Abstract
A novel air-assisted dispersive solid-phase extraction method based on nickel/aluminum-layered double hydroxides combined with high performance liquid chromatography was established for the simultaneous determination of ofloxacin, norfloxacin, ciprofloxacin and enrofloxacin in honey. With the assistance of air, nickel/aluminum-layered double hydroxides extracted quinolones from the matrix under alkaline conditions via the coordination reaction between their surface layer metal cations and quinolones, and their interlayer exchange reaction toward anionic quinolones. Afterwards, the adsorbents were dissolved with acidic solutions and the analytes were detected by high performance liquid chromatography-fluorescence detector. Under optimization, the method achieved low detection limits of 0.25–0.82 ng/g and quantification limits of 0.82–2.73 ng/g. High accuracy were obtained with recoveries of 82.3–90.5%. The intra-day and inter-day relative standard deviations were 4.39–11.0% and 2.16–17.5%, respectively. The method was simple, sensitive, convenient and environment friendly. It was suitable for the determination of quinolones in honey.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Honey is a natural product with high nutritional value produced by bees from nectar and secretions of the plants. It’s widely consumed in the world [1, 2]. Quinolone veterinary drugs are frequently detected in bee products for diseases prevention and control [3]. However, long term intake of quinolones (QNs) leads to drug resistance, allergic reaction, carcinogenesis, teratogenesis, mutation, and side effects to the liver and kidney [4]. Therefore, the use of typical QNs like ofloxacin (OFL), norfloxacin (NOR), lomefloxacin (LOM), pefloxacin (PEF) and their salts, esters and preparations in food animals was banned by the Announcement No. 2292 of the Ministry of Agriculture of China in 2015 [5]. However, OFL and NOR are frequently reported being found in honey in China. Meanwhile, enrofloxacin (ENR) and ciprofloxacin (CIP) are usually detected in honey [6]. To ensure the safety of honey, the accurate, sensitive and efficient determination methods for QNs such as OFL, NOR, ENR and CIP are of great significance.
The complex food matrix and low contents of QNs make it difficult to detect residues of QNs in food. Sample pretreatment is a crucial step to obtain high sensitivity and accuracy. The reported sample pretreatment technologies for QNs in food include solid-phase extraction (SPE) [7], solid-phase microextraction (SPME) [8], dispersive solid-phase extraction (dSPE) [2, 9], magnetic solid-phase extraction (MSPE) [10], QuEChERS (quick, easy, cheap, effective, rugged, safe) [11, 12], etc. SPE is the most commonly used method, but it needs tedious equilibration, washing, elution steps etc. Besides, it requires large amounts of organic reagents, long pretreatment time and high cost. QuEChERS is widely used in recent years. It uses the matrix dispersive extraction to remove most of the interfering substances in the matrix, then the extract is directly injected for instrumental analysis. However, the biggest disadvantage is that the adsorbent lacks selectivity for impurities, which makes it difficult for the purification and enrichment of analytes in complex samples. Meanwhile, excessive use of adsorbents may lead to the adsorption of target compounds, resulting in reduced recoveries. On the other hand, the purification ability is poor when insufficient adsorbents are used. SPME, dSPE and MSPE require only a small amount of solid-phase adsorbents and organic solvents. However, SPME often takes a long extraction time. MSPE and dSPE are rapid as they can simplify the tedious sample preparation steps. However, MSPE needs to synthesize specific magnetic adsorbents, while dSPE utilizes regular adsorbents and needs no specific synthesis procedure.
