Abstract
Sesame meal with high protein content is the by-product of sesame oil extraction. Using sesame meal protein in food formulations enhances the functional and nutritional attributes of food. In the present study, the effect of salt concentration (18% and 22% w/v) and drying temperature of sesame seeds (45 ºC and 180 ºC) on functional attributes (solubility, water, and oil hold capacity, stability and capacity of foam, stability and activity index of emulsion, and the least gelation concentration (LGC) at different pH) were investigated. Sesame protein isolate (SPI) samples revealed the lowest changes in functional attributes at isoelectric pH, and the highest effect was obtained at pH 11. Salt concentration and drying temperature of sesame also significantly affected the functional and structural attributes of SPI (p < 0.05). The highest salt concentration and drying temperature of sesame are 22% and 180 °C, respectively, increasing the solubility, foam capacity and stability, oil and water hold capacity, emulsion activity, and stability. In addition, at pH 7, 9, and 11, the control sample and sample treated with 22% of salt concentration at 180 ºC had the lowest and highest ability to form a gel as compared to other treatments. The structural attributes showed the presence of protein and denaturation in the structure of all samples. Given the good functional attributes of protein treated at higher salt concentration and drying temperature, this SPI can improve functional attributes in different food formulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sesame seed (Sesamum indicum L.) is a most vital oilseed crop belonging to the Pedaliaceae family [1]. It is one of the most important oilseed plants worldwide [2, 3], containing about 50%, 20%, and 15% of fats, protein, and carbohydrates, respectively [4]. Sesame oil production ranks eighth in the world’s oil market [1].
Sesame oil and seeds are used in the pharmaceutical, food, and cosmetic industries [5]. Sesame meal is a by-product obtained from oil extraction factories, and it contains about 50% proteins [2]. Protein extracted from sesame meal is a valuable source of leucine, arginine, and methionine, however, it is relatively poor in lysine [3].
The growing global population and limited environmental resources lead to a constant search for new plant protein sources [6, 7]. Plant proteins play significant roles in human nutrition as an alternative to animal proteins [8], and they are gaining increased interest as ingredients in food systems during manufacturing [9].
Proteins extracted from different plant sources display various functional properties like solubility, water, and oil holding capacity, gelation, foam, and emulsion stability, which is caused by their heterogeneous composition and structure [10]. The conclusive success of utilizing plant proteins in food systems during manufacturing, processing, and storage, largely depends on the favorable attributes they show to foods. Therefore, the processing parameters greatly influence the functional characteristics of plant protein products. Different extraction and drying procedures would assign proteins with varying functional properties [11]. Drying techniques and seed peeling are necessary for the preparation of plant protein isolates. Both parameters could improve storage stability or form insoluble aggregates caused by partial protein denaturation, thereby shifting the functional properties of plant proteins.
Various researchers assessed oilseed by-products proteins as an alternative to animal proteins, such as sunflower seed, rapeseed [12], flaxseed, sesame seed [13], pumpkin seed [14], mustard seed [15], grapeseed [16, 17], cottonseed [6], and peanut [13]. However, the effects of drying temperature and salt concentration on functional attributes of sesame seed have not been investigated. The present research aimed to determine the extractability of SPI from sesame meal in the presence of various concentrations of NaCl (0%, 18%, and 22% w/v) and study the effect of different drying temperatures of the sesame seed (25 ºC, 45 ºC, and 180 ºC) on functional attributes of SPI.
Materials and methods
Materials
All chemicals and reagents with analytical grade (purity > 99%) were purchased from Merck (Darmstadt, Germany). Sesame (Sesamum indicum L.) seeds variety Yekta were obtained from the local market (Yazd, Iran).
Preparation of sesame meal
Sesame seeds were manually cleaned and peeled using a peeler (Himura, Japan). Then, the sesame seeds were immersed in the salt solution at three concentration levels (0%, 18%, and 22% w/v) for 10 min. Subsequently, sesame seeds were washed twice with distilled water and dried in the oven (VO200, Memmert, Germany) at three different temperature levels (25 °C for 24 h, 45 °C for 48 h, and 180 °C for 5 min). The whole flour was prepared by milling the dried seeds (B.G 300P, Pars-Khazar, Iran), and it was defatted with n-hexane at a 1 to 10 w/v ratio (sesame to solvent) with constant stirring at room temperature for 2 h. Then, the slurry was defatted twice in the same ratio, and it was dried at 25 °C for 24 h after filtering. The defatted sesame meal (DSM) was ground and passed through a sieve with 80 meshes to the inch for protein extraction. Table 1 shows the different conditions of sesame meal preparation.
