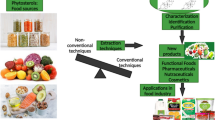
Abstract
The phytonutrient extract helps in the development and manufacturing of nutraceuticals and food additives. Some of these secondary metabolites, such as polyphenol, anthocyanins, carotenoids, flavonoids, phytosterols, terpenoids, and others, are extracted from plants. These phytonutrients provide many health benefits like prevention and treatment of a variety of diseases such as cardiovascular disease, neurological disorders, atherosclerosis, certain cancers, and diabetes. Traditional extraction methods such as Soxhlet, maceration, decoction, percolation and infusions are still used, but significant improvements can be made by using novel or greener extraction methods, including accelerated solvent extraction, microwave-assisted extraction, ultrasound-assisted extraction, supercritical fluid extraction, and enzyme-assisted extraction. Once extracted, these phytonutrients can be utilised in the cosmetic, nutraceuticals, pharmaceutical, or food industries, with the later focusing on improving food quality. This article aims to provide a comprehensive overview of the various techniques used in the extraction and isolation of phytonutrients. Additionally, this article describes the advantages, disadvantages, practical examples, and a comparative study of these processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytonutrients are phytochemicals (from the Greek word “phyto”, meaning plant) or secondary metabolites present in plant-based foods and have nutritional value, and their biological activities help in providing various good health benefits and prevention of diseases for humans than those attributed to micronutrients and macronutrients [1]. In general, the plant chemicals that protect plant cells from damage and diseases caused by environmental hazards such as pathogenic attacks, droughts, pollution, UV exposure, stress, and drought are called phytochemicals.
These phytochemicals also contribute to color, flavor, and aroma [2]. According to epidemiological recommendations, phytonutrient intake is advised since it has been employed in pharmaceutics and has a positive influence on the healthcare due to its antioxidant, anticancer, anti-inflammatory, and other properties. Food bioactive such as polyphenolics and phenolics have high antioxidant activity [3, 4]. According to the WHO, approximately 21,000 plant species are capable of being used as traditional medicines, and 80 percent of people worldwide rely on herbal medicines for some of their basic health care needs. Plant pharmaceuticals are estimated to make up to 25% of total drugs in developed nations, while they account for up to 80% in rapidly developing nations like India and China [5]. The bioactive compounds that are produced by the biological system are typically divided into primary metabolites (chemical substances like sugar, amino acids, fatty acids, protein, and lipids) vital for growth and development, and polyphenols, glycosides, quinones, terpenoids, saponins, allicin, flavonoids, and scopolamine are the secondary metabolites that have antimicrobial properties since their use is increasing rapidly for the management of diseases [6, 7]. Natural bioactive compounds are essential in the development of novel drugs. According to research, fruits and vegetables have antiplatelet activity, which may impact the development of CVD [8].
The extraction and purification of phytonutrients are the most significant processing stages in the production of phytonutrient compounds. Various solvents are employed in the extraction procedure, depending on the target compound and solvent toxicity. Novel technologies for extracting phytonutrients from plants, which include microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), accelerated solvent extraction (ASE), enzyme-assisted extraction (EAE), and supercritical fluid extraction (SFE), have been developed to fulfill the increasing demand for nutraceuticals. These methods shorten extraction times, utilize less solvent, and improve extraction efficiency. Many advanced and effective extraction methods have been developed in recent years to overcome the limitations of conventional extraction methods. These techniques have benefits over traditional methods, such as shorter extraction times and lower solvent volumes with higher extraction yields. These new techniques are also known as “Green Extraction” methods [9].
Phenolic compounds (PCs)
Phenolics are a group of chemical compounds occurring in all plants in variable amounts as bioactive compounds. As a result, the three types of PCs are those that have (a) a single benzene ring (C6), (b) a C6–Cn class, or (c) a more complex skeleton, the C6–Cn–C6 class (Table 1). In their structure, all PCs have at least one aromatic ring and one hydroxyl group [10, 11]. PCs derived from plants can be monomeric or polymeric. Flavonoids subtypes are the most prevalent monomeric compounds, whereas hydroxycinnamic and hydroxybenzoic acids are non-flavonoids. Polymeric PCs are derived from phenolic acids (gallotannins and ellagitannins) and flavan-3-ols (proanthocyanidins) [12].
Flavonoids
Flavonoids have the phenyl chromanone structure C15 (C6–C3–C6). Flavonoids have a phenyl benzopyran skeleton with two chromane rings joined by a heterocyclic pyran ring at C2 and isoflavonoids have a phenyl benzopyran skeleton with two chromane rings connected by a heterocyclic pyran ring at C3 [13, 14]. Another important flavonoid is kaempferol, which is derived from kalanchoe. Flavonoids occur in nearly all species of plants, accounting for almost two-thirds of dietary PCs. Flavanone, flavonol, isoflavone, flavone and anthocyanin are the subclasses of flavonoids (Fig. 1). Golden berry, waxy barley, Dracocephalun kotschyi, Lippia citriodora, Moringa oleifera leaves, grape skin (Tintilla de Rota), apple (Molus domestica) roots, and black rice (Oryza sativa cv. Poireton) husk [14, 15].
Flavonoid compounds help in the development of different pharmacological activities. These compounds have also attracted the interest of extraction because they possess antioxidant, anti-inflammatory, antibacterial, anticancer, and antiviral effects [16,17,18]. When these compounds are added to food products, they help in preventing fat oxidation, protecting enzymes and vitamins, and also colour and aroma [19].
Isoflavones
These are the subclass of flavonoids and have a similar structure to estrogen. Isoflavones or phytoestrogens help in the prevention of atherosclerosis, heart diseases, and cancer [18]. Isoflavones are produced by Fabaceae species.
Anthocyanins
Anthocyanins possess the characteristic C6C3C6 carbon skeleton (Fig. 2). Anthocyanins are the natural pigments that give plants their red, violet, orange, purple, blue, and magenta colors, whereas betalain is made up of yellow–orange betaxanthin and red–violet betacyanin [20]. The anthocyanin can be extracted from many different plant species, including red radish (Raphanus sativus) root, rhubarb (Rheum rhaponticum) stem, blackcurrant (Ribes nigrum), cranberry (Vaccinium common), purple corn (Zea mays), grape (Vitis spp.), and red onions (Alium cepa). Temperature, pH, oxygen, and light are the factors that influence anthocyanin stability and cold.
Besides, these pigments have shown therapeutic or medicinal properties such as antioxidant or antilipoperoxidant activity [21, 22], anti-inflammatory activity [23], anticonvulsant activity [24], the ability to lower serum cholesterol [25] and serum lipid levels [26], and the ability to act as chemoprotective in certain cancer therapies [27].
Polyphenols (tannins)
Tannins are sometimes referred to as polyphenols. Tannins are a heterogeneous group of water-soluble polyphenolic chemicals found in plants, foods, and beverages, their molecular weights range from 500 to 3000 Da and up to 20 hydroxyl groups. Complex, hydrolyzable, proanthocyanins, or condensed, and phlorotannins are types of tannin [28] (Fig. 3). They are biogenetically derived from two major biosynthetic pathways: the shikimate pathway and the acetate pathway [29, 30]. Tannin may be derived from a variety of plants including Rhizophora apiculate, Limonium delicatulum, pomegranate (Punica granatum L.), persimmon (Diospyros kaki L.), Vaccinium vitis-idaea L., green tea (Camellia sinensis), and sericea lespedeza (Lespedeza cuneata), etc.
They are beneficial for the treatment of diabetes, diarrhea, accelerated blood clotting, and bleeding gums. They also reduce blood pressure and skin injuries. Tannin has antitumor [31], antimutagenic, antinutrient effect, diabetes mellitus, anticarcinogenic properties, antioxidant properties [32], antimicrobial activity, and antiplasmodial [31,32,33,34,35].
Lignans and neolignans
Lignan and neolignans are secondary metabolites and a large group of dimeric phenylpropanoids as ββ′-dimers of phenylpropane (C6C3) unit. Furofuran, furan, dibenzylbutyrolactol, aryltetralin, dibenzylbutyrolactone, arylnaphthalene, dibenzylbutane, and dibenzocyclooctadiene are the 8 subgroups of lignans while neolignans consist of 15 subtypes (NL) [36] (Fig. 4a, b). Dihydrobenzofuran neolignans, podophyllotoxin, 1-arylnaphthalene lignans, and naphthalenic lignans are some examples of lignans and neolignans used as lead compounds. Their main economic sources include Podophyllum peltatum L., Virola surinamensis, Podophyllum emodi, Linum album, Linum usitatissimum, Linum bienne, Anthriscus sylvestris L., Linum linearifolium L., Linum setaceum Brot. [37,38,39]. Lignan possesses various biological activities, including antitumor, antiangiogenic, antiviral, antifungal, hypolipidemic, and antirheumatic agents [37].
Phytosterols
Plant sterols, also known as phytosterols, are bioactive compounds derived from plants that have a 1,2-cyclopentanophenanthrene (5α- or 5β-gonane) carbon skeleton with methyl substituents at C-10 and C-13 and an alkyl substituent (side-chain) at C-17 [40, 41]. Nonconjugated free sterols and conjugated glycosylated such as steryl esters, acylated steryl glycosides, and steryl glycosides are examples of phytosterols [42]. Phytosterols found in plants include stigmasterol, 5-avenasterol, -sitosterol, and campesterol (Fig. 5). They are distinguished from cholesterol by the presence of an alkyl group at C-24. Grape seeds, caraway, roselle seeds, sea buckthorn seeds, anise, white mustard, nutmeg seeds, rapeseed, tomato seeds, corn, sesame, coriander, oat, peanut, pumpkin seeds, goldenberry pomace, and lotus bee pollen can all be used to extract phytosterols [43].