The dispersion step is very important for the extraction of analytes during the process of dSPE. Air-assisted extraction has been reported can improve the dispersion of extractants, increase the mass transfer of analytes to the extractants, and improve the extraction efficiency in a short time [13,14,15]. For instance, Wang et al. [16] applied an air-assisted dispersive liquid–liquid microextraction (AA-DLLME) method for the preconcentration of six fluoroquinolones in milk powder and egg samples without the use of any disperser. Rajabi et al. [17] applied an efficient, rapid and reliable air-assisted dispersive micro-solid phase extraction (AA-dμ-SPE) method to extract the polycyclic aromatic hydrocarbons from biological samples. These studies showed that the use of air-assistance could increase the contact between the extractants and samples, resulting in higher extraction efficiency than vortex, ultrasound and other auxiliary reaction methods. Besides, owing to the availability and low cost of air-assistance, it can be applied as a routine practice. However, to our knowledge, the application of air-assisted dSPE in the determination of QNs has not been reported.
Recently, layered double hydroxides (LDHs) are considered as a new kind of green adsorbent. They have been used in various sample pretreatment methods, such as SPE [18], dSPE [2], SPME [8] and MSPE [10], because of their excellent anion exchange capacity, high thermal stability, low cost, large surface area, high porosity, high adsorption efficiency and good water resistance [19, 20]. LDHs are a class of inorganic material with two-dimensional nanostructure, which are composed of multiple layers of positively charged mixed metal hydroxides and large interlayer space with high anion exchange activity [19, 21]. It is notable that LDHs can be dissolved in solutions of pH less than 4, which have been well explored in sample extraction processes to omit the elution procedure [22,23,24]. For instance, magnesium/aluminum (Mg/Al)-LDHs have been applied for the dSPE of tetracyclines in milk and water [8, 18], and for chloramphenicol veterinary drugs in milk powder, fish and chicken in combination of aptamer-functionalized magnetic mesoporous carbon [10]. It has been reported that zinc/aluminum (Zn/Al)-LDHs can be used for the dSPE of acid quinolones, including oxolinic acid, nalidixic acid, and flumequine in honey [2], and the SPME of penicillin in milk in combination of ZIF-8 [25]. In addition, nickel/aluminum (Ni/Al)-LDHs have been used for the SPE of QNs in eggs and pig liver, including NOR, CIP and ENR [7]. However, the exploration of the adsorption mechanism between these veterinary drugs and LDHs is still needed for better method performance. QNs are anionic under the condition of pH 8.28–8.89 [26, 27], and can coordinate with a variety of metal ions like Al3+, Mg2+, Ni2+, Ca2+, Fe3+, Cu2+, Sc3+, Ga3+, In3+ and Tb3+ [28, 29]. Therefore, it is possible that LDHs can adsorb and extract QNs via the coordination reaction between the surface metal cations of LDHs and QNs, as well as the interlayer anions exchange of LDHs on anionic QNs.
In this work, an air-assisted dSPE combined with HPLC method was established for the determination of QNs in honey using LDHs as the adsorbents after revealing the reaction mechanism of LDHs and QNs for the first time. Factors influencing the extraction and detection performance were optimized. The developed method was successfully applied for the determination of QNs in honey.
Materials and methods
Chemicals and reagents
The standards of OFL (99.7%), NOR (99.6%), CIP (83.1%), ENR (98%)were all purchased from National Institutes for Food and Drug Control (Beijing, China). HPLC-grade acetonitrile (ACN, ≥ 99.9%) and methanol (MeOH, ≥ 99.9%) were purchased from Macklin Biochemical Co., Ltd (Shanghai, China). Ni/Al-LDHs and Zn/Ni/Al-LDHs were purchased from Jiangsu XFNANO Materials Tech. Co., Ltd (Nanjing, China). Both Ni/Al-LDHs and Zn/Ni/Al-LDHs were prepared with ultra-pure water at the concentration of 10 mg/mL and were used after ultrasonication for 5 min. HPLC-grade orthophosphoric acid (H3PO4, 85–90%) and triethylamine (TEA, ≥ 99.5%) were purchased from Macklin Biochemical Co., Ltd (Shanghai, China). Other reagents were analytical grade. Hydrochloric acid (HCl, 36–38%), nitric acid (HNO3, 65–68%) and sodium hydroxide (NaOH, 95%) were purchased from Jinshan Chemical Reagent Co., Ltd (Chengdu, China). Ultra-pure water was prepared using a Milli-Q purification system (18.2 MΩ cm, Merck KGaA, Darmstadt, Germany). The software SPSS version 26.0 was used for the statistical analysis.