Isolation of protein from sesame meal
The alkali method developed by Sharma et al. (2016) with slight modification was used to prepare SPI [18]. DSM was mixed with water at a ratio of 1:10 w/v, and the pH was adjusted to 11 using 1 M NaOH with continuous stirring for 1 h at 35 ºC and centrifuged (z200A, Hermle, Germany) at 4500 rpm for 20 min. The supernatant was collected and adjusted to pH 4.5 using 1 M HCL. The suspension was centrifuged at 4500 rpm for 20 min. Subsequently, the precipitate was neutralized to pH 7 using 1 M NaOH and dialyzed using distilled water for 48 h at 4 ºC. Then it was vacuum-dried (VO200, Memmert, Germany) and passed through a sieve with 80 meshes to the inch. The content of protein was determined by the Kjeldahl method using an automatic Kjeldahl system (230-Hjeltec Analyser, Hoganas, Sweden). The SPI was weighed and transferred into a Kjeldahl digestion flask containing 10 g of catalyst (1 g of CuSO4.5H2O + 9 g of K2SO4) and 25 mL of H2SO4 (98%) and digestion was done using an electrical heater for 2.5 g. Afterward, it was cooled to room temperature and 80 mL of NaOH was added to the flask. Ammonium hydroxide was trapped as ammonium borate in a boric acid solution during distillation. Total nitrogen (N × 6.25) was determined by titration with HCL [19].
Determination of functional attributes
Protein solubility
The solubility of SPI samples was determined over a pH of 4, 5, 7, 9, and 11. Briefly, protein solutions were prepared by dispersing powdered protein into distilled water (1% w/v), and their pH was adjusted from 4 to 11. The protein solutions were stirred at room temperature for 2 h and centrifuged at 5175 rpm for 30 min. The protein content of the supernatants was determined using the Bradford method [20]. Bovine serum albumin was used as a standard protein. Total protein content in the sample was determined after solubilization using 0.5 N NaOH. Protein solubility was calculated as Eq. 1:
Where PS is the protein content present in the supernatant after centrifugation and filtration, and PT is the total protein content present in the protein sample.
Water Hold Capacity (WHC) and Oil Hold Capacity (OHC)
The WHC and OHC were determined using the method described by Elsohaimy et al. [21]. SPI sample of 1 g was mixed with 10 mL distilled water for WHC, and soybean oil for OHC in the centrifuge tubes. After storing for 30 min at room temperature, samples were then centrifuged at 7900 rpm for 25 min. The WHC and OHC were expressed as the weight of water or oil held by 1 g of the SPI sample.
Foam attributes
Foam capacity (FC) and foam stability (FS) were determined based on the method of Fathollahy et al. [22]. SPI solution of 2.5 g/L was dissolved in 10 mL distilled water and whipped for 10 min using a homogenizer (T-25, IKA, India) at the speed of 20,000 rpm. Subsequently, the whipped SPI sample was transferred to a 50 mL cylinder and the total volume was measured. In addition, the whipped samples were allowed to stand for 5, 10, 20, 30, 45, and 60 min at room temperature, and the volume was recorded. FC and FS were calculated using Eqs. 2 and 3:
Emulsion capacity (EC) and stability (ES)
The emulsion capacity (EC) and stability (ES) were determined based on the method of Elsohaimy et al. (2015) [21]. A total of 15 mL of soybean oil was added to 45 mL SPI suspension (0.2% w/v) at different pH levels (4–11) and homogenized using a homogenizer (T-25, IKA, India) for 1 min at 20,000 rpm. Then, 50 µL of emulsion at 0 and 10 min after homogenization was mixed with 5 mL of 0.1% SDS and the absorbance was immediately measured at 500 nm (A0) and after 10 min (A10). The emulsion activity index (EAI) and the emulsion stability index (ESI) were calculated based on Eqs. 4 and 5:
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
According to Laemmli’s (1970) method [23], SDS-PAGE performed the protein profile of sesame meal with 12% and 5% of separating gel and shaking gel, respectively. SPI samples (20 µl) with optimized characteristics were prepared from 500 µl protein solution and were added to 1 mL buffer (1% bromophenol blue and β-mercaptoethanol, 10% SDS, glycerol, distilled water, and 0.5 M Tri-HCl pH 6.8). Subsequently, it was heated at 98 ºC for 10 min and applied to the sample wells. Electrophoretic migration was monitored at 14 mA/gel for 2 h. The gel was fixed using a fixing solution and then stained using the Coomassie Brilliant Blue for 1 h.