They are anti-polymerization properties [44], antioxidant activity [45], anti-inflammatory [46], anticancer [47], reduction in blood cholesterol levels, obesity, immunity-stimulating properties, anti-osteoarthritis [48, 49].
Terpenoids
Terpenoids are the most abundant secondary metabolite group. Terpenoids, also referred to as isoprenoids, are formed by combining two or more isoprene molecules (a five-carbon unit, chemically known as 2-methyl-1,3-butadiene) and are classified in terms of C10-units (Table 2). Terpenoids possess a broad range of biological activities anticancer activity [50], anti-inflammatory [51], antimicrobial activity, antinociceptive effects, and antifoaming and carminative activity [52].
Limonoids (tetranortriterpenoids)
Limonoids are a class of highly oxygenated modified triterpenoids with a diverse range of biological activities. Although restricted occurrences in the plant kingdom, these compounds are found extensively in the Meliaceae and Rutaceae families. Limonoids are of great interest in science given that the small number of plant families where they occur exhibit a broad range of medicinal properties that promote health and prevent diseases [53]. Limonoids comprise a large class of C26 degraded triterpenes, as opposed to intact triterpenes which possess thirty carbon atoms (C30H48) derived from six isoprene (C5H8) units [54] (Fig. 6).
Besides their health-promoting effects, various limonoids have been shown to possess antioxidative, anti-inflammatory, immunomodulatory, insecticidal, antiviral, anti-hyperglycaemic activities, neuroprotective, anti-obesity, antitumor and anti-bacteria [55]. Limonoids are widely distributed in many Citrus fruits such as lime (Citrus aurantiifolia), pummelo (Citrus maxima), sweet orange (Citrus sinensis), lemon (Citrus limon), grapefruit (Citrus paradisi) and sour orange (Citrus aurantium) [56]. The antioxidant properties of the main component of the genus Citrus, limonene, may be useful to prevent neuronal suffering induced by Aβ1–42 oligomers, preventing the hyperactivity of KV3.4 [56].
Carotenoids
Carotenoids are naturally occurring pigments (red, yellow, and orange) that are divided into two main categories depending on their function: xanthophylls, which include lutein and zeaxanthin, and carotenes, which also include (α-carotene, β-carotene, and lycopene) [57] (Fig. 7). The main sources of carotenoids include tomato (Solanum lycopersicum), carrot (Daucus carota), corn (Zea mays), citrus fruits, watermelon (Citrullus lanatus), peas, and saffron, pumpkins (Cucurbita pepo). Zeaxanthin and lutein possess antioxidant properties [58]. Epidemiological studies suggest that the consumption of rich carotenoids helps in eye health [59, 60], cognitive performance [61, 62], cardiovascular health [63,64,65], cancer prevention [66,67,68], and infant nutrition [69, 70].
Phenolic acids
Phenolic acids are comprised of two large classes: hydroxycinnamic acid (or cinnamic acid derivatives) and hydroxybenzoic acid (or benzoic acid derivatives) [71] (Fig. 8). The hydroxybenzoic acid and hydroxycinnamates derivatives are mostly found in plants as glucosides (or esters). Hydroxycinnamic acid derivatives are mainly represented with hydroxy carboxylic acids or glucose as malic acid, tartaric acid, α-hydroxy-hydrocaffeic acid, hydroxycitric acid, gluconic acid, tartronic acid, galactaric acid, glucaric acid, and 4-methoxyaldaric acid. Benzoic acid derivatives occur mostly in three forms: p-hydroxybenzoic, vanillic, and protocatechuic acid derivatives [72]. According to Naczk and Shahidi [73], phenolic acids are phenylpropanoid and cinnamic acid derivatives.
The primary sources of phenolic acid include are Vitis rotundifolia, rapeseed (Brassica napus), Vitis labrusca, linseed (L. usitatissimum), pepper (Piper nigrum), sunflower fruits (Helianthus annuus), small radish (R. sativus), sweet cherries (Bigarreau Napoleon), yellow mustard seed (Sinapis alba), Dendropanax morbifera, Cinnamomum cassia (Chinese cinnamon) Panax ginseng, grapes, orange, carrot, caraway, and pineapple stems. They possess various biological activities like antimicrobial activity [74], antifungal activity [75, 76], anticancer activity [77], antiplasmodial activity [78], neurological activity [79], antidiabetic activity, antioxidant activity [80, 81] and anti-inflammatory activity [82, 83].
Glucosinolates
Glucosinolates are common anionic and sulfur-rich plant secondary metabolites found in the Brassica genus [84]. The highest quantities of dietary isothiocyanates are found in cruciferous vegetables. 2-Propenyl (allyl), indole-3-methyl, 4-methylsulfinylbutyl (sulforaphane), 3-methylsulfinylpropyl, 3-butenyl, and 2-hydroxyl-3-butenyl are some of the most significant isothiocyanates found in the Brassica genus [85]. Field mustard (Brassica rapa), broccoli (Brassica oleracea var. italica), maca (Lepidium meyenii), canola (B. napus L.), cabbage (Brassica oleracea var. capitata), cauliflower (Brassica oleracea var. botrytis), mustard green (Brassica junicea), turnip (Brassica rapa ssp. rapa), kale (Brassica oleracea var. acephala), and Brussels sprout (Brassica oleracea var. gemmifera). The chemical structure of glucosinolates and isothiocyanates is determined by the molecular structure of the sidechains of the glucone moiety and the aglucone sidechain. This decides whether the glucosinolates are aromatic (Sinigrin), aliphatic (Glucobrassicin), or indole (Gluconasturtiin) [86] (Fig. 9).
They possess various health benefits like antioxidant activity [87,88,89], anticancer activity [90, 91], anti-inflammatory activity [92, 93], and other activities [94,95,96] (Table 3).
Technological methods for extraction of phytonutrients from different plant sources
The initial step in acquiring bioactive phytonutrients from plant sources is extraction. The right extraction solvent, as well as parameters such as temperature, exposure times, solid–liquid ratio, and so on, are critical for extracting and isolating phytonutrients from a complex and wide variety of substances. Traditional methods, as well as non-conventional methods, are being used to extract plant phytonutrients. Traditional extraction methods include SE, ME, decoction, and infusions, whereas newer or greener extraction methods include ASE, EAE, MAE, and SFE. In addition, some of the principles and mechanisms of traditional methods are explained.
Conventional/traditional extraction methodologies for phytonutrients
Maceration
Maceration is one of the simplest extraction methods and involves heat transfer (conduction and convection). Maceration is a commonly used process for isolating nonvolatile plant compounds for use in the pharma industry [106]. The plant materials are mixed or soaked with the solvent in a jar with regular agitation and allowed to stand at room temperature for 2–5 days in these methods. This process softens and breaks down the plant cell walls, causing the phytonutrients to be liberated. The mixture materials are then pressed, filtered, or centrifuged at the end of the operation. If the plant material is too small to be filtered, centrifugation is frequently required. The remaining plant matter is repeatedly extracted using new solvents to achieve complete extraction [107]. Maceration has some limitations such as long duration time, extended concentration steps, and large volumes of hazardous solvents [108]. When the extraction was carried out by macerating with 50% acetone for 30 min. As a result, maceration proved to be the most effective method for extracting flavonoids [109]. The extraction of orientoside and luteolin from Cajanus cajan leaves [110]. The maceration extracted from Arbutus unedo L. fruits was discovered to be the most successful approach capable of generating catechin under optimal extraction conditions [111].
Infusions
The term “infusion” is mainly used for the preparation of dilute solutions of readily soluble phytonutrients from crude drugs. Infusion is a simple chemical process used to extract plant material that is volatile and dissolves readily or releases its active ingredients easily in organic solvents [112]. Infusion uses the same principle as maceration; in this method, plant materials are prepared by macerating them for a short period in a specific volume of cold or boiling water (e.g., 1:4 or 1:6, for 15 min) and then filtering them through a filter or filter paper. The maceration time for infusion is, however, shorter. The liquid may then be separated and concentrated under a vacuum using a rotary evaporator [108]. The plant material is ground into a fine powder, placed in a glass container, and either a hot or cold extraction solvent is applied on top of the material for a few minutes before being left alone. This method is particularly effective for obtaining readily soluble secondary metabolites [113]. Infusion finds its application in tea preparation and consumption prescribed for psychophysical asthenia, diarrhea, bronchitis, asthma, etc. In tropical Africa, the infusion of the bark of Prunus africana (pygeum) is taken orally to increase the ease of urination and reduce inflammation and cholesterol deposits [114]. Ethanolic extraction from Carapa guianensis leaves [115].
Decoction
The decoction technique entails either boiling the plant samples for a short period of time or pouring hot water over the plant samples and leaving the combination to stand for a period of time [107]. Decoction operates on the same concept as maceration. In this method, the plant materials are boiled in a certain volume of water (e.g., 1:4 or 1:20) for a set period. The concentrated extract is then cooled and filtered. This method is best for extracting water-soluble, as well as heat-stable components as well as hard plant materials. The main drawbacks are that it takes time, and that the extraction is incomplete. This method is commonly utilized in the production of Ayurvedic extracts known as “quath” or “kawath” [108]. In phytotherapy, the decoction is used when the components are more difficult to extract from plant parts such as roots, rhizomes, bark, etc. [116]. Methanol and aqueous extracts were extracted from Ocimum sanctum leaves. [117].