The stock standard solutions were prepared by dissolving each standard in MeOH at 1.00 mg/mL, sealed and stored in a refrigerator at 4 ℃. The middle concentration mixed standard solution was prepared by diluting the stock standards with ultra-pure water to 1.00 μg/mL and kept at 4 ℃.
Instrumentation
The chromatographic analysis was carried out using an Agilent 1260 HPLC system (Agilent technologies, Waldbronn, Germany) equipped with a 1260 Quat Pump VL, a G7129A 1260 auto-sampler and a G7121B 1260 fluorescence detector. The morphology of Ni/Al-LDHs was characterized by transmission electron microscope (TEM, FEI Talos F200X, Thermo Fisher Scientific, Hillsboro, Oregon, USA) and scanning electron microscope (SEM, Apreo 2C, Thermo Fisher Scientific, Hillsboro, Oregon, USA). X-ray diffraction patterns were obtained on an X-ray powder diffractometer (XRD, Rigaku Ultima IV, Cu Kα radiation, Rigaku Corporation, Tokyo, Japan). Fourier transform infrared (FTIR) spectra were collected on a Nicolet LS5 FTIR spectrometer (Thermo Fisher Scientific, Hillsboro, Oregon, USA). Other instruments used in the experiment included a RADWAG Wagi Elektroniczne balance (Toruńska, Radom, Poland), a 5430R centrifuge (Eppendorf AG, Hamburg, Germany), a GL-88B vortex mixer (Haimen Qilinbeier Instrument Manufacturing Co., Ltd, Haimen, China), and a SB-800 DTD ultrasonic cleaner (Ningbo Scientz Biotechnology co., Ltd, Ningbo, China).
The HPLC conditions were adopted from a reported method with modifications [30]. HPLC separation was accomplished using a ZORBAX SB-C18 column (4.6 × 150 mm, 5 µm) (Agilent Technologies, New Port, USA) thermostated at 35 °C. The fluorescence excitation wavelength was 280 nm and the emission wavelength was 460 nm. The mobile phase consisted of MeOH, ACN, and water containing 3.4 mL/L H3PO4 and 6 mL/L TEA with their volume ratio set at 18: 6: 76. The flow rate was 0.9 mL/min. The injection volume was 20 μL.
Samples
Twenty-five brands of multifloral honey, jujube honey, chaste honey, linden honey, gallnut honey and acacia honey samples were purchased in 2022. These honey samples were produced from Shandong, Fujian, Shanghai, Zhejiang, Jiangxi, Sichuan, Hubei, Jiangsu and other provinces in China.
Sample preparation
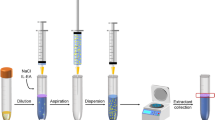
As shown in Fig. 1 1 g honey was weighed into a 15-mL centrifuge tube for each sample and water was added to a total volume of 6 mL for dilution. After vortex mixing, the pH of the aqueous honey solution was adjusted to 8 with 1 mol/L NaOH solution to form anionic QNs. Then 1 mL of the solution was taken and 200 μL Ni/Al-LDHs (10 mg/mL) was added. Air-assisted extraction was conducted for 50 times by consecutive aspirating and dispensing the solution. Afterwards, the solution became increasingly turbid and was centrifuged at 10,621×g for 5 min. Subsequently, the supernatant was decanted and 250 μL 0.1% H3PO4 solution was added to dissolve the precipitate by vortex mixing for 30 s. The final pH of the solution was 3. Then the solution was filtered with a 0.45 μm organic filter membrane, and 20 μL was injected into HPLC for analysis. Each run was injected as triplicate.