Fourier transform infrared analysis (FT-IR)
The SPI samples were dispersed in water at 35 ºC for 2 h. Fourier transform infrared (FT-IR) spectrum was recorded using FT-IR spectrometer (870 Thermo Nicolet Nexus, Madison, USA) at the 4000–500 cm− 1. The instrument is equipped with a KBr beam splitter and GaAs detector [24].
The LGC measurement
The LGC was determined by preparing 10 mL dispersion between 2% and 20% (w/) solids concentration in test tubes at different pH levels (4–11). The dispersion was mixed for 5 min using a vortex and then heated in a 95 °C water bath for 1 h. Then, the mixtures were subsequently cooled in a refrigerator at 4 ºC for 2 h. The lowest concentration at which the sample did not fall or slip from an inverted tube was taken as the LGC [25].
Statistical analysis
The data were analyzed using the SPSS software version 22. One-way analysis of variance (ANOVA) using a post hoc test (Duncan) with p < 0.05 was performed to identify significant differences among all study parameters. All experiments were carried out in triplicates.
Results and discussion
Protein solubility
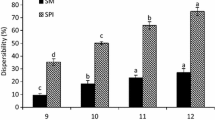
Figure 1 shows the results of protein solubility as a function of pH. The lowest protein solubility was observed in the control sample which ranged from 3.5 to 22.4 at pH 5 and pH 11, respectively. It is because salt ions interact with oppositely charged groups and decrease electrostatic attraction between protein molecules [26]. The salt concentration and drying temperature at different pH levels had a significant effect on the solubility of the samples (p < 0.05). An increase in drying temperature from 45 to 180 °C and a reduction in salt concentration from 22 to 18% caused protein solubility to increase. The highest solubility was obtained in the sample treated with 18% salt solution at 180 °C. The increase in solubility of protein to 18% of salt concentration can be attributed to the decrease in the electrical charge of the protein network and the surface activity of the soluble proteins. In addition, the reduction in electrostatic interactions or binding between hydrophilic domains within the sesame proteins increases the solubility. The presence of different ions in protein solution causes changes in the solubility of the protein. Meanwhile, the interaction between salt ions and some charged groups of protein is one of the important reasons for proteins’ solubility to increase. At the concentration of 18–22% of NaCl solution, peeling of sesame is well done, and a concentration higher than 22% of NaCl hurts protein properties. In other words, 18% is the minimum concentration at which sesame peeling can be well done, and a concentration of more than 22%, in addition to adverse effects on protein properties, also causes environmental pollution. Therefore, these two concentrations were selected for sesame peeling based on the pre-treatment tests performed.
An increase in pH from 5 to 11 causes protein solubility to increase, which is similar to the results observed by [25] for pea protein isolate. The lower protein solubility in pH 5 may be due to the balance between positive and negative electric charges and generally a decrease in repulsive forces. These results coincide with those reported for Persian lime seed protein [22], cowpea (Vigna unguiculata) and pea (Pisum sativum) protein isolate [27], fava beans, chickpeas and fenugreek protein [28], mung bean (Vigna radiata) protein isolate [11], and fenugreek (Trigonella foenum graecum) protein concentrate [29]. They reported lower protein solubility at a pH of around 5.
