Abstract
Active packaging which deliberately incorporated with releasing or absorbing components into or from packaged foods, was developed in order to extend the shelf life of products by stalling the degradative reactions The most significant factor affecting the shelf life of olive oil is oxidation. In this study a new oxygen scavenger called cobalt (II) oxalate nanoparticles powder was in the incorporated in glass and PET bottle screw-caps. It improves the shelf life of retailed olive oil packaging during storage. The effect of oxygen scavenger system on olive oil acidity, peroxide value and spectrophotometric indices (K232, K270) was evaluated at 6 intervals (monthly) at 25 ° C in light conditions. Chemical and physical properties of cobalt (II) oxalate nanoparticles adsorbent were analyzed by Scanning Electron Microscope and X-ray Diffraction analyses.
The results showed that 1 g of cobalt (II) oxalate nanoparticles powder decreased packaged olive oil peroxide value and acidity by about 30%. Olive oil packaged in glass containers (sealed with cobalt (II) oxalate nanoparticles), significantly, showed less peroxide value and acidity by 95% more likely than in PET containers. Physical properties of synthesized cobalt (II) oxalate nanoparticles approved the pure β-cobalt oxalate formation.
This study showed that cobalt (II) oxalate nanoparticles have potential to be used in the food packaging industry as a new oxygen scavenger.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many food products are damaged by oxygen. Oxygen scavengers effectively prevent oxidative damage in a wide range of food constituents, such as oils and fats. Damage might be in the form of discoloration, change in texture, loss of flavor, or the generation of off-flavors. Also, there is some evidence that the reduction of oxygen in a package can reduce spoilage caused by organisms [1]. Metallic scavengers are the largest section of the market and have expanded economically [1].
In 1970, the first commercial oxygen scavenger was produced and marketed by Mitsubishi Company in Japan. The oxygen in the package or the one entering it oxidizes the iron in the presence of moisture adsorbed by the food. These adsorbents reduce the oxygen concentration inside the package to 0.1% of the initial concentration [2]. The commercially available oxygen scavengers are in form of small sachets containing metallic reducing agents, such as powder iron oxide, ferrous carbonate and metallic platinum. The majority of these scavengers are based on the principle of iron oxidation in water presence [2]. Lee et al. [3] investigated the effect of light on the structure of titanium dioxide as an oxygen scavenger in nanocomposite films. They found that titanium dioxide formed electron-holes in the presence of ultraviolet light. Mills et al. [4] investigated the photocatalyst properties of oxygen-inhibiting flexible polymer films. They used titania nanoparticles in a film with an ethyl cellulose polymer substrate with tri-ethanolamine as an electron reducing agent and also without using it, in a glass container coated with octadyl porphyrin platinum. They found that this method is more desirable than the traditional oxygen containment methods. Furthermore, it has good potential for use in the packaging industry [4]. Lee et al. [3] investigated the reduction of oxygen content using sodium ascorbate and modified iron in polyethylene matrix films [5]. In addition, in another study conducted by Shin et al. [6], they investigated two types of polyethylene films containing ascorbic acid and sodium ascorbate as oxygen adsorbents with modified iron. In these studies, several types of compounds are commonly used to produce oxygen-absorbing films [6].
Durec et al. [7] investigated the effect of screw-cap with oxygen scavenger bound inside the bore seal of the closure of polyethylene terephthalate (PET) bottles on the quality of pineapple juices. For the beer market, BERICAP (Budenheim, Germany) has developed a crown design to differentiate from the context sensitive design (CSD) version resembling the crown cork used in the past. The Super Shorty® is equipped with an oxygen scavenger for the beer market. Removal of oxygen from a bottle by a closure requires that a component reacts with gaseous oxygen in the headspace of the bottle. Darex oxygen scavenging technology utilizes a material that can be incorporated into barrier packaging such as crowns, cans and a broad variety of plastic and metal closures. The basic reaction of their oxygen scavenging technique is ascorbate oxidizing to dehydroascorbic acid and sulphite to sulphate. Darex Dar Extend is designed to be incorporated as an integral part of traditional barrier packaging such as the aluminium roll-on closures as well as in plastic closures and crowns. Darex oxygen scavenging technology utilizes a material that can be incorporated into the barrier packaging, such as crowns, cans and a broad variety of plastic and metal closures. The basic reaction of their oxygen scavenging technique is ascorbate oxidizing to dehydroascorbic acid and sulphite to sulphate. Darex Dar Extend is designed to be incorporated as an integral part of traditional barrier packaging, such as the aluminium roll-on closures as well as in the plastic closures and crowns. Darex Dar Eval is an EVOH-based oxygen scavenging barrier resin used as an inner layer in multi-layer PET [8]. Dar Eval enables multi-layer PET bottles to have a glass-like performance [8, 9].
Cobalt (II) oxalate is an inorganic compound with the formula CoC2O4. The cobalt metal is silver white and easily polished. It dissolves in dilute acids and oxidizes slowly in the air. Highly absorbs oxygen on the surface [10]. Cobalt (II) oxalate has been applied as a electrocatalyst for oxygen evolution reaction [9, 11], as a precursor for cobalt oxide preparation [12, 10], as a catalyst [13, 11], and as a negative-electrode material for lithium-ion batteries and high-capacity lithium storage materials [12, 14].