Results and discussion
Optimization of HPLC conditions
The maximum excitation wavelength and emission wavelength of the four QNs were 280 nm and 460 nm, respectively. Other HPLC conditions were adopted from a reported method [30] with modifications. In order to separate all the analytes and the interferences and to reduce run time, the mobile phase, column temperature, and flow rate were optimized. When the volume ratio of MeOH varied from 15 to 20%, the retention time decreased but the separation of the QNs and the interferents from the matrix got worse. The separation of the QNs and the interferents from the matrix was achieved with the volume ratio of MeOH, ACN, and water consisting of 3.4 mL/L H3PO4 and 6 mL/L TEA was set at 18: 6: 76. The addition of H3PO4 and TEA can improve the chromatographic peak shapes of QNs. With the increase of the column oven temperature from 30 to 40 °C, the retention time of the QNs was shortened while the separation of the QNs and the matrix became worse. Considering the retention time and the resolution of the peaks concurrently, the column oven temperature was determined at 35 °C. When the flow rate varied from 0.8 to 1.2 mL/min, the retention time of the analytes was shortened. However, though the resolution of the analyte peaks increased with the flow rate varied from 0.8 to 0.9 mL/min, it then decreased when the flow rate increased from 0.9 to 1.2 mL/min. Finally, the optimized conditions were set as follows: the mobile phase was composed of MeOH, ACN, and water containing 3.4 mL/L H3PO4 and 6 mL/L TEA (18: 6: 76, v/v). The column oven temperature was 35 °C and the flow rate was maintaining at 0.9 mL/min. The fluorescence excitation and emission wavelengths were 280 nm and 460 nm, respectively.
Characterization of Ni/Al-LDHs
Ni/Al-LDHs were characterized by SEM, TEM, FTIR and XRD. The morphology of LDHs is shown in SEM and TEM images (Fig. 2a, b). Ni/Al-LDHs have uniform distribution and flake polygonal structures with a lateral size of appropriately 10–40 nm. The FITR spectrum is shown in Fig. 2c. Due to the hydrogen and oxygen stretching vibration of metal hydroxide layer and interlayer water molecules [7, 19, 24], a significant absorption band can be observed at about 3519 cm−1. The absorption peak at about 1382 cm−1 is due to the bending vibration of C–O–H bond of interlayer carbonates [23, 31,32,33]. Consistent with previous reports [33], peaks appeared in the range of about 400–700 cm−1 are attributed to the stretching vibration of Ni–O and Al–O bonds. As shown in Fig. 2d, the crystal structure of Ni/Al-LDHs was studied by XRD. Four obvious diffraction peaks are observed near 001, 002, 003, 004. Besides, and the 001 position is moved to a higher d value, which is consistent with the previous report [33], indicating that the Ni/Al-LDHs are relatively pure and have good crystal structure with a layered structure. The first basal reflection of Ni/Al-LDHs (001) appearing at 2θ is 11.40°, corresponding to an interlayer distance of 0.78 nm.
Optimization of sample preparation
Volume of the dilution water
Honey is a viscous foodstuff which can present a homogenous liquid when dissolved in water. The viscosity of aqueous honey solution under different honey-water ratio may affect the extraction of QNs in honey. Therefore, we compared the extraction efficiencies of 10 ng QNs in 1 g honey samples dissolved in different volumes of water. As shown in Fig. S1, when the total volume of the aqueous solution was 6 mL, the extraction efficiencies of most QNs reached the highest level. It was suggested that insufficient volume of water lead to the high viscosity of the aqueous solution that affected the extraction. On the other hand, when excessive volume of water was added, the honey sample was over diluted and resulted in low sensitivity since the constant volume (1 mL) of the aqueous honey solution was taken for the subsequence extraction procedure.