Water and oil hold capacity
Table 2 presents the results of WHC and OHC. The highest WHC and OHC were observed in the control sample which were 2.22 and 1.18 g/g, respectively. In addition, the lowest value was observed in the sample treated at a higher concentration of the salt solution and drying temperature. When the concentration of salt increases, protein ions promote bond formation between water and salt ions, thereby leading to dehydration of proteins and reduction of the WHC [30]. A similar result was reported by Adubiaro et al. (2009) [31], who stated that a high concentration of salt leads to a decrease in the WHC of bean flour. Ogungbenle (2008) [32] also reported that the WHC of different types of legume flour decreased when exposed to NaCl at a concentration between 0.2 and 0.4 M, which coincides with the results of our study.
Hydrated salt ions form weak bonds with the carboxyl and amine polar groups of the proteins and don’t show any protein dehydration at the low salt concentrations. Therefore, water binding depends on the hold of proteins by ions. However, salt ions compete with protein to hold water and prevent the hold of water by protein at high salt concentrations [33]. Based on the results found in Table 2, the control and sample treated with 22% of salt solution at 180 °C showed the highest and lowest OHC. It may be due to the degradation of hydrophilic and hydrophobic groups during heat processing [34] which decreases the protein’s ability to absorb and retain water or oil [35, 36]. Adebowale et al. (2004) also reported an increase in OHC by increasing non-polar amino acids in the protein side-chain [37].
Foam attributes
Results in Table 3 indicate no significant difference between samples at pH 4–7 (p > 0.05). However, with increasing pH from 7 to 11, a statistically significant difference was observed between samples (p < 0.05). The lowest and highest FC was observed in control and sample treated at higher temperature and salt concentration, respectively. Proteins play a major role in foam formation as a surfactant by reducing surface tension and creating an adhesive film on the middle surface of the water-air [38]. Therefore, the control sample with lower protein solubility exhibited higher WHC and OHC [26].
The highest FC was provided by protein meal prepared at 22% salt concentration and dried at 180 °C. Benelhadj et al. (2016) showed an increasing rate in algae protein isolate foam capacity by raising the pH level [39]. Mazloomi et al. [40] investigated the influence of salt concentration on pumpkin meal protein functional attributes. They found that the FC decreased with an increase in salt concentration, which coincide with the results of this study. Nasri et al. reported an increase in FC of fenugreek meal protein by increasing the ionic strength of the salt solution, which was attributed to the precipitation of proteins by salt [29]. In addition, the protein type and salt concentration can be a reason for differences. Ragab et al. [41], showed in another study that FC of chickpea protein isolates increased by increasing salt concentration.
The foam stability (FS) is achieved by reducing the interfacial tension of gas-liquid and the formation of a resistant protein layer to rupture with elasticity around the bubbles and changing the viscosity of the liquid phase. The results of FS of different samples in Fig. 2 revealed that FS in all samples decreased over time at pH 9 and 11. The FS of all samples at pH lower than 9 was 0.5% and it was completely affected by pH. The most important factor that causes the protein to act as a favorable foaming agent at different pH levels is its ability for fast absorption on the surface between the air and water and rapid structural changes of protein surface [42].
The FS increased with an increase in temperature from 45 ºC to 180 ºC. Factors such as pH, salt concentration, and surface tension of proteins affect the FS. It may be due to the formation of a stronger layer between the air and water that generates a stable foam. Researchers believe that this effect is attributed to the critical concentration of salt [30]. The highest FS was observed at pH 11 and this result can be attributed to the excessive increase of hydrophilicity attributes at alkaline pHs. Therefore, it does not allow the optimal interaction of the protein with air, which acts as a hydrophobic phase in the foam system. Creating a stable foam also depends on the type of protein, temperature, degree of protein denaturation, pH, and method used.
Emulsion attributes
Salt concentrations, pH, and drying temperatures had a significant (p < 0.05) effect on stability (ES) and activity of (EA) of emulsion, as shown in Fig. 3. The higher ESI and EAI were observed in the SPI sample treated with higher drying temperature and salt solution followed by S18T180, S22T45, and S18T45, respectively. The control sample showed the least ESI and EAI, and an increase in pH from 5 to 9, also increase the ESI and EAI of the SPI. The minimum ESI and EAI were observed at pH 5, this pH is the isoelectric pH of SPI at which protein has the lowest solubility. Similar results were observed by other researchers for Persian lime seed protein [22], Hemp seed protein [43], and Chinese quince seed protein isolate [44]. Regarding the effect of different pH as shown in Fig. 3, the ESI and EAI of SPI were dependent on pH changes, which is caused by the effect of pH on the hydrophobic-hydrophilic protein balance.