The aim of this study was to investigate the PET and glass bottles screw-cap containing oxygen scavenger to absorb the oxygen in the headspace of the bottle for improving the extra virgin olive oil stability and shelf-life.
Materials and methods
Materials
Cobalt (II) nitrate hexahydrate, ammonium oxalate, ethanol, phenolphtalein acetic acid-chloroform and thiosulfate was purchased from Merck (Germany). Olive oil donated by Danzeh Co. (Manjil, Iran). 100 mL glass and PET bottle were commercially prepared.
Cobalt (II) oxalate nanoparticles synthesis
To prepare cobalt (II) oxalate nanoparticles, an aqueous solution of ammonium oxalate (10 mmol) was slowly added to an aqueous solution of cobalt (II) nitrate hexahydrate. The resulted mixture was agitated at 60 °C for 2 h. The formed precipitate was filtered, washed with distilled water and dried at 80 °C.
Sample preparation
Olive oil was filled aseptically into the PET and glass bottles (100 mL volume) and split into 4 sub-groups which contain 100, 75, 50 and 25 mL extra virgin olive oil (0.0, 25, 50 and 75% headspace, respectively). Full filled containers (100 mL olive oil) screw-capped without scavenger were controlled. All packaged olive oil (glass and PET) screwed with caps containing 1 g cobalt (II) oxalate nanoparticles. The cobalt (II) oxalate nanoparticle powder was placed in the screw-cap in such a way that it did not come into contact with the oil by an air permeable fabric (cotton) septum.
Packaged olive oil samples were stored at the room temperature (25 ± 1 °C) for 6 months to cover the expiration period set by the producer. The samples were analyzed monthly.
Characterization of nanoparticles of cobalt (II) oxalate
X-ray diffraction (XRD) analysis was carried out by Gomes et al. [12, 14]. Field-emission scanning electron microscopy (FE-SEM) and energy dispersive X-ray spectroscopy (EDX) were performed using Zeiss Sigma VP SEM equipped with Oxford EDX/WDX detectors.
Measurement of olive oil acidity
Olive oil acidity determined according to the method of Hidayah et al.[13, 15]. In summary, the acidity is carried out by titrating hydroxide (KOH) against the sample using the principle amount of potassium hydroxide (KOH) needed (mg) to neutralize 1 gram of fat to the following Eq. 1.
Acid Value = \(\frac{V\times N\times K}{10G}\) (1)
Where V: KOH titration volume (mL) N: KOH normality (meq/L) K: KOH molecular weight (56.1) G: sample weight (g).
Measurement of olive oil peroxide value
Peroxide value was determined according to Hidayah et al. [13, 15]. In briefly, peroxide value determined using the principle of titration of iodine released from potassium iodide compounds by peroxide using a standard solution of thiosulfate (0,1 N) as titrant and starch solution as indicator. The peroxide value is calculated from the Eq. 2.
Peroxide value = \(\frac{S\times M\times 1000}{\text{S}\text{a}\text{m}\text{p}\text{l}\text{e} \text{W}\text{e}\text{i}\text{g}\text{h}\text{t} \left(g\right)}\) (2)
where S is the amount of sodium thiosulfate in mL, and M is sodium thiosulfate concentration (0.01 N).
Spectrophotometric indices
Κ232 and Κ270 show UV absorption in 232 and 270 nm and can give us information about quality and oxidative alteration. More specifically, in 232 nm primary oxidation products show absorption (conjugated peroxides) and in 270 nm secondary oxidation products show absorption (aldehydes and ketones). K232 is considered a critical marker for good quality extra virgin olive oil. Oxidation can be the result of natural aging or indicative of poor handling or heating during the refining process. K270 increases when extra virgin olive oil is not fresh meaning that our product consists of extra virgin olive oil of a previous harvesting or a blend of extra virgin olive oil of previous harvesting along with the fresh one.
The extinction coefficients, K270 and K232, were calculated by absorption at 270 and 232 nm respectively, using a UV spectrophotometer (Hewlett-Packard, HP 8452 A) were determined following the analytical methods described in Regulation (EEC) no°/2568/91 of the Commission of the European Union [14, 16].
Statistical analysis
Results were expressed as the mean and standard deviation of three independent replicates. All the data were statistically analyzed using one-way analysis of variance (ANOVA) through Duncan post hoc (p < 0.05). All of the statistical analyses were performed using SAS software version 9.3 (SAS Institute Inc.).