Percolation
Percolation is the process by which the active principles are extracted from plants using a solvent in countercurrent. Percolation consists of three steps: wetting and soaking the raw material, followed by percolating the extractant. Wetting is carried out for 4–5 h using half or an equal amount of extractant. During soaking, the raw material swells and opens up more for extractant penetration. In addition, within the raw-material cells, a concentrated solution of extracted compounds is formed. The soaking stage then begins and lasts 24–48 h. Similar to maceration, this process has extraction efficiency. After soaking, percolation begins at 1/48 of the percolator volume per hour, with continued passing of extractant over the raw material at the same rate. Percolation is more efficient than maceration because the internal diffusion rate remains high due to the percolating extractant [116]. The technique has been used for isolating a variety of polyphenolics from food matrices [118]. The extraction of fucoxanthin from dried Undaria pinnatifida using percolation extraction and the refluxing method was compared. The purity of fucoxanthin extracted by percolation was higher than that extracted by refluxing [119]. Percolation technique uses the extraction rates of sinomenine and ephedrine hydrochloride as the index and involves soaking the drug in 70% ethanol for 24 h before percolating with 20 time the amount of 70% ethanol. The transfer rates for sonomenine and ephedrine hydrochlorides were 78.23 and 76.92%, respectively [120].
Soxhlet extraction
Soxhlet extraction is a common conventional method used for extracting heat-stable compounds. Soxhlet extraction, also known as continuous extraction, is a technique used to separate compounds from solid materials. Soxhlet is vital equipment used in pharmacognosy and the extraction of phytonutrients and pharmacological bioactive compounds. The Soxhlet apparatus is made up of four major components: a round bottom flask, a drug chamber, a vapor, a siphon tube, a condenser unit, and a water pipe [121] (Fig. 10). Plant samples are placed in a thimble-holder or porous bag (mainly of cellulose) and filled with condensed non-polar solvents (hexane, ether, and petroleum ether) to the distillation flask in the Soxhlet apparatus or hot continuous extraction. The solvents vaporize into the thimble and condense into the condenser when heated. When the level of solvent is raised sufficiently, it flows to the round bottom flask through a siphon tube, and this process is repeated until the solvent is concentrated [122].
Conventional Soxhlet apparatus [123]
When compared to maceration or percolation, the SE method is a continuous automated extraction process with high extraction efficiency that consumes less time and solvent. The advantage of this method is that it can extract significant amounts of drugs with a considerably less amount of solvent. This is extremely resource-efficient in terms of time, effort, and consequently financial inputs [124]. SE is suitable for both preliminary and bulk extraction. Andrographolide and deoxyandrographolide were extracted from the leaves of Andrographis paniculata using a solid–liquid extraction method [125]. The high temperature and longer extraction duration of the SE will enhance the probability of thermal (Table 4).
Novel technique extraction methodologies for phytonutrient
Ultrasounds assisted extraction or sonication
Ultrasound is a mechanical or ultrasonic wave where the frequency wave ranges from 20 to 100 MHz, and the ultrasound frequency ranges 20 GHz to 10 MHz. The two frequencies are diagnostic and power ultrasound. Diagnostic ultrasounds can also be called high frequency (2 MHz to 10 MHz where I < 1 cm−2), whereas power ultrasound is low-frequency ultrasound (20 MHz to 100 MHz where I > 1 W/cm2) [6]. Ultrasound causes acoustic cavitation by increasing the surface contact between solvents and samples and increasing the permeability of cell walls. A negative pressure is applied during the rarefaction phase, creating a void in the liquid. When the bubbles reach a critical point and collapse, this is referred to as compression (temperature 5000 K and pressure 5000 atm) [6, 9, 126, 127].
There are various possibilities for ultrasound extraction enhancement, including cell wall breakdown, increased penetration and swelling, hydration mechanisms, and others. The intensity of ultrasound lowers intermolecular forces, causing the molecular structure to break down [128]. This is known as cavitation, and it involves the production, growth, and collapse of bubbles. Cell membranes are disrupted by the eruption of bubbles, enabling extractable compounds to be discharged. It also enhances solvent penetration and mass transfer into cellular materials and finally releases the desired compound into the solvents [129]. However, it was also suggested that high power intensity could have the opposite effect, resulting in a decrease in phytonutrient yields. This is due to high bubble volume concentrations, which reduce cavitation activity; the “saturation effect,” caused by a large number of cavitation bubbles surrounding the probe tip; bubble deformation and non-spherical collapse; and reduced yield, which may be caused by ultrasonic degradation of the extracted phytonutrient [130] (Fig. 11).
Mechanisms of UAE method [131]
In comparison to other modern extraction techniques, such as MAE, ultrasonic equipment is less costly and simpler to use, and it can extract a wide range of natural compounds using any solvent. While the main disadvantages are the creation of free radicals on viable cells and enzymes, the action of 'jet' impact on the surface, the use of high-power ultrasound causes damage or rupture of the biological cell wall [132, 133] (Table 5).
Supercritical fluids extraction
SFE is termed as “Green Technology”. At its critical point, a supercritical fluid has both the physical properties of a gas and a liquid. Because SC-CO2 is non-toxic, non-corrosive, non-flammable, simple to handle, and operates at low pressure and room temperature. This technique with the help of two parameters, i.e., pressure and CO2 destroys microorganisms without affecting the nutritional contents and organoleptic attributes [134]. Because of its excellent extraction yields, short reduction time, and use of minimal volumes of solvent, SFE is widely used in the extraction of bioactive chemicals from natural sources. Carbon dioxide, ethylene, nitrogen, xenon or fluorocarbon, and methane are the most common solvents employed in the extraction process [135]. SC-CO2 is the most often utilized solvent since it is simple to handle and use (low pressure and room temperature). CO2 is inexpensive, widely available, and generally regarded as safe, GRAS [136]. Raising the pressure can increase solubility, whilst increasing the temperature reduces the SC-CO2 density. The solvent–solute and solute–solute interactions influence the solubility of compounds in SC-CO2. Non-polar solutes can solubilize SC-CO2 at high pressure at low density, but at high density, less volatile solutes are required [137]. This method entails extracting plant components using supercritical CO2 in a high-pressure tank and separating them with a solid phase separator. SFE is a non-hazardous technique. This extraction is used in a variety of sectors, including food, pharmaceuticals, and so on [134, 138] (Table 6).
The selected raw materials are placed in extractor vessels that are maintained at a consistent temperature and pressure during this procedure. The extractor is then filled with fluid through the pump. After the fluid and the chemicals have been dissolved, they are separated using a solid phase separator. SFE has two stages: extraction (from the solid substrate) and separation from the supercritical solvent. First, the solvent is poured into the extraction vessels (solvent pump and heat exchange) and uniformly dispersed throughout the fixed bed produced by the solid substratum. The solvent dissolves with the soluble components during the extraction. In the flash tanks, the solute–solvent combination is separated by rapidly lowering the pressure or raising the temperature of the fluids. The solvents should be cooled by the cooler and recompressed by the compressor before being returned to the extractor [133, 139, 140]. The SFE apparatus is shown in (Fig. 12).
Schematic diagram of SFE apparatus [141]
Microwave-assisted extraction
MAE uses electromagnetic radiation (transmitted as a wave) for the extraction of the phytonutrients at the frequency ranges from 300 MHz to 300 GHz. When heat is generated by continuous dipole rotation (dielectric heating) and frictional resistance, the extraction of phytonutrients increases. MAE instruments are classified into two types: closed vessel and open vessel (Fig. 13a) Closed MAEs are utilized for extraction at controlled pressure and temperature, whereas focused microwave ovens are used for extraction at atmospheric pressure [6, 9, 142].
The various steps of the extraction process are affected by the electromagnetic wave.
-
(a)
The process involves penetration (inside the solid or plants matrix) and transportation (out of the solid matrix),
-
(b)
Solubilization and breakdown,
-
(c)
Separation and discharge of bioactive compounds extracted and
-
(d)
Migration of the extracted solutes to the bulk solution.
During microwave extraction, heat is also generated via ionic conduction, which occurs when dissolved positively and negatively charged ions migrate in the direction of oppositely charged regions. Since a higher temperature raises the diffusion rate, which in turn increases extraction, heat generation is a key component in the extraction process. The solubility of the target phytonutrients in the solvent and the dielectric properties of the sample and solvent will also influence the extraction rate and quality of the extracted phytonutrients [144]. The mechanisms of MAE involve the molecular interactions between the microwave field with the solvent-carrying sample present in the extraction mixtures leading to the absorption of microwave energy by the sample without being absorbed by the solvents and converted into thermal energy. The heat energy causes vapor moisture evaporation due to high vapor pressure, resulting in the breakdown of the cell wall matrix and the release of phytonutrient or phytochemicals within the material matrix into the solution [131, 145] (Fig. 13b).
In comparison to SFE, MAE is a low-cost, simple process that is also a powerful innovative approach for nutraceutical extraction. However, this technique does not adequately extract tannins and anthocyanins. MAE has certain disadvantages for removing solid particles and when the solvents are non-polar or volatile as compared to SFE [133, 146] (Table 7).