According to Nasiri and EI Tinay (2007), high pH has a greater effect on the increase of emulsion capacity of fenugreek protein. This effect can be attributed to the effect of the isolation production process and protein composition. Proteins contain polar, uncharged, and non-polar charged amino acids, thereby leading proteins to act as stabilizers in enhancing emulsion stability. Having both hydrophilic and hydrophobic molecules increase the binding of water/oil in food systems using protein [45]. In addition, proteins tend to absorb at the surface of oil and water and form a layer around the oil droplets [46]. The reduction of interfacial tension of water and oil affects the emulsion activity.
The sample treated with 22% salt solution at 180 °C had the highest reduction in interfacial tension. In addition to pH, other factors such as nitrogen, salt concentration, protein shape, surface tension, and protein concentration are also effective in changing the emulsion capacity [30, 37]. This is because of the reduction of solubility and flexibility of the protein at isoelectric pH. Fat globules were surrounded by a charged layer of proteins at a high salt concentration and a two-way repulsion was created between them. The number of available proteins is also reduced, thereby limiting the absorption of protein against water and oil. According to Kinsella et al. [34], protein solubility is a necessary condition in forming suitable emulsions. They concluded that ionic strength affects solubility and emulsion activity by increasing salt concentration which may be due to the formation of a cohesive protein network. Khattab et al. [47] also examined the functional attributes of canola, soybean, and flaxseed protein meal and reported that canola meal had the best emulsion capacity in 0.1 M salt concentration. In addition, the emulsion activity of the SPI sample that was heated to 180 °C at all pH levels was higher than others. The effect of drying temperature on emulsion attributes can be attributed to the form of protein caused by the denaturation at high temperatures.
Protein profiling using SDS-PAGE
SDS-PAGE was performed to evaluate the change in the protein composition of a sesame seed. Sesame protein includes globulin (60–70%), albumin (15–25%), prolamine (1.4%), gluten (6.9%) and oleosin (1.5%) [5]. Figure 4 presents the protein molecular weight distribution profiles of sesame isolates. As observed in the pattern, SPI samples had three polypeptides (17, 30, and 50 kDa) with the 50 kDa band in the lowest proportion. The main bands were found at about 17, 35, and 50 kDa, which shows that these can be the main components of our samples. Despite different drying temperatures and salt concentrations, all samples depicted identical band patterns, which consisted of some polypeptides linked to disulfide bonds. This is in agreement with Brishti et al. [11], who reported that different drying techniques do not cause dissociation of mung bean protein subunits.
The SPI treated at 180 °C and 22% salt solution formed the highest proportion band. The dried samples have various protein subunits with low molecular weight, and this result may be attributed to the high temperature of the drying process [48]. The type of protein and heating methods influenced the secondary structure of the protein. The polypeptide composition of the SPI samples coincides with the results of Singharaj & Onsaard [49], who stated that sesame protein concentrate had protein bands with molecular weight of 12–39 kDa.
Fourier transform infrared analysis (FT-IR)
The protein structure shifted caused by the changes in drying temperature and salt concentration. Amide II was observed at the main peak of 1534 cm− 1 as shown in Fig. 5. The range of this peak is extended from 1500 cm− 1 to 1580 cm− 1. Amide I and II bands are the main peaks that indicate the presence of protein. Changes in the intensity of these peaks indicate denaturation in the structure of each sample [50]. Amide I was observed at the main peak of 1647 cm− 1 and the range of this peak is from 1600 cm− 1 to 1700 cm− 1. In this band, the C = O is detected in the α-helix structure. The amide I protein bond is a complex structure that carries various components such as α-helix, β-sheet, random ring, or β-rotation. Based on the comparison of all spectra of samples treated with 18% and 22% of salt solution at different drying temperatures, their protein structure appeared with a slight difference in peak intensity; Sample treated at a higher salt concentration (22%) and drying temperature (180 °C) showed the shortest peak of amide I in structure and it is more strongly denatured than other samples.