Selection of the LDH adsorbent
QNs can form complexes with various cations of transition metals, such as Mg2+, Al3+, Ca2+, Fe3+, Cu2+, Ni2+, and Zn2+ [28, 29]. The extraction performance of the two-dimensional layered bimetallic hydroxides (Ni/Al-LDHs) and the two-dimensional layered trimetallic hydroxides (Zn/Ni/Al-LDHs) on 10 ng/g QNs spiked in honey were compared. As shown in Fig. S2, the peak areas of the four QNs extracted by Ni/Al-LDHs were greater than those extracted by Zn/Ni/Al-LDHs. The contents of Ni and Al elements in Ni/Al-LDHs were 5 w/w and 42 w/w, respectively. The contents of Zn, Ni and Al elements in Zn/Ni/Al-LDHs were 34 w/w, 9 w/w and 13 w/w, respectively. We conjectured that the difference in adsorption capacities of Ni/Al-LDHs and Zn/Ni/Al-LDHs might be attributed to the different element contents of these two LDH adsorbents. So, Ni/Al-LDHs were chosen as the adsorbent.
The pH of the solution
The pH of the solution is an important factor affecting the extraction efficiency. In order to obtain higher extraction efficiency, the pH value of the solution was optimized. QNs possess two pKas (pKa1 at 5.90~6.23, pKa2 at 8.28~8.29) and become anionic QNs when the pH is greater than about 8, in which they can be exchanged by the LDHs and co-precipitated with alkalized LDHs [26]. We used 10 mol/L NaOH and 0.6% H3PO4 solutions to adjust the pH of 1 mL aqueous honey solution spiked with 10 ng/g QNs to the values of 6–11. As shown in Fig. 3A, the peak areas of the four QNs increased from pH 6 to 8, possibly owing to the increasing ionization of the QNs that made them be prone to coordinate with the surface metal ions on LDHs and be exchanged by the interlayers of LDHs. However, the peak areas decreased from pH 8 to 11 since high contents of OH– competed with QNs for the anion exchange sites of LDHs [2, 7]. So, the pH of the aqueous honey solution was adjusted to 8 for further research.
The amount of adsorbents
In the process of dispersive solid-phase extraction, the amount of adsorbents determined the complete extraction of QNs. Therefore, the effect of the amounts of Ni/Al-LDH adsorbents ranging from 1 to 30 mg on the extraction efficiencies of QNs was investigated. As shown in Fig. 3b, the peak area of ENR reached the largest when the amount of Ni/Al-LDHs was 1 mg, and the peak areas of OFL, NOR and CIP reached the largest when the amount of Ni/Al-LDHs was 2 mg. This difference might be related to the slight difference in the structures and properties of the four QNs. The result indicated that 2 mg Ni/Al-LDHs had enough coordination and anion exchange sites to extract the QNs. Besides, incomplete dissolution of the LDHs led to the release difficulty of the analytes when too many LDHs were added. Therefore, 2 mg of Ni/Al-LDHs was used.
The auxiliary extraction method
Both air-assisted extraction and vortex could increase the contact between LDHs and the analytes in honey samples. To ensure the complete extraction of QNs by LDHs, the effects of vortex time and air-assisted times on extraction efficiencies of QNs were studied. The aqueous honey solution (1 mL) spiked with 10 ng/g QNs was extracted with 1–30 min of vortex mixing and 5–150 times of air-assisted extraction. Air-assisted extraction was accomplished by consecutive aspirating and dispensing the solution in the centrifuge tube with a syringe. As shown in Fig. S3, the peak areas of OFL, NOR, CIP and ENR reached the largest at 10 min, 30 min, 15 min, and 10 min by vortex mixing, respectively. As shown in Fig. 3c, the peak areas of OFL, NOR and ENR were the largest with 50 times of air-assisted extraction, and CIP had the largest peak area with 75 times of air-assisted extraction. Then we compared the extraction efficiencies of the four QNs by air-assisted extraction with vortex. As shown in Fig. 3d, the peak areas of the four QNs with 50 times of air-assistance were larger than those with 10 min of vortex. The results were in accordance with the reports [13], suggesting that air-assisted extraction could increase the mass transfer and accelerate the speed of migration of the analytes to the adsorbent surface, reduce the consumption of adsorbent, improve the extraction efficiency and shorten the extraction time. Therefore, 50 times of air-assisted extraction was selected.