Accelerated solvent extraction
ASE is a novel extraction technique that employs high temperatures and pressure to accelerate dissolution kinetics and break analytical interaction bonds [147]. The ASE method involves packing plant samples in the extraction cell with an inert material, such as sand, to prevent sample aggregation and system tubing blockage [148]. ASE is an efficient form of a solid–liquid extraction process performed at increased temperature and elevated pressure (50 °C to 200 °C and 10 to 15 MPa) to keep the solvent at a liquid state (below its critical condition) for further extraction processes [133]. For extraction of bioactive compounds or phytonutrients by ASE method, the mechanisms process are similar to pressurized fluid extraction where both are employed the use of solvents at high temperatures [149]. At high or elevated temperature it accelerated the extraction kinetics and reduces or decrease the viscosity so that the solvents can easily penetrate the pores of the matrix and at elevated pressure, the pressurized solvent increases the solubility with the analytes resulting in increased extraction speed [133, 142]. The diagram displays both the ASE mechanism and a schematic illustration of the PLE system (Fig. 14a, b).
When compared to traditional SE, there is a significant reduction in solvent amounts utilized and extraction time, as well as a wide variety of applications dealing with acidic and alkaline matrices. This automated extraction technology's strength resides in its capacity to regulate the temperature and pressure of each individual sample layer as well as the extraction period [122]. The ASE technique greatly increases the extraction of PCs from black sorghum at temperatures well over 100 °C using water and ethanol as solvents when compared to traditional extractions using the same solvents [150]. At present, ASE is considered an alternative approach to SFE for the extraction of polar molecules, with the primary disadvantage being that at high temperatures it causes thermolabile substances to degrade [133]. Other advantages of ASE include high reproducibility, rapid sample throughput, and reduced solvent usage (e.g., 50 mL solvent for 20 g material) [151] (Table 8).
Enzyme assisted extraction
The EAE method falls under the category of green extraction technologies since it encompasses the advantages of being environmentally friendly [152]. EAE technique enhanced compound extraction by rupturing the cell wall and cleaving the structural integrity with enzymes such as cellulase, hemicellulase, α-amylase, and pectinase [153]. Some phytochemicals in plant cells are bound to cell wall components, making them difficult to remove using traditional solvent extraction procedures. However, releasing some bound molecules into the solvent and so increasing the overall yield of the desired chemical can be accomplished by pretreating the sample with an enzyme [154]. The plant cell wall is broken down by enzymatic treatment, the phytonutrient present in the cell are easily released into the solvent [155] (Fig. 15).
Mechanisms of EAE method [131]
EAE is affected by operating parameters such as extraction time, temperature, system pH, enzyme mode of action, enzyme loading, and substrate availability [156]. The important features of EAE extract of natural plant metabolites are reduced extraction time, reduced solvent consumption, and higher yield and product quality (Table 9).
The EAE of bioactive compounds from plants has some limitations.
-
(a)
The cost of enzymes is rather high when processing large quantities of raw materials.
-
(b)
Current enzyme preparations cannot easily break down plant cell walls, limiting component extraction yields and
-
(c)
EAE is not viable for industrial use because enzymes behave differently and are strictly controlled by environmental factors such as dissolved oxygen percentage, temperature, and nutrition availability [155, 157].
Comparison between non-conventional extraction and conventional extraction method of phytonutrients
Traditional extraction methods such as SE, PE, and ME are time-consuming, require enormous bulks of solvents, and, in the end, may result in some target molecule degradation and fractional volatile loss [158]. Great improvements can be accomplished using non-traditional trials such as supercritical CO2 extraction, UAE, EAE and MAE. Traditional procedures such as distillation, solvent extraction, and cold compression are still utilized, but major advances can be made by merging ultrasound and microwave technology [159]. Modern green extraction techniques are rapid, suitable, inexpensive, sustainable, environmentally friendly, and have an overall beneficial influence on climate change by minimizing CO2 emissions and toxic solvent consumption As a result, researchers all around the world are attempting to reduce the problems associated with global warming by developing more eco-friendly and sustainable modern green processes for effective phytonutrient extraction from plant matrices that meet international regulatory criteria [160, 161].
According to the rate constant obtained during the first 10 min of extraction for carvone and limonene, UAE was 1.3–2 times faster, than a traditional method [162]. The 15-min MAE yielded 0.28% Rg1 and 1.31% Rb1 under 150 W microwave power, outperforming the 10-h conventional solvent extraction yielding 0.22% Rg1 and 0.87% Rb1 in 70% water–ethanol [163]. For its prospective applications in the nutrition and pharmacological industries, the UAE was confirmed as an efficient, green, and sustainable technology for extracting phenolics from Eucalyptus robusta leaf yield of 163.68 + 2.13 mg GAE/g using 250 W for 90 min at 60 °C with water [164]. MAE for 5 min extracted more flavonoid and phenolic components from Eucalyptus camaldulensis Dehn leaves and anthraquinones from the roots of Morinda citrifolia than UAE solvent extraction for 24 h for 60 min [165]. The TPC of the MAE extract achieved under optimal conditions was 24.6–42.36 mg of GAE/g of dry material, which was significantly higher than that obtained using the conventional method using the same solid-to-solvent ratio (1:30) [166]. UAE is a more effective method than the traditional way, although it depends on the solvent and temperature used. The effectiveness of PC extraction for all solvents is improved by increasing the sonication temperature. When compared to the traditional approach, only water extraction yielded lower levels of phenolic chemicals [167]. A 5-min extraction at 50 °C and 800 W yielded a maximum of chlorogenic acids (8.40%) and caffeine (7.25%), which were higher than those obtained after a heat reflux extraction at the same condition [168]. In a comparison of these two methods, MAE had a better extraction efficiency at 15 min (88.298 mg TAE/g) than the maceration method at 24 h (69.027 mg TAE/g) of exposure to ethanol extract of the Koroneiki variety [169]. MAE of flavonoids from Radix astragali yielded a high yield of 1.234–0.031 mg/g, which was comparable to Soxhlet (1.292–0.033 mg/g), UAE (0.736–0.038), and HRE (0.934–0.021 mg/g) [170]. Using MAE reduces process time and solvent consumption while enhancing extraction yields. The extraction effectiveness of the four approaches varies greatly and follows the sequence MAE > RFX > UE > RTE [171] (Table 10).
Conclusion and Future recommendations
This article provides a brief overview of the advancements in extraction technologies that are currently being used to recover phytonutrients. The demand for green extraction techniques and the rising market demand for phytonutrients are the main factors driving the continuous rise in the number of articles discussing the scaling-up of un-conventional extraction techniques. Extraction methods for phytonutrients have been subjected to a variety of optimization models. The traditional extraction methods widely employed for extracting phytonutrients are Soxhlet, percolation, infusion, and maceration. However, the traditional methods still have their own values, but they have some drawbacks, such as limited yield recovery, higher extraction solvent consumption, longer extraction time, and massive residue accumulation. Extraction optimization is necessary to determine optimal parameters for the efficient extraction of natural substances utilizing innovative extraction technologies. To overcome the limitations of conventional techniques, innovative techniques such as MAE, UAE, SC-CO2, EAE, and PLE, etc., have been developed.
Recent advancements in non-conventional techniques and innovative technology solutions for pilot and industrial applications should drive up interest in this area. As a result, for multistep and hyphenated extraction processes which are crucial in the development of “zero waste” biorefining processes some future issues and recommendations might be emphasized. All of these green extraction technologies are “mature,” with significant intellectual property prospects for such applications, but they are underutilized due to a lack of data on investment return. In order to scale up, a comprehensive approach spanning several processes is essential, including methodology, equipment design, bioanalytical, etc. Furthermore, the development of multidisciplinary skills is regarded as vital for a future in which the knowledge and integration of multiple technologies will be crucial. For instance, the processing time is a crucial factor that influences extraction costs in the case of industrial SFE-CO2, whereas microwave-assisted SFE-CO2 and ultrasonic can reach maximum yields in less time. EAE methods have the potential to not only produce better yields of diverse ingredients with fewer by-products but also to improve their bioavailability, which is one of the limiting factors in providing effective pharmacological activities.
Data availability
Not applicable.
