Aziz, J. Biol Sci. 19, 65–73 (2019)
AOAC, Association of Official Analytical Chemists, Official Method of Analysis, 18th edn. (Benjamin Franklin Station, Washington, 2007)
J.C. Ribas, P.T. Matumoto-Pintro, A.C.P. Vital, B.R. Saraiva, F.A. Anjo, R.L. Alves, L.M. Zeoula, J. Food Sci. Technol. 56, 5214–5224 (2019)
S.P. Tan, T.C. Kha, S. Parks, C. Stathopoulos, P.D. Roach, Foods. 4, 400–419 (2015)
A.A. Hayaloglu, M. Guven, P.F. Fox, P.L.H. McSweeney, J. Dairy Sci. 88, 3460–3474 (2005)
S.L. Shalabi, P.F. Fox, Irish. J. Food Sci. Technol. 11, 135–151 (1987)
R.W. Blakesley, J.A. Boezi, Annu Rev. Biochem. 82, 580–581 (1977)
A. Madadlou, A. Khosroshahi, M.E. Mousavi, J. Farmani, Int Dairy J. 17, 547–554 (2007)
M. Afzaal, F. Saeed, H. Ateeq, A. Ahmed, A. Ahmed, T. Tufail, Z. Ismail, F.M. Anjum, Food Sci & Nutr. 8, 2739–2747 (2020)
P. Solhi, S. Azadmard-Damirchi, J. Hesari, H. Hamishehkar, Int J. Dairy Tecnol. 73, 226–233 (2020)
G.G. Amador-Espejo, I.I. Ruiz-Lopez, P.J. Gibbens-Bandala, R.J. Delgado-Macuil, H. Ruiz-Espinosa, Ultrason Sonochem. 76, 105621 (2021)
S.P. Silva, S.C. Ribeiro, J.A. Teixeira, C.C. Silva, LWT 153, 112486 (2022)
Y.E. Gezmiş, Z. Tarakçı, J. Food Process. Preserv. 44, e14868 (2020)
C.C. Mureşan, R.A.V. Marc, C. Anamaria Semeniuc, S. Ancuţs Socaci, A. Fărcaş, D. Fracisc, C.R. Pop, A. Rotar, A. Dodan, V. Muresan, A.E. Muresan, Foods 10, 258 (2021)
M.A. Murtaza, S.U. Rehman, F.M. Anjum, N. Huma, O.M. Tarar, G. Mueen-ud-din, J. Food Biochem. 36, 99–106 (2012)
A. Selahvarzi, M.R. Sanjabi, Y. Ramezan, H. Mirsaeedghazi, F. Azarikia, A. Abedinia, J. Food Process. Preserv. 45, e15726 (2021)
A. Selahvarzi, M.R. Sanjabi, Y. Ramezan, H. Mirsaeedghazi, F. Azarikia, A. Abedinia, J. Food Meas. Charact. 15, 5683–5694 (2021)
R. Ghobadi, R. Mohammadi, J. Chabavizade, M. Sami, Adv. Biomed. Res. 7, 24–28 (2018)
S.M. El-Sayed, A.M. Youssef, Heliyon. 5, e01989 (2019)
S.A. Tabibian, M. Labbafi, G.H. Askari, A.R. Rezaeinezhad, H. Ghomi, J. Food Eng. 270, 109766 (2020)
Y. Ardö, P.L.H. McSweeney, A.A.A. Magboul, V.K. Upadhyay, P.F. Fox, in Biochemistry of Cheese Ripening. ed. by P.L.H. McSweeney, P.F. Fox, P.D. Cotter, D.W. Everett (Elsevier Academic Press, London, 2017), p.445
J. Hesari, M.R. Ehsani, A. Khosroshahi, P.L.H. McSweeney, Lait 86, 291–302 (2006)
A.A. Hayaloglu, B. Karatekin, H. Gurkan, Int Dairy J. 38, 136–144 (2014)
Y.K. Kim, M.S. Nam, H.C. Bae, Korean J. Food Sci. Anim Resour. 37, 833–839 (2017)
M. Soltani, D. Sahingil, Y. Gokce, A.A. Hayaloglu, J. Food Sci. Technol. 56, 589–598 (2019)
M. Abd El-Aziz, S.H.S. Mohamed, F.L. Seleet, M.A.L. Abd El-Gawad, Am J. Food Technol. 10, 37–47 (2015)
S. Niro, A. Fratianni, A. D’Agostino, I. Notardonato, G. Panfili, Italian J. Food Sci. 33, 46–51 (2021)
E.A. Romeih, A. Michaelidou, C.G. Biliaderis, G.K. Zerfiridis, Int Dairy J. 12, 525–540 (2002)
N. Gutiérrez-Méndez, A. Balderrama-Carmona, S.E. García-Sandoval, P. Ramírez-Vigil, M.Y. Leal-Ramos, A. García-Triana, Foods. 8, 44 (2019)
K. Kumakura, C. Hori, H. Matsuoka, K. Igarashi, M. Samejima, J. Sci. Food. Agric. 99, 529–535 (2019)
A. Madadlou, A. Khosroshahi, M.E. Mousavi, J. Dairy Sci. 88, 3052–3062 (2005)
H.J. Giroux, G. De Grandpre, P. Fustier, C.P. Champagne, D. St-Gelais, M. Lacroix, M. Britten, Dairy Sci. Technol. 93, 241–254 (2013)
M.G. Wilkinson, G. LaPointe, J. Dairy Sci. 103, 10963–10985 (2020)
J.A. Adesokan, O.P. Abiola, M.O. Ogundiya, Afr J. Biotechnol. 9, 1803–1808 (2010)
M. Abd El-Aziz, S.H.S. Mohamed, F.L. Seleet, Pol. J. Food Nutr. Sci. 62, 77–83 (2012)
Acknowledgements
The authors would like to thank Mr. Ahmad Paidar Roodmajani (director manager of Sharifabad Agro-Industry Co.) for providing raw materials and production equipment of the cheeses.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mousavi, R.S., Nateghi, L., Soltani, M. et al. Innovative UF-white cheese fortified with Ganoderma lucidum extract: antioxidant capacity, proteolysis, microstructure and sensory characteristics. Food Measure 17, 1651–1661 (2023). https://doi.org/10.1007/s11694-022-01732-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01732-4