The type and volume of the acidic solution
Since the LDH adsorbents could be dissolved in acidic solutions with pH lower than 4 [22], the adsorbed analytes could be released, avoiding the elution procedure with organic solvents. Therefore, we compared the detected peak areas of 10 ng/g QNs in honey by dissolving with 0.1% H3PO4 (pH 3–4), 0.1% HNO3 (pH 3–4) and 0.1% HCl (pH 3–4). As shown in Fig. 4a, the peak areas of the four QNs in 0.1% H3PO4 were the largest. Besides, the mobile phase contained H3PO4. Therefore, 0.1% H3PO4 was used to dissolve the Ni/Al-LDHs. As shown in Fig. 4b, when the volume of 0.1% H3PO4 was 250 μL, the peak areas of the four QNs reached the largest. So, 250 μL of 0.1% H3PO4 was used.
Method validation
The method validation was based on the "Guidelines for the Validation of Chemical Methods for the FDA Foods Program, 3rd Edition" and the reference approaches [34]. Standard and matrix-matched calibration curves were obtained by analyzing three replicates of standard solutions with concentration points of 1, 4, 10, 25, 50, and 100 ng/g. A comparison of the standard calibration curves and the matrix-matched calibration curves was conducted by t-test. The results indicated that the slopes of the standard calibration curves and the matrix-matched calibration curves were statistically different for all the four QNs (P < 0.05). Therefore, QNs in honey samples were quantified by matrix-matched calibration curves which were prepared by adding the standards into a blank sample and treated according to the sample preparation procedures to avoid introducing systematic errors and ensure the accuracy of the analysis results. As shown in Table 1, the linearities of the four QNs were in the range of 1–100 ng/g with their coefficients of determination (R2) greater than 0.9996. The limits of detection (LODs) and limits of quantification (LOQs) were calculated as 3Sb/m and 10Sb/m, respectively. Where Sb was the response standard deviation of 6 blank honey samples, and m was the slope of the calibration curve. The LODs and LOQs of the four QNs were 0.25–0.82 ng/g and 0.82–2.73 ng/g, respectively. Figure 5 showed the chromatograms of (A) a mixed 50 ng/g QNs standard solution, (B) a blank honey sample and the honey sample spiked with 10 ng/g QNs. No peaks of interferences appeared in the region of interest where the target analytes were expected to elute, indicating satisfactory specificity of this method. The accuracy and precision of the method were measured by analyzing three concentration levels (5, 20, 50 ng/g) of QNs spiked into a blank honey sample in three-parallel experiments in a day (intra-day) and three consecutive days (inter-day). As shown in Table 2, the average recoveries of OFL, NOR, CIP and ENR were 86.6%, 90.5%, 85.9% and 82.3%, respectively. The intra-day and inter-day RSDs were 4.39–11.0% and 2.16–17.5%, respectively. The acceptable recoveries and precision indicated that the applied method was highly efficient for the determination of QNs in honey samples.
Method application
The method was applied to the detection of 25 brands of genuine honey samples. The detected contents of OFL, NOR, CIP and ENR in these honey samples were all lower than the LODs. Compared with reported dSPE, SPME, MSPE and DLLME methods for the determination of QNs using adsorbents and extractants like dissolvable Zn/Al-LDHs, magnetic materials, deep eutectic solvents, etc. [2, 35,36,37,38], this developed method was sensitive and accurate. Meanwhile, since this method needed no organic solvent to conduct the elution procedure, it was simple, convenient and environment friendly.
Conclusions
In this work, an air-assisted dispersive solid-phase extraction combined with HPLC method for the simultaneous determination of four QNs in honey was established for the first time. Anionic QNs were extracted by Ni/Al-LDHs under alkaline conditions via the coordination reaction and the interlayer exchange reaction. The usage of Ni/Al-LDHs as the adsorbents which could be dissolved with acids avoided the use of organic solvents for elution. In addition, the application of air-assisted extraction significantly enhanced the extraction efficiencies. This method showed advantages of high extraction efficiency, simplicity, convenience, environment-friendliness, low cost and good accessibility. Therefore, it can be applied as a practical analytical method for the analysis of QNs in honey. Further research can be conducted to explore the application of this method for other matrices and for different classes of compounds.