Abbreviations
- MAE:
-
Microwave assisted extraction
- SFE:
-
Supercritical fluid extraction
- UAE:
-
Ultrasound assisted extraction
- CSE:
-
Conventional solvent extraction
- SE:
-
Soxhlet extraction
- SC-CO2 :
-
Supercritical carbon dioxide
- ME:
-
Maceration extraction
- MP:
-
Microwave power
- W:
-
Watt
- SE:
-
Soxhlet Extraction
- CS:
-
Co-solvent
- UP:
-
Ultrasonic power
- RE:
-
Reflux extraction
- S/F:
-
Solvent-to-feed ratio
- F:
-
Frequency
- Tc:
-
Critical temperature
- Rpm:
-
Revolutions per minute
- RFX:
-
Reflux temperature extraction
- Ar:
-
Aryl
- NL:
-
Neolignans
- TPC:
-
Total phenolic content
- TFC:
-
Total flavonoid contents
- RSM:
-
Response surface methodology
- BBD:
-
Box–Behnken Design
- GC-FID:
-
Gas chromatography coupled with flame-ionization detection
- UHPLC–QTOF/MS:
-
Ultra-high-performance liquid–chromatography coupled with quadrupole time of-flight mass spectrometry
- HPLC–ESI-QTOF-MS:
-
High performance liquid chromatography coupled to electrospray quadrupole-time of flight mass spectrometry
- GC:
-
Gas–liquid chromatograph
- ORAC:
-
Oxygen radical absorbance capacity
- MTT:
-
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- SEM:
-
Scanning electron microscope
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- HPLC:
-
High performance liquid chromatography
- FTIR:
-
Fourier transform infrared spectroscopy
- HPLC-DAD:
-
High performance liquid chromatography with diode-array detection
- GCMS:
-
Gas-chromatography coupled with mass spectrometry
- ABTS:
-
2,2-Azinobis (3-ethyl-benzothiazoline-6-sulfonic acid)
- FRAP:
-
Ferric reducing antioxidant power
- TLC:
-
Thin layer chromatography
- LC–MS:
-
Liquid chromatography and mass spectrometry
- LC–MS/MS:
-
Liquid chromatography/tandem mass spectrometry
References
C.M. Hasler, J.B. Blumberg, J. Mayer, In Symposium on Phytochemicals: Biochemistry and Physiology Introduction (1999), p. 1
K. Mathai, In: Krause’s Food, Nutrition and Diet Therapy, ed. by L.K. Mahan, S. Escott-Stump, vol. 271, 10th edn. (W.B. Saunders Co, London, 2000), p. 274
M. Bansal, N. Singh, S. Pal, I. Dev, K.M. Ansari, Adv. Mol. Toxicol. 12, 69–121 (2018)
M. Naczk, F. Shahidi, J. Pharm. Biomed. Anal. 41, 1523 (2006)
M.A. Khan, Unani. Zahid, India (2016)
J. Azmir, I.S.M. Zaidul, M.M. Rahman, K.M. Sharif, A. Mohamed, F. Sahena, M.H.A. Jahurul, K. Ghafoor, N.A.N. Norulaini, A.K.M. Omar, J. Food Eng. 117, 426 (2013)
M.M. Cowan, Clin. Microbiol. Rev. 12, 564 (1999)
E. Fuentes, I. Palomo, J. Funct. Foods 6, 73 (2014)
T. Belwal, H.P. Devkota, S. Ramola, H.C. Andola, I.D. Bhatt, Phytonutrients in Food (Elsevier, Inc., Amsterdam, 2019)
D. Tsimogiannis, V. Oreopoulou, Polyphenols in Plants (Elsevier, Amsterdam, 2019), pp.263–284
B. Vieira da Silva, J.C.M. Barreira, M.B.P.P. Oliveira, Trends Food Sci. Technol. 50, 144 (2016)
A. Laura, J.O. Moreno-Escamilla, J. Rodrigo-García, E. Alvarez-Parrilla, Postharvest Physiology and Biochemistry of Fruits and Vegetables (Elsevier, Amsterdam, 2019), pp.253–271
D.M. Pereira, P. Valentão, J.A. Pereira, P.B. Andrade, Molecules 14, 2202 (2009)
C. Andrés-Lacueva, A. Medina-Remon, R. Llorach, M. Urpi-Sarda, N. Khan, G. Chiva-Blanch, R. Zamora-Ros, M. Rotches-Ribalta, R.M. Lamuela-Raventos, Fruit and Vegetable Phytochemicals Chemistry, Nutritional Value and Stability, vol. 1 (Wiley, London, 2010)
S. Jan, N. Abbas, Himalayan Phytochemicals: Sustainable Options for Sourcing and Developing Bioactive Compounds (Elsevier, Amsterdam, 2018)
A. Di Costanzo, R. Angelico, Molecules 24, 2155 (2019)
A. Durazzo, M. Lucarini, E.B. Souto, C. Cicala, E. Caiazzo, A.A. Izzo, E. Novellino, A. Santini, Phytother. Res. 33, 2221 (2019)
L.H. Yao, Y.M. Jiang, J. Shi, F.A. Tomás-Barberán, N. Datta, R. Singanusong, S.S. Chen, Plant Foods Hum. Nutr. 59, 113–122 (2004)
A. Martínez-Sánchez, A. Gil-Izquierdo, M.I. Gil, F. Ferreres, J. Agric. Food Chem. 56, 2330 (2008)
R.L. Jackman, J.L. Smith, Natural Food Colors (Springer, Berlin, 1996), pp.244–309
H. Tamura, A. Yamagami, J. Agric. Food Chem. 42, 1612 (1994)
T. Tsuda, T. Osawa, K. Ohshima, S. Kawakishi, J. Agric. Food Chem. 42, 248 (1994)
M. Vlaskovska, D. Drenska, R. Ovcharov, Probl. Vutr. Med. 18, 19 (1990)
D. Drenska, I. Bantutova, R. Ovcharov, Fomatsiya (Sofia) 39, 33 (1989)
K. Igarashi, S. Abe, J. Satoh, Agric. Biol. Chem. 54, 171 (1990)
K. Igarashi, K. Inagaki, Agric. Biol. Chem. 55, 285 (1991)
P. Jing, J.A. Bomser, S.J. Schwartz, J. He, B.A. Magnuson, M.M. Giusti, J. Agric. Food Chem. 56, 9391 (2008)
J. Serrano, R. Puupponen-Pimiä, A. Dauer, A.M. Aura, F. Saura-Calixto, Mol. Nutr. Food Res. 53, S310 (2009)
A. Smeriglio, D. Barreca, E. Bellocco, D. Trombetta, Br. J. Pharmacol. 174, 1244 (2017)
L. Bravo, Nutr. Rev. 56(11), 317–333 (1998)
S. Yoshizawa, T. Horiuchi, H. Fujiki, T. Yoshida, T. Okuda, T. Sugimura, Phytother. Res. 1, 44 (1987)
H.-F. Gu, C.-M. Li, Y. Xu, W. Hu, M. Chen, Q. Wan, Food Res. Int. 41, 208 (2008)
K.Y. Ho, C.C. Tsai, J.S. Huang, C.P. Chen, T.C. Lin, C.C. Lin, J. Pharm. Pharmacol. 53, 187 (2001)
M.K. Reddy, S.K. Gupta, M.R. Jacob, S.I. Khan, D. Ferreira, Planta Med. 53, 461 (2007)
Z.Y. Wang, S.J. Cheng, Z.C. Zhou, M. Athar, W.A. Khan, D.R. Bickers, H. Mukhtar, Mutat. Res. Toxicol. 223, 273 (1989)
A. Tsopmo, F.M. Awah, V. Kuete, Medicinal Plant Research in Africa (Elsevier, Oxford, 2013), pp.435–478
S. Apers, A. Vlietinck, L. Pieters, Phytochem. Rev. 2, 201 (2003)
I. Ionkova, Mini Rev. Med. Chem. 11, 843 (2011)
N. Vasilev, I. Ionkova, Pharm. Biol. 43, 509 (2005)
G.M.K.B. Gunaherath, A.A.L. Gunatilaka, Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation (Wiley, New York, 2006), p.1
C. Grunwald, Annu. Rev. Plant Physiol. 26, 209 (1975)
L. Nyström, A. Schär, A. Lampi, Eur. J. Lipid Sci. Technol. 114, 656 (2012)
M.S. Uddin, S. Ferdosh, M.J. Haque Akanda, K. Ghafoor, A.H. Rukshana, M.E. Ali, B.Y. Kamaruzzaman, M.B. Fauzi, S. Shaarani, M.Z. Islam Sarker, Sep. Sci. Technol. 53, 2206 (2018)
L.L. Tian, P.J. White, J. Am. Oil Chem. Soc. 71, 1087 (1994)
L.L. Tian, P.J. White, J. Am. Oil Chem. Soc. 71, 1079 (1994)
S. Loizou, S. Paraschos, S. Mitakou, G.P. Chrousos, I. Lekakis, P. Moutsatsou, Exp. Biol. Med. 234, 553 (2009)
A.B. Awad, A. Downie, C.S. Fink, U. Kim, Anticancer Res. 20, 821 (2000)
R. John Ogbe, R.J. Ogbe, D.O. Ochalefu, S.G. Mafulul, O.B. Olaniru, Pelagia Res. Libr. Asian J. Plant Sci. Res. 5, 10 (2015)