Least gelation concentration
Table 4 shows the results of LGC. The drying temperature and pH are effective factors in gel formation and the control sample had the lowest ability to form a gel at pH 7, 9, and 11. There was no gel formed at pH 4 and 5 because of an insufficient electrical charge on the protein surface and protein solubility [51]. The LGC in all samples decreased by increasing the pH. Given that the formation of a gel by proteins is a complex process that involves various reactions such as aggregation, network formation, or denaturation [25], the SPI samples treated at 180 ºC had the highest ability to form a gel as compared to other treatments. The gel is formed through intermolecular reactions that display a 3D network structure. Chemical, physical, and enzymatic processes can intensify the gelation of proteins. Although the salt concentration does not affect the LGC of samples in this study, the gel formation characteristics of proteins largely depend on hydrogen and covalent bonding and interaction [25]. The interactions between disulfide bridges and hydrophobic bonds during the drying process caused protein denaturation and gel formation [52].
Conclusion
Current work reports the thorough functional properties of sesame seed isolate that was obtained using different processing conditions. The salt concentration and drying temperature of sesame at different pH levels had a significant effect on the functional and structural attributes of sesame meal protein isolate. The highest drying temperature )180 °C) increased the solubility, EA and ES, FC and FS, and decreased OHC and WHC. Meanwhile, the highest salt concentration )22%) decreased the solubility, OHC, and WHC, and increased FC and FS, EA, and ES. Suitable functional attributes were also observed at pH 9 and 11. Considering the good functional attributes of SPI, which is dried at 180 °C and 18% of salt concentration, this protein is suggested as an essential functional ingredient for general protein-based food to improve some functional attributes of new food products.
References
R. Esmaeilzadeh Kenari, R. Razavi, Phenolic profile and antioxidant activity of free/bound phenolic compounds of sesame and properties of encapsulated nanoparticles in different wall materials (Food Science & Nutrition, 2022)
W. Liu, G. Cheng, H. Liu, Y. Kong, Purification and identification of a novel angiotensin I-converting enzyme inhibitory peptide from sesame meal. International Journal of Peptide Research and Therapeutics 21(4),433 – 42 (2015)
V. Rezaeipour, A. Barsalani, R. Abdullahpour, Effects of phytase supplementation on growth performance, jejunum morphology, liver health, and serum metabolites of Japanese quails fed sesame (Sesamum indicum) meal-based diets containing graded levels of protein. Trop. Anim. Health Prod. 48(6), 1141–1146 (2016)
K. Yamauchi, M. Samanya, K. Seki, N. Ijiri, N. Thongwittaya, Influence of dietary sesame meal level on histological alterations of the intestinal mucosa and growth performance of chickens. Journal of applied poultry research 15(2),266 – 73 (2006)
A. Achouri, V. Nail, J.I. Boye, Sesame protein isolate: Fractionation, secondary structure and functional properties. Food Res. Int. 46(1), 360–369 (2012)
M. Kumar, M. Tomar, J. Potkule, R. Verma, S. Punia, A. Mahapatra, T. Belwal, A. Dahuja, S. Joshi, M. K. Berwal, Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 115, 106595 (2021)
O. Yuliarti, T.J.K. Kovis, N.J. Yi, Structuring the meat analogue by using plant-based derived composites. J. Food Eng. 288, 110138 (2021)
A.G.A. Sá, Y.M.F. Moreno, B.A.M. Carciofi, Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 60(20), 3367–3386 (2020)
S. Ekramian, H. Abbaspour, B. Roudi, L. Amjad, A. Mohammadi, Nafchi, An experimental study on characteristics of sago starch film treated with methanol extract from Artemisia sieberi Besser. J. Food Meas. Charact. 15(4), ,3298–3306 (2021). https://doi.org/10.1007/s11694-021-00895-w