Results and Discussion
Characterization of nanoparticles of cobalt (II) oxalate
X-ray diffraction (XRD), field-emission scanning electron microscopy (FE-SEM) and energy dispersive X-ray spectroscopy (EDX) analysis techniques were utilized to characterize the synthesized cobalt oxalate. The X-ray diffraction (XRD) pattern of the synthesized sample is exhibited in Fig. 1. Generally, cobalt (II) oxalate dihydrate displays two allotropic forms of α-monoclinic (Spacegroup C2/c), and β-orthorhombic (Spacegroup Cccm)[14, 17]. Depending on the precipitation conditions, the crystallization can cause various allotropes. The stable (α) and metastable (β) forms with varied crystal lattices are distinguished from each other by the position of the cobalt-oxalate chains [15, 18]. The XRD pattern of the sample contains the diffraction peaks at 2θ of 18.8, 22.8, 30.0, 35.0, 37.9, 40.6, 43.3 and 47.4° corresponding to the crystal planes (202), (004), (400), (022), (206), (315), (224), and (602), respectively. These reflections can be attributed to the orthorhombic β-cobalt oxalate with lattice constants a = 11.855 Å: b = 5.412 Å: c = 15.578 Å in agreement with the previous reported results. [14, 16, 17].
The FE-SEM micrographs for the synthesized sample are presented in Fig. 2. Asshown in Fig. 2, the sample exhibits a rod-like morphology which is a characteristic of β-cobalt oxalate [14, 17]. A narrow particle size distribution is observable from FE-SEM images for the sample. Energy-dispersive X-ray spectroscopy (EDX) analysis was applied to determine the sample composition. The EDX results for the synthesized sample (Fig. 3) specify that the Co:O molar ratio is 3.96. This ratio is close to the theoretical value, indicating the formation of pure β-cobalt oxalate.
Olive oil acidity
Table 1 shows the acidity changes in PET and glass containers in the different volumes over a period of 6 months storage times. According to Table 1, the acidity during 6 months in containers (PET and glass) containing cobalt (II) oxalate nanoparticles and control sample (sample without headspace) does not show a significant difference (p > 0.05). The level of free acidity changed significantly during the 6 months of storage in all oils ranging from 0.3 to 0.8%, and the official limits for this parameter 0.8% for extra virgin olive oil (EVOO) – European Economic Community Regulation 1989/2003;18 have not been exceeded. The results revealed that incorporating the cobalt (II) oxalate nanoparticles in olive oil packaging could prevent the increased acidity during consumption simulation storage. However, the amount of acidity over time in the different volumes showed a significant difference and the least change in acidity was in the volume of 50 mL. The results of this study are similar to the results of Sacchi et al. [18, 19] in which the amount of acidity in the glass, polyethylene terephthalate (PET), PET including 1% of oxygen-scavengers (PET 1%), and PET including 5% of oxygen-scavengers (PET 5%) container were not significantly different.
Values quoted are mean values ± standard deviations of results for the three experiments. In the same row, different superscript letters (a, b, c, d) mean that values are significantly different (P ≤ 0.05), and different uppercase letters in each row indicate a significant difference in PET and glass containers.
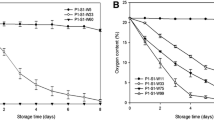
Olive oil peroxide value
Table 2. shows the changes in the peroxide value in the olive oil during 6 months of storage. In the 3 treated volumes of package, a decrease in the peroxide value was observed as headspace decreased. The changes in peroxide value in the glass and PET bottles were different Also, in all 3 volumes studied, glass bottles had lower peroxide values than PET bottles as lipid oxidation progresses during storage and over time, the amount of oxygen in the main space continuously increases. Nanoparticles of cobalt (II) oxalate reduce the peroxide value by absorbing this oxygen. In fact, nanoparticles of cobalt (II) oxalate reduced peroxides formation and increased the shelf life of olive oil by absorbing oxygen in the headspace of the container. Andersson & Lingnert [18, 20] showed that lowering the headspace oxygen level down to 0.03% above powdered cream greatly reduced peroxide value. Hidayah et al. [13, 15] studied the effect of iron powder as an oxygen scavenger on fish oil oxidation. Their findings showed that the number of oil peroxide decreased compared to the control sample but did not have a linear trend and that is similar to the present study. The problem of oxygen permeability of plastic bottles compared to the glass bottles is recognized by the industry. In the study of Michiels et al. [19, 21], the oxygen permeability of PET containers was estimated to be 1–5 (cm3 mm m− 2 day− 1 atm− 1). In this study, oxygen permeability in the glass containers was lower than PET containers and also. olive oil peroxide value in the glass containers was lower. The best oxygen scavengering in terms of reducing the peroxide value was determined in the glass containers containing 50 mL olive oil. It was found that using nanoparticles of cobalt (II) oxalate in the glass containers can obtain less peroxide and increase the shelf life of olive oil.
Values quoted are mean values ± standard deviations of results for three experiments. In the same row, different superscript letters (a, b, c,d) mean that values are significantly different (P ≤ 0.05), and also, different uppercase letters in each row indicate a significant difference in PET and glass containers.
Spectrophotometric indices
Κ232 & Κ270 show UV absorption in 232 and 270 nm and can give us information about quality and oxidative alteration. More specifically, in 232 nm primary oxidation products show absorption (conjugated peroxides) and in 270 nm secondary oxidation products show absorption (aldehydes and ketones) [20]. Table 3 shows spectrophotometric indices after 6 months storage in PET and glass containers. The amount of K232, which indicates the initial oxidation in all samples up to the first 4 months has a decreasing trend and