E.A. Trautwein, I. Demonty, Ol. Corps Gras Lipides 14, 259 (2007)
J. Lesgards, N. Baldovini, N. Vidal, S. Pietri, Phytother. Res. 28, 1423 (2014)
M.I. Georgiev, N. Ivanovska, K. Alipieva, P. Dimitrova, R. Verpoorte, Phytochemistry 92, 8 (2013)
A. Ludwiczuk, K. Skalicka-Woźniak, M.I. Georgiev, Pharmacognosy (Elsevier, Boston, 2017), pp.233–266
T.L. Olatunji, C.A. Odebunmi, A.E. Adetunji, Future J. Pharm. Sci. 7, 1 (2021)
B. Heasley, Eur. J. Org. Chem. 2011, 19 (2011)
Y.-S. Shi, Y. Zhang, H.-T. Li, C.-H. Wu, H.R. El-Seedi, W.-K. Ye, Z.-W. Wang, C.-B. Li, X.-F. Zhang, G.-Y. Kai, J. Funct. Foods 75, 104213 (2020)
I. Piccialli, V. Tedeschi, L. Caputo, G. Amato, L. De Martino, V. De Feo, A. Secondo, A. Pannaccione, Antioxidants 10, 937 (2021)
P. Langi, S. Kiokias, T. Varzakas, C. Proestos, Microbial Carotenoids (Humana Press, New York, 2018), p.57
D. Prakash, C. Gupta, G. Sharma, J. Chin. Med. Res. Dev. 1, 70–78 (2012)
P.S. Bernstein, B. Li, P.P. Vachali, A. Gorusupudi, R. Shyam, B.S. Henriksen, J.M. Nolan, Prog. Retin. Eye Res. 50, 34 (2016)
B. Li, G.T. Rognon, T. Mattinson, P.P. Vachali, A. Gorusupudi, F.-Y. Chang, A. Ranganathan, K. Nelson, E.W. George, J.M. Frederick, Arch. Biochem. Biophys. 649, 22 (2018)
B.R. Hammond Jr., L.S. Miller, M.O. Bello, C.A. Lindbergh, C. Mewborn, L.M. Renzi-Hammond, Front. Aging Neurosci. 9, 254 (2017)
J.N. Keller, F.A. Schmitt, S.W. Scheff, Q. Ding, Q. Chen, D.A. Butterfield, W.R. Markesbery, Neurology 64, 1152 (2005)
T. Iwamoto, K. Hosoda, R. Hirano, H. Kurata, A. Matsumoto, W. Miki, M. Kamiyama, H. Itakura, S. Yamamoto, K. Kondo, J. Atheroscler. Thromb. 7, 216 (2000)
H. Yoshida, H. Yanai, K. Ito, Y. Tomono, T. Koikeda, H. Tsukahara, N. Tada, Atherosclerosis 209, 520 (2010)
K. Nakagawa, T. Kiko, T. Miyazawa, G.C. Burdeos, F. Kimura, A. Satoh, T. Miyazawa, Br. J. Nutr. 105, 1563 (2011)
S.K. Clinton, C. Emenhiser, S.J. Schwartz, D.G. Bostwick, A.W. Williams, B.J. Moore, J.W. Erdman Jr., Cancer Epidemiol. Prev. Biomark. 5, 823 (1996)
J.L. Rowles, K.M. Ranard, J.W. Smith, R. An, J.W. Erdman, Prostate Cancer Prostatic Dis. 20, 361 (2017)
A. Erhardt, W. Stahl, H. Sies, F. Lirussi, A. Donner, D. Häussinger, Eur. J. Med. Res. 16, 76 (2011)
L. Johnson, E. Norkus, S. Abbasi, J.S. Gerdes, V.K. Bhutani, FASEB J. 9, 1869 (1995)
J. Bettler, J.P. Zimmer, M. Neuringer, P.A. DeRusso, Eur. J. Nutr. 49, 45 (2010)
A. Septembre-Malaterre, F. Remize, P. Poucheret, Food Res. Int. 104, 86 (2018)
K. Herrmann, C.W. Nagel, Crit. Rev. Food Sci. Nutr. 28, 315 (1989)
M. Naczk, F. Shahidi, J. Chromatogr. A 1054, 95 (2004)
J.D. Guzman, Molecules 19, 19292 (2014)
B. Korošec, M. Sova, S. Turk, N. Kraševec, M. Novak, L. Lah, J. Stojan, B. Podobnik, S. Berne, N. Zupanec, J. Appl. Microbiol. 116, 955 (2014)
B. Podobnik, J. Stojan, L. Lah, N. Krasevec, M. Seliskar, T.L. Rizner, D. Rozman, R. Komel, J. Med. Chem. 51, 3480 (2008)
X. Liang, C. Ma, X. Yan, X. Liu, F. Liu, Trends Food Sci. Technol. 93, 185 (2019)
I. Perković, S. Raić-Malić, D. Fontinha, M. Prudêncio, L.P. de Carvalho, J. Held, T. Tandarić, R. Vianello, B. Zorc, Z. Rajić, Eur. J. Med. Chem. 187, 111927 (2020)
W. Löscher, H. Potschka, Nat. Rev. Neurosci. 6, 591 (2005)
O.M. Abd El-Raaouf, E.M. El-Sayed, M.F. Manie, J. Biochem. Mol. Toxicol. 29, 426 (2015)
V.D. Kortenska, M.P. Velikova, N.V. Yanishlieva, I.R. Totzeva, V.S. Bankova, M.C. Marcucci, Eur. J. Lipid Sci. Technol. 104, 19 (2002)
T.K. Hyun, Y.-J. Ko, E.-H. Kim, I.-M. Chung, J.-S. Kim, Ind. Crops Prod. 74, 263 (2015)
A. García-Lafuente, C. Moro, N. Manchón, A. Gonzalo-Ruiz, A. Villares, E. Guillamón, M. Rostagno, L. Mateo-Vivaracho, Food Chem. 161, 216 (2014)
Z. Ladak, M. Khairy, E.A. Armstrong, J.Y. Yager, Nutraceuticals Brain Health and Beyond (Elsevier, Amsterdam, 2021), pp.155–167
R. Thirumdas, A. Kothakota, Indian Food Ind. Mag. 56, 31 (2017)
M. Ishida, M. Hara, N. Fukino, T. Kakizaki, Y. Morimitsu, Breed. Sci. 64, 48 (2014)
G. Yuan, X. Wang, R. Guo, Q. Wang, Food Chem. 121, 1014 (2010)
S. Oh, C. Tsukamoto, K. Kim, M. Choi, J. Food Biochem. 41, e12366 (2017)
R. Biegańska-Marecik, E. Radziejewska-Kubzdela, R. Marecik, Food Chem. 230, 271 (2017)
N. Förster, I. Mewis, H. Glatt, M. Haack, R. Brigelius-Flohé, M. Schreiner, C. Ulrichs, Food Funct. 7, 4660 (2016)
V. Boscaro, L. Boffa, A. Binello, G. Amisano, S. Fornasero, G. Cravotto, M. Gallicchio, Molecules 23, 3004 (2018)
Y.M. Lee, H.J. Cho, S.P. Ponnuraj, J. Kim, J.-S. Kim, S.G. Kim, J.H.Y. Park, J. Med. Food 14, 377 (2011)
F. Burčul, I. Generalić Mekinić, M. Radan, P. Rollin, I. Blažević, J. Enzyme Inhib. Med. Chem. 33, 577 (2018)
S. Awasthi, N.T. Saraswathi, Int. J. Biol. Macromol. 83, 410 (2016)
N. Baenas, S. Piegholdt, A. Schloesser, D.A. Moreno, C. García-Viguera, G. Rimbach, A.E. Wagner, Int. J. Mol. Sci. 17, 251 (2016)
N. Hanlon, N. Konsue, N. Coldham, M.J. Sauer, C. Ioannides, Nutr. Cancer 63, 300 (2011)
S.D.S. Banjarnahor, N. Artanti, Med. J. Indones. 23, 239 (2014)
S.J. Maleki, J.F. Crespo, B. Cabanillas, Food Chem. 299, 125124 (2019)
A. Rees, G.F. Dodd, J.P.E. Spencer, Nutrients 10, 1852 (2018)
D. Li, P. Wang, Y. Luo, M. Zhao, F. Chen, Crit. Rev. Food Sci. Nutr. 57, 1729 (2017)
T. Uemura, T. Goto, M-S. Kang, N. Mizoguchi, S. Hirai, J-Y. Lee, Y. Nakano, J. Shono, S. Hoshino, K. Taketani, N Tsuge, T. Narukami, M. Makishima, N. Takahashi, T. Kawada, J. Nutr. 141(1), 17–23 (2011)
T. Rabi, S. Gupta, Front. Biosci. 13, 3457 (2008)
M.S.A. Khan, S.U.K. Khundmiri, S.R. Khundmiri, M.M. Al-Sanea, P.L. Mok, Front. Pharmacol. 9, 569 (2018)
R. Tundis, M.R. Loizzo, F. Menichini, Crit. Rev. Food Sci. Nutr. 54, 225 (2014)
M.E. Cartea, P. Velasco, Phytochem. Rev. 7, 213 (2008)
Q.-W. Zhang, L.-G. Lin, W.-C. Ye, Chin. Med. 13, 1 (2018)
O. Sticher, Nat. Prod. Rep. 25, 517 (2008)
S.S. Handa, S.P.S. Khanuja, G. Longo, D.D. Rakesh, Extraction Technologies for Medicinal and Aromatic Plants, No. 66, 1st edn. (United Nations Industrial Development Organization and the International Centre for Science and High Technology, Rome, 2008)
N. Nastić, I. Borrás-Linares, J. Lozano-Sánchez, J. Švarc-Gajić, A. Segura-Carretero, J. Ind. Eng. Chem. 68, 282 (2018)
T. Uemura, T. Goto, M. Kang, N. Mizoguchi, S. Hirai, J. Lee, Y. Nakano, J. Shono, S. Hoshino, K. Taketani, J. Nutr. 141, 17 (2011)
B.R. Albuquerque, M.A. Prieto, M.F. Barreiro, A. Rodrigues, T.P. Curran, L. Barros, I.C.F.R. Ferreira, Ind. Crops Prod. 95, 404 (2017)