E. Parsaei, A. Mohammadi Nafchi, L. Nouri, A.A. Al-Hassan, The effects of tannic and caffeic acid as cross-linking agents on the physicochemical, barrier, and mechanical characteristics of cold-water fish gelatin films. J. Food Meas. Charact. (2022). https://doi.org/10.1007/s11694-022-01495-y
F.H. Brishti, S.Y. Chay, K. Muhammad, M.R. Ismail-Fitry, M. Zarei, S. Karthikeyan, N. Saari, Effects of drying techniques on the physicochemical, functional, thermal, structural and rheological properties of mung bean (Vigna radiata) protein isolate powder. Food Res. Int. 138, 109783 (2020)
W. Jia, E. Rodriguez-Alonso, M. Bianeis, J.K. Keppler, A.J. van der Goot, Assessing functional properties of rapeseed protein concentrate versus isolate for food applications. Innovative Food Science & Emerging Technologies 68, 102636 (2021)
C. Terrien, 3-Available protein substitutes. Meat Analogs,65–124 (2017)
S. Vinayashree, P. Vasu, Biochemical, nutritional and functional properties of protein isolate and fractions from pumpkin (Cucurbita moschata var. Kashi Harit) seeds. Food Chem. 340, 128177 (2021)
P. Chakraborty, D.K. Bhattacharyya, M. Ghosh, Extrusion treated meal concentrates of Brassica juncea as functionally improved ingredient in protein and fiber rich breadstick preparation. LWT 142, 111039 (2021)
P. Fantozzi, Grape seed: A potential source of protein. J. Am. Oil Chem. Soc. 58(12), 1027–1031 (1981)
B.S. Kamel, H. Dawson, Y. Kakuda, Characteristics and composition of melon and grape seed oils and cakes. J. Am. Oil Chem. Soc. 62(5), 881–883 (1985)
L. Sharma, C. Singh, H.K. Sharma, Assessment of functionality of sesame meal and sesame protein isolate from Indian cultivar. J. Food Meas. Charact. 10(3), 520–526 (2016)
B. Beljkaš, J. Matić, I. Milovanović, P. Jovanov, A. Mišan, L. Šarić, Rapid method for determination of protein content in cereals and oilseeds: validation, measurement uncertainty and comparison with the Kjeldahl method. Accred. Qual. Assur. 15(10), 555–561 (2010)
M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry 72(1–2),248 – 54 (1976)
S. Elsohaimy, T. Refaay, M. Zaytoun, Physicochemical and functional properties of quinoa protein isolate. Annals of Agricultural Sciences 60(2), 297–305 (2015)
I. Fathollahy, J. Farmani, M.R. Kasaai, H. Hamishehkar, Characteristics and functional properties of Persian lime (Citrus latifolia) seed protein isolate and enzymatic hydrolysates. LWT 140, 110765 (2021)
U.K. Laemmli, Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 227(5259), 680–685 (1970). https://doi.org/10.1038/227680a0
A. Achouri, J.I. Boye, Thermal processing, salt and high pressure treatment effects on molecular structure and antigenicity of sesame protein isolate. Food Research International 53(1),240 – 51 (2013)
A.P. Adebiyi, R.E. Aluko, Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem. 128(4), 902–908 (2011)
D.R. Brewer, J.M. Franco, L.A. GARCIA-ZAPATEIRO, Rheological properties of oil-in-water emulsions prepared with oil and protein isolates from sesame (Sesamum Indicum). Food Science and Technology 36,64 – 9 (2016)
A. Rangel, G.B. Domont, C. Pedrosa, S.T. Ferreira, Functional properties of purified vicilins from cowpea (Vigna unguiculata) and pea (Pisum sativum) and cowpea protein isolate. J. Agric. Food Chem. 51(19), 5792–5797 (2003)
E.S.M. Abdel-Aal, A.A. Shehata, A.R. El‐Mahdy, M.M. Youssef, Extractability and functional properties of some legume proteins isolated by three different methods. J. Sci. Food. Agric. 37(6), 553–559 (1986)
N.A. El Nasri, A. El, Tinay, Functional properties of fenugreek (Trigonella foenum graecum) protein concentrate. Food Chem. 103(2), 582–589 (2007)
O.S. Lawal, Functionality of African locust bean (Parkia biglobossa) protein isolate: effects of pH, ionic strength and various protein concentrations. Food Chem. 86(3), ,345–355 (2004)