References
L. Ceballos, J.A. Pino, C.E. Quijano-Celis, A. Dago, J. Food Quality (2010). https://doi.org/10.1111/j.1745-4557.2010.00330.x
X. Di, X. Wang, Y. Liu, X. Guo, X. Di, J. Sep. Sci. (2019). https://doi.org/10.1002/jssc.201801009
Ż Barganska, J. Namiesnik, M. Ślebioda, Trac-Trend Anal. Chem. (2011). https://doi.org/10.1016/j.trac.2011.02.014
Q. Tang, T. Yang, X. Tan, J. Luo, J. Agric. Food Chem. (2009). https://doi.org/10.1021/jf900513b
Ministry of Agriculture of the people's republic of China, Notice No. 2292 of the People's Republic of China. (2015) http://www.moa.gov.cn/nybgb/2015/jiuqi/201712/t20171219_6103873.htm. Accessed 23 March 2022
A.İ Emir, Y.K. Ece, R. Sinem, A. Sezer, E. Özge, Food Addit. Contamin. Part A (2021). https://doi.org/10.1080/19440049.2021.1881621
X. Wang, W. Zhou, C. Wang, Z. Chen, Talanta (2018). https://doi.org/10.1016/j.talanta.2018.04.100
N. Phiroonsoontorn, S. Sansuk, Y. Santaladchaiyakit, S. Srijaranai, J. Chromatogr. A (2017). https://doi.org/10.1016/j.chroma.2017.09.005
R. Wang, S. Li, D. Chen, Y. Zhao, Y. Wu, K. Qi, Food Chem. (2021). https://doi.org/10.1016/j.foodchem.2020.128269
Q. Zhang, Q. Zhou, L. Yang, X. Wang, Y. Zheng, L. Bao, J. Sep. Sci. (2020). https://doi.org/10.1002/jssc.201901189
A.H. Shendy, M.A. Al-Ghobashy, S.A.G. Alla, H.M. Lotfy, Food Chem. (2016). https://doi.org/10.1016/j.foodchem.2015.06.048
J. Wang, D. Leung, Drug Test. Anal. (2012). https://doi.org/10.1002/dta.1355
M. Ezoddin, L. Adlnasab, A.A. Kaveh, M.A. Karimi, B. Mahjoob, Biomed. Chromatogr. (2019). https://doi.org/10.1002/bmc.4572
L. Adlnasab, M. Ezoddin, M. Shabanian, B. Mahjoob, Microchem. J. (2019). https://doi.org/10.1016/j.microc.2018.12.020
Y. Yang, S. Yin, L. Wu, Y. Li, C. Sun, Eur. Food Res. Technol. (2021). https://doi.org/10.1007/s00217-021-03838-3
L. Wang, T. Huang, H. Cao, Q. Yuan, Z. Liang, G. Liang, Food Anal. Methods (2016). https://doi.org/10.1007/s12161-016-0409-6
M. Rajabi, A.G. Moghadam, B. Barfi, A. Asghari, Microchim. Acta (2016). https://doi.org/10.1007/s00604-016-1780-0
Y. Yang, J. Zhang, Y. Li, S. Yin, Y. Jiang, C. Sun, Anal. Methods (2021). https://doi.org/10.1039/d1ay00154j
H. Abdolmohammad-Zadeh, Z. Rezvani, G.H. Sadeghi, E. Zorufi, Anal. Chim. Acta (2011). https://doi.org/10.1016/j.aca.2010.11.035
B. Wang, J. Qu, X. Li, X. He, Q. Zhang, J. Am. Ceram. Soc. (2016). https://doi.org/10.1111/jace.14404
C. Taviot-Guého, V. Prévot, C. Forano, G. Renaudin, C. Mousty, F. Leroux, Adv. Funct. Mater. (2018). https://doi.org/10.1002/adfm.201703868
S. Tang, H.K. Lee, Anal. Chem. (2013). https://doi.org/10.1021/ac4013573
M. Rajabi, S. Arghavani-Beydokhti, B. Barfi, A. Asghari, Anal. Chim. Acta (2017). https://doi.org/10.1016/j.aca.2016.12.041