M. Kamil Hussain, M. Saquib, M. Faheem Khan, Natural Bio-active Compounds (Springer, Berlin, 2019), pp.179–200
S.O. Majekodunmi, Merit Res. J. Med. 3, 521 (2015)
F. Fotsing Yannick Stéphane, B. Kezetas Jean Jules, G. El-Saber Batiha, I. Ali, L. Ndjakou Bruno, Complementary Medicine (IntechOpen, London, 2022), p.1
B.S. Nayak, J. Kanhai, D.M. Milne, L.P. Pereira, W.H. Swanston, Evid. Based Complement. Altern. Med. 2011, 419612 (2011)
A.-M. Tabaraşu, S.Ş Biriş, I. Gageanu, D. Anghelache, C. Bălţatu, C. Persu, Acta Tech. Corviniensis Bull. Eng. 15, 23 (2022)
S. Shetty, S. Udupa, L. Udupa, Evid. Based Complement. Altern. Med. 5, 95 (2008)
A. Sridhar, M. Ponnuchamy, P.S. Kumar, A. Kapoor, D.-V.N. Vo, S. Prabhakar, Environ. Chem. Lett. 19, 3409 (2021)
H. Zhang, W. Wang, Z.M. Fu, C.C. Han, Y. Song, Zhongguo Shipin Tianjiaji 9, 91 (2014)
X. Gao, J. Han, H. Dai, L. Xiang, Zhongguo Yaoshi 12, 1395 (2009)
M.D. Luque de Castro, L.E. García-Ayuso, Anal. Chim. Acta 369, 1 (1998)
N.N. Azwanida, Med. Aromat. Plants 4, 412 (2015)
M.A. López-Bascón, M.D.L. De Castro, Liquid-Phase Extraction (Elsevier, Amsterdam, 2020), pp.327–354
W.P. Jones, A.D. Kinghorn, Natural Products Isolation (Springer, Berlin, 2006), pp.323–351
A.C. Kumoro, M. Hasan, H. Singh, Sci. Asia 35, 306 (2009)
D. Pingret, A.S. Fabiano-Tixier, C. Le Bourvellec, C.M.G.C. Renard, F. Chemat, J. Food Eng. 111, 73 (2012)
M.G. Cares, Y. Vargas, L. Gaete, J. Sainz, J. Alarcon, Phys. Procedia 3, 169 (2010)
A. Ilghami, S. Ghanbarzadeh, H. Hamishehkar, Pharm. Sci. 21, 46 (2015)
S. Baig, R. Farooq, F. Rehman, World Appl. Sci. J. 10, 936 (2010)
M.C.N. Picot-Allain, B. Ramasawmy, M.N. Emmambux, Food Rev. Int. 38, 282 (2022)
M.S. Uddin, S. Ferdosh, M.J. Haque Akanda, K. Ghafoor, A.H. Rukshana, M.E. Ali, B.Y. Kamaruzzaman, M.B. Fauzi, S. Hadijah, S. Shaarani, M.Z. Islam Sarker, Sep. Sci. Technol. 53, 2206 (2018)
Y. Pico, Trends Anal. Chem. 43, 84 (2013)
L. Wang, C.L. Weller, Trends Food Sci. Technol. 17, 300 (2006)
T. Ahmad, F.A. Masoodi, S.A. Rather, S.M. Wani, A. Gull, J. Biol. Chem. Chron. 5, 114 (2019)
L.D. Kagliwal, S.C. Patil, A.S. Pol, R.S. Singhal, V.B. Patravale, Sep. Purif. Technol. 80, 533 (2011)
K. Ghafoor, J. Park, Y.H. Choi, Innov. Food Sci. Emerg. Technol. 11, 485 (2010)
M. Sun, F. Temelli, J. Supercrit. Fluids 37, 397 (2006)
B.A.S. Machado, C.G. Pereira, S.B. Nunes, F.F. Padilha, M.A. Umsza-Guez, Sep. Sci. Technol. 48, 2741 (2013)
M. Bimakr, R.A. Rahman, F.S. Taip, A. Ganjloo, L.M. Salleh, J. Selamat, A. Hamid, I.S.M. Zaidul, Food Bioprod. Process. 89, 67 (2011)
C.G. Pereira, M.A.A. Meireles, Food Bioprocess. Technol. 3, 340 (2010)
S.K. Mahawer, S.A. Himani, R. Kumar, O. Prakash, Essential Oils (IntechOpen, London, 2022), p.3
P. Christen, B. Kaufmann, Phytochem. Anal. 113, 105 (2002)
C.H. Chan, R. Yusoff, G.C. Ngoh, F.W.L. Kung, J. Chromatogr. A 1218, 6213 (2011)
A. Kute, D. Mohapatra, B. Babu, B.P. Sawant, J. Food Res. Technol. 3, 62 (2015)
M. Mirzadeh, M.R. Arianejad, L. Khedmat, Carbohydr. Polym. 229, 115421 (2020)
X. Pan, G. Niu, H. Liu, Biochem. Eng. J. 12(1), 71–77 (2002)
A. Popova, D. Mihaylova, Asian J. Pharm. Clin. Res. 11, 28 (2018)
M.K. Khan, L. Paniwnyk, S. Hassan, Plant Based “Green Chem. 2.0” (Springer, Singapore, 2019), pp.197–235
G. Alvarez-Rivera, M. Bueno, D. Ballesteros-Vivas, J.A. Mendiola, E. Ibañez, Liquid-Phase Extraction (Elsevier, Amsterdam, 2019), p.375
F. Barros, L. Dykes, J.M. Awika, L.W. Rooney, J. Cereal Sci. 58, 305 (2013)
B.J. Deans, J. Just, J.A. Smith, A.C. Bissember, Asian J. Org. Chem. 9, 1144 (2020)
O.R. Alara, N.H. Abdurahman, C.I. Ukaegbu, Curr. Res. Food Sci. 4, 200 (2021)
M. Manzoor, J. Singh, A. Gani, N. Noor, Food Chem. 362, 130141 (2021)
S. Latif, F. Anwar, Eur. J. Lipid Sci. Technol. 111, 1042 (2009)
M. Puri, D. Sharma, C.J. Barrow, Trends Biotechnol. 30, 37 (2012)
H.B. Sowbhagya, V.N. Chitra, Crit. Rev. Food Sci. Nutr. 50, 146 (2010)
X. Cheng, L. Bi, Z. Zhao, Y. Chen, Advances in Enzyme Assisted Extraction of Natural Products (Ic3me) (2015), pp. 371–375. https://doi.org/10.2991/ic3me-15.2015.72
L. Alexandru, G. Cravotto, L. Giordana, A. Binello, F. Chemat, Innov. Food Sci. Emerg. Technol. 20, 167 (2013)
M. Vinatoru, T.J. Mason, I. Calinescu, Trends Anal. Chem. 97, 159 (2017)
M.S. Easmin, M.Z.I. Sarker, S. Ferdosh, S.H. Shamsudin, K. Bin Yunus, M.S. Uddin, M.M.R. Sarker, M.J.H. Akanda, M.S. Hossain, H.P.S.A. Khalil, J. Chem. Technol. Biotechnol. 90, 981 (2015)
N. Mahato, M. Sinha, K. Sharma, R. Koteswararao, M.H. Cho, Foods 8, 523 (2019)
S. Chemat, A. Lagha, H. AitAmar, P.V. Bartels, F. Chemat, Flavour Fragr. J. 19, 188 (2004)
Y.Y. Shu, M.Y. Ko, Y.S. Chang, Microchem. J. 74, 131 (2003)
D.J. Bhuyan, Q.V. Vuong, A.C. Chalmers, I.A. van Altena, M.C. Bowyer, C.J. Scarlett, Sep. Sci. Technol. 52, 100 (2017)
M. Gharekhani, M. Ghorbani, N. Rasoulnejad, Lat. Am. Appl. Res. 42, 305 (2012)
A.E. Ince, S. Sahin, G. Sumnu, J. Food Sci. Technol. 51, 2776 (2014)
C. Proestos, M. Komaitis, J. Food Qual. 29, 567 (2006)
R. Upadhyay, K. Ramalakshmi, L.J.M. Rao, Food Chem. 130, 184 (2012)
Z. Rafiee, S.M. Jafari, M. Alami, M. Khomeiri, J. Anim. Plant Sci. 21, 738 (2011)
W. Xiao, L. Han, B. Shi, Sep. Sci. Technol. 43, 671 (2008)
J. Dai, V. Orsat, G.S.V. Raghavan, V. Yaylayan, J. Food Eng. 96, 540 (2010)
E.I. Oikeh, F.E. Oviasogie, E.S. Omoregie, Clin. Phytosci. 6, 1 (2020)
O.R. Alara, N.H. Abdurahman, C.I. Ukaegbu, J. Appl. Res. Med. Aromat. Plants 11, 12 (2018)
E. Aspé, K. Fernández, Ind. Crops Prod. 34, 838 (2011)
M.N.H. Daud, D.N. Fatanah, N. Abdullah, R. Ahmad, Food Chem. 232, 621 (2017)
K. Hartonen, J. Parshintsev, K. Sandberg, E. Bergelin, L. Nisula, M.L. Riekkola, Talanta 74, 32 (2007)
K. Szewczyk, M. Olech, Phytochem. Lett. 20, 322 (2017)
G. Zu, R. Zhang, L. Yang, C. Ma, Y. Zu, W. Wang, C. Zhao, Int. J. Mol. Sci. 13, 11027 (2012)
I. Alzorqi, A. Singh, S. Manickam, H.F. Al-Qrimli, Resour. Technol. 3, 46 (2017)
G.F. Barbero, A. Liazid, M. Palma, C.G. Barroso, Talanta 75, 1332 (2008)
E.N.A. Abd Rahim, A. Ismail, M.N. Omar, U.N. Rahmat, W.A.N.W. Ahmad, Pharmacogn. J. 10(1), 110–119 (2018)
M. Corrales, S. Toepfl, P. Butz, D. Knorr, B. Tauscher, Innov. Food Sci. Emerg. Technol. 9, 85 (2008)
G. Pan, G. Yu, C. Zhu, J. Qiao, Ultrason. Sonochem. 19, 486 (2012)
R. Tabaraki, A. Nateghi, Ultrason. Sonochem. 18, 1279 (2011)
A. Tomšik, B. Pavlić, J. Vladić, M. Ramić, J. Brindza, S. Vidović, Ultrason. Sonochem. 29, 502 (2016)