H. Adubiaro, O. Olaofe, E. Akintayo, Effects of salts on the functional properties of Albizzia lebbeck seed flour. Electronic Journal of Environmental, Agricultural & Food Chemistry 8(8) (2009)
H.N. Ogungbenle, Effects of salt concentrations on the functional properties of some legume flours. Pakistan J. Nutr. 7(3), 453–458 (2008)
U. Inyang, C. Nwadimkpa, Functional properties of dehulled sesame (Sesamum indicum L.) seed flour. J. Am. Oil Chem. Soc. 69(8), ,819–822 (1992)
J. Kinsella, Relationships Between Structure and Functional. Food proteins,51 (1982)
U. Chavan, D. McKenzie, F. Shahidi, Functional properties of protein isolates from beach pea (Lathyrus maritimus L.). Food Chem. 74(2), 177–187 (2001)
E. Khalid, E. Babiker, A.E. Tinay, Solubility and functional properties of sesame seed proteins as influenced by pH and/or salt concentration. Food Chem. 82(3), 361–366 (2003)
K. Adebowale, O. Lawal, Comparative study of the functional properties of bambarra groundnut (Voandzeia subterranean), jack bean (Canavalia ensiformis) and mucuna bean (Mucuna pruriens) flours. Food Research International 37(4),355 – 65 (2004)
S. Mostofi, Oiseed market and its products (Institute for planning Research and Agricultural Economics, 2008)
S. Benelhadj, A. Gharsallaoui, P. Degraeve, H. Attia, D. Ghorbel, Effect of pH on the functional properties of Arthrospira (Spirulina) platensis protein isolate. Food Chem. 194, 1056–1063 (2016)
N. Mazloomi, M.A. Sadeghi, M. Ghorbani, M. Aalami, M. Kashaninjad, Evaluation of the effect of salt, temperature and pH on the funtional propes of defatted pumpkin seed flour and its protein isolate. (2017)
D.M. Ragab, E.E. Babiker, A.H. Eltinay, Fractionation, solubility and functional properties of cowpea (Vigna unguiculata) proteins as affected by pH and/or salt concentration. Food chemistry 84(2),207 – 12 (2004)
A. Fidantsi, G. Doxastakis, Emulsifying and foaming properties of amaranth seed protein isolates. Colloids and surfaces B: Biointerfaces 21(1–3),119 – 24 (2001)
C.-H. Tang, Z. Ten, X.-S. Wang, X.-Q. Yang, Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J. Agric. Food Chem. 54(23), 8945–8950 (2006)
Y. Deng, L. Huang, C. Zhang, P. Xie, J. Cheng, X. Wang, S. Li, Physicochemical and functional properties of Chinese quince seed protein isolate. Food Chem. 283, 539–548 (2019)
J. Yu, M. Ahmedna, I. Goktepe, Peanut protein concentrate: Production and functional properties as affected by processing. Food Chem. 103(1), 121–129 (2007)
E. Dickinson, G. Stainsby, Advances in food emulsions and foams. sole distributor in the USA and Canada (Elsevier Science Publishers, 1988)
R. Khattab, S. Arntfield, Functional properties of raw and processed canola meal. LWT-Food Sci. Technol. 42(6), 1119–1124 (2009)
Q.-T. Zhang, Z.-C. Tu, H. Xiao, H. Wang, X.-Q. Huang, G.-X. Liu, C.-M. Liu, Y. Shi, L.-L. Fan, D.-R. Lin, Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food Bioprod. Process. 92(1), 30–37 (2014)
S. Singharaj, E. Onsaard, Production and characteristic of sesame proteins. Journal of Food Science and Agricultural Technology (JFAT) 1,188 – 92 (2015)
M. Ma, Y. Ren, W. Xie, D. Zhou, S. Tang, M. Kuang, Y. Wang, S.-k. Du, Physicochemical and functional properties of protein isolate obtained from cottonseed meal. Food Chem. 240, 856–862 (2018)
S. Jovanović, M. Barać, O. Maćej, Whey proteins-properties and possibility of application. Mljekarstvo 55(3), 215–233 (2005)
A. Maghazechi, A.M. Nafchi, T.-C. Tan, E.-K. Seow, A.M. Easa, Rheological characterization of coconut cream emulsion using steady-state shear and time-dependent modeling. J. Food Eng. 306,110642 (2021) https://doi.org/10.1016/j.jfoodeng.2021.110642
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rezaei, M., Nouri, L., Daneshi, M. et al. Effect of salt concentration and drying temperature on functional properties of sesame (Sesamum indicum L.) meal protein isolate. Food Measure 16, 4665–4674 (2022). https://doi.org/10.1007/s11694-022-01561-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01561-5