S. Sansuk, S. Nanan, S. Srijaranai, Green Chem. (2015). https://doi.org/10.1039/c5gc00713e
A. Khoobi, M. Salavati-Niasari, M. Ghani, S.M. Ghoreishi, A. Gholami, Food Chem. (2019). https://doi.org/10.1016/j.foodchem.2019.02.118
Y. Picó, V. Andreu, Anal. Bioanal. Chem. (2007). https://doi.org/10.1007/s00216-006-0843-1
H. Park, C. Oh, H. Lee, J. Lee, K. Yang, K. Bark, Photochem. Photobiol. (2002). https://doi.org/10.1562/0031-8655(2002)0750237SPOFAI2.0.CO2
A. Albini, S. Monti, Chem. Soc. Rev. (2003). https://doi.org/10.1039/b209220b
H. Park, T.H. Kim, K. Bark, Eur. J. Med. Chem. (2002). https://doi.org/10.1016/S0223-5234(02)01361-2
M. Gao, H. Wang, M. Ma, Y. Zhang, X. Yin, R.A. Dahlgren, D. Du, X. Wang, Food Chem. (2015). https://doi.org/10.1016/j.foodchem.2014.11.132
M.N. Sepehr, T.J. Al-Musawi, E. Ghahramani, H. Kazemian, M. Zarrabi, Arab. J. Chem. (2017). https://doi.org/10.1016/j.arabjc.2016.07.003
F. Beigi, M.S.S. Mousavi, F. Manteghi, M. Kolahdouz, Appl. Clay Sci. (2018). https://doi.org/10.1016/j.clay.2018.09.006
S. Mallakpour, M. Dinari, RSC Adv. (2015). https://doi.org/10.1039/c5ra03383g
B. Barfi, A. Asghari, M. Rajabi, Arab. J. Chem. (2020). https://doi.org/10.1016/j.arabjc.2017.06.002
Q. Gao, H. Zheng, D. Luo, J. Ding, Y. Feng, Anal. Chim. Acta (2012). https://doi.org/10.1016/j.aca.2011.12.067
X. Cui, P. Zhang, X. Yang, M. Yang, W. Zhou, S. Zhang, H. Gao, R. Lu, Anal. Chim. Acta (2015). https://doi.org/10.1016/j.aca.2015.03.056
K. Yu, M. Yue, J. Xu, T. Jiang, Food Chem. (2020). https://doi.org/10.1016/j.foodchem.2020.127371
L. Chen, X. Huang, J. Agric. Food Chem. (2016). https://doi.org/10.1021/acs.jafc.6b03965
Acknowledgements
This work was financially supported by the Science and Technology Project of Luzhou (No. 2021-JYJ-72), the Natural Science Foundation of Sichuan Province (No. 2022NSFSC1490) and the Applied Basic Research Foundation of Southwest Medical University (No. 2021ZKQN003).
Funding
This work was supported by the Science and Technology Project of Luzhou (No. 2021-JYJ-72), the Natural Science Foundation of Sichuan Province (No. 2022NSFSC1490) and the Applied Basic Research Foundation of Southwest Medical University (No. 2021ZKQN003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This study does not contain human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, T., Ma, L., Jiang, Y. et al. Simultaneous determination of four quinolones in honey by nickel/aluminum-layered double hydroxide-based air-assisted dispersive solid-phase extraction and high-performance liquid chromatography. Food Measure 16, 5023–5031 (2022). https://doi.org/10.1007/s11694-022-01595-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01595-9