P. Sharayei, E. Azarpazhooh, S. Zomorodi, H.S. Ramaswamy, LWT 101, 342 (2019)
L. Zhang, Y. Jiang, X. Pang, P. Hua, X. Gao, Q. Li, Z. Li, Molecules 24, 3461 (2019)
C.P. Passos, R.M. Silva, F.A. Da Silva, M.A. Coimbra, C.M. Silva, Chem. Eng. J. 160, 634 (2010)
S. MacHmudah, S. Zakaria, S. Winardi, M. Sasaki, M. Goto, N. Kusumoto, K. Hayakawa, J. Food Eng. 108, 290 (2012)
M. Kehili, M. Kammlott, S. Choura, A. Zammel, C. Zetzl, I. Smirnova, N. Allouche, S. Sayadi, Food Bioprod. Process. 102, 340 (2017)
B. Pavlić, O. Bera, N. Teslić, S. Vidović, G. Parpinello, Z. Zeković, Ind. Crops Prod. 120, 305 (2018)
A. Calvo, J. Morante, S. Plánder, E. Székely, Acta Aliment. 46, 27 (2017)
K. Ghafoor, F.Y. Al-Juhaimi, Y.H. Choi, Plant Foods Hum. Nutr. 67, 407 (2012)
L.A. Conde-Hernández, J.R. Espinosa-Victoria, A. Trejo, J. Guerrero-Beltrán, J. Food Eng. 200, 81 (2017)
M.A. Falcão, R. Scopel, R.N. Almeida, A.T. do Espirito Santo, G. Franceschini, J.J. Garcez, R.M.F. Vargas, E. Cassel, J. Supercrit. Fluids 129, 9 (2017)
A.M. Farías-Campomanes, M.A. Rostagno, M.A.A. Meireles, J. Supercrit. Fluids 77, 70 (2013)
N.W. Ismail-Suhaimy, S.S.A. Gani, U.H. Zaidan, M.I.E. Halmi, P. Bawon, Plants 10(4), 682 (2021)
L.G.G. Rodrigues, S. Mazzutti, I. Siddique, M. da Silva, L. Vitali, S.R.S. Ferreira, J. Supercrit. Fluids 165, 104976 (2020)
J. Huang, W. He, C. Yan, X. Du, X. Shi, BIO Web Conf. 8, 03008 (2017)
I. Calinescu, I. Asofiei, A.I. Gavrila, A. Trifan, D. Ighigeanu, D. Martin, C. Matei, M. Buleandra, Chem. Eng. Commun. 204, 965 (2017)
W. Xiong, X. Chen, G. Lv, D. Hu, J. Zhao, S. Li, J. Pharm. Anal. 6, 382 (2016)
I. Borrás-Linares, Z. Stojanović, R. Quirantes-Piné, D. Arráez-Román, J. Švarc-Gajić, A. Fernández-Gutiérrez, A. Segura-Carretero, Int. J. Mol. Sci. 15, 20585 (2014)
Z. Karami, Z. Emam-Djomeh, H.A. Mirzaee, M. Khomeiri, A.S. Mahoonak, E. Aydani, J. Food Sci. Technol. 52, 3242 (2015)
H. Li, Z. Deng, T. Wu, R. Liu, S. Loewen, R. Tsao, Food Chem. 130, 928 (2012)
T.S. Ballard, P. Mallikarjunan, K. Zhou, S. O’Keefe, Food Chem. 120, 1185 (2010)
Y. Kong, Y.G. Zu, Y.J. Fu, W. Liu, F.R. Chang, J. Li, Y.H. Chen, S. Zhang, C.B. Gu, J. Food Compos. Anal. 23, 382 (2010)
O.J. Williams, G.S. Vdaya Raghavan, V. Orsat, J. Dai, J. Food Biochem. 28(2), 113–122 (2004)
R. Ahmad, N. Ahmad, M. Riaz, M. Al-Tarouti, F. Aloufi, A. AlDarwish, B. Alalaq, B. Alhanfoush, Z. Khan, Biomed. Chromatogr. 34(11), e4942 (2020)
J. Andrew, J. Masetlwa, T. Tesfaye, B. Sithole, Sustain. Chem. Pharm. 18, 100327 (2020)
M. Repajić, E. Cegledi, V. Kruk, S. Pedisić, F. Çinar, D.B. Kovačević, I. Žutić, V. Dragović-Uzelac, Processes 8, 803 (2020)
W.Y.J. Kam, F. Abas, N. Hussain, H. Mirhosseini, Nat. Prod. Res. 34, 1937 (2020)
C.Y. Gan, N.H. Abdul Manaf, A.A. Latiff, Food Hydrocoll. 24, 471 (2010)
K. Rafińska, P. Pomastowski, J. Rudnicka, A. Krakowska, A. Maruśka, M. Narkute, B. Buszewski, Food Chem. 289, 16 (2019)
J.K.U. Ling, Y.S. Chan, J. Nandong, SN Appl. Sci. 2, 1365 (2020)
B. Nayak, F. Dahmoune, K. Moussi, H. Remini, S. Dairi, O. Aoun, M. Khodir, Food Chem. 187, 507 (2015)
A. Toubane, S.A. Rezzoug, C. Besombes, K. Daoud, Ind. Crops Prod. 97, 620 (2017)
H. Chen, X. Zhou, J. Zhang, Carbohydr. Polym. 111, 567 (2014)
Y.J. Fu, W. Liu, Y.G. Zu, M.H. Tong, S.M. Li, M.M. Yan, T. Efferth, H. Luo, Food Chem. 111, 508 (2008)
T. Liu, X. Sui, L. Li, J. Zhang, X. Liang, W. Li, H. Zhang, S. Fu, Anal. Chim. Acta 903, 91 (2016)
T. Maier, A. Göppert, D.R. Kammerer, A. Schieber, R. Carle, Eur. Food Res. Technol. 227, 267 (2008)
I.F. Strati, E. Gogou, V. Oreopoulou, Food Bioprod. Process. 94, 668 (2015)
W. Tchabo, Y. Ma, F.N. Engmann, H. Zhang, Ind. Crops Prod. 63, 214 (2015)
I. Tomaz, L. Maslov, D. Stupić, D. Preiner, D. Ašperger, J.K. Kontić, Sep. Sci. Technol. 51, 255 (2016)
D. Wu, T. Gao, H. Yang, Y. Du, C. Li, L. Wei, T. Zhou, J. Lu, H. Bi, Ind. Crops Prod. 66, 229 (2015)
S. Pan, S. Wu, Carbohydr. Polym. 111, 606 (2014)
J. Jiao, Z.G. Li, Q.Y. Gai, X.J. Li, F.Y. Wei, Y.J. Fu, W. Ma, Food Chem. 147, 17 (2014)
J.M. Billakanti, O.J. Catchpole, T.A. Fenton, K.A. Mitchell, A.D. Mackenzie, Process Biochem. 48, 1999 (2013)
S. Chen, X.H. Xing, J.J. Huang, M.S. Xu, Enzyme Microb. Technol. 48, 100 (2011)
F. Sahne, M. Mohammadi, G.D. Najafpour, A.A. Moghadamnia, Ind. Crops Prod. 95, 686 (2017)
V. Briones-Labarca, M. Plaza-Morales, C. Giovagnoli-Vicuña, F. Jamett, LWT Food Sci. Technol. 60, 525 (2015)
H.İ Odabaş, I. Koca, Ind. Crops Prod. 91, 114 (2016)
S. Plazzotta, R. Ibarz, L. Manzocco, O. Martín-Belloso, Ultrason. Sonochem. 63, 104954 (2020)
H. Van Le, V.V.M. Le, Int. J. Food Sci. Technol. 47, 1206 (2012)
K.S. Andrade, R.T. Gonçalvez, M. Maraschin, R.M. Ribeiro-do-Valle, J. Martínez, S.R.S. Ferreira, Talanta 88, 544 (2012)
J. Švarc-Gajić, Z. Stojanović, A.S. Carretero, D.A. Román, I. Borrás, I. Vasiljević, J. Food Eng. 119, 525 (2013)
S. Akay, I. Alpak, O. Yesil-Celiktas, J. Sep. Sci. 34, 1925 (2011)
M.J. García-Sarrió, M.L. Sanz, J. Sanz, A. González-Coloma, A. Cristina Soria, Electrophoresis 39, 1957 (2018)
M. Radojković, Z. Zeković, P. Mašković, S. Vidović, A. Mandić, A. Mišan, S. Đurović, J. Supercrit. Fluids 117, 50 (2016)
Z. Zhang, G. Lv, W. He, L. Shi, H. Pan, L. Fan, Carbohydr. Polym. 98, 1524 (2013)
M.-M. Yan, W. Liu, Y.-J. Fu, Y.-G. Zu, C.-Y. Chen, M. Luo, Food Chem. 119, 1663 (2010)
M.C. Bubalo, N. Ćurko, M. Tomašević, K.K. Ganić, I.R. Redovniković, Food Chem. 200, 159 (2016)
A. Elik, D.K. Yanık, F. Göğüş, LWT 123, 109100 (2020)
A.M. Goula, M. Ververi, A. Adamopoulou, K. Kaderides, Ultrason. Sonochem. 34, 821 (2017)
P. Tatke, M. Rajan, Indian J. Pharm. Educ. Res. 48, 27 (2014)
Funding
No research grant was used for this work.
Author information
Authors and Affiliations
Contributions
BK conceptualized the review. BK, PKC, KD and VK developed the protocol and wrote the review. PKC, KD and VK supervised the writing. JOI reviewed the technical content, developed and improved the paper structure. This paper has been supported by the RUDN University Strategic, Academic Leadership Program.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khongthaw, B., Chauhan, P.K., Dulta, K. et al. A comparison of conventional and novel phytonutrient extraction techniques from various sources and their potential applications. Food Measure 17, 1317–1342 (2023). https://doi.org/10.1007/s11694-022-01697-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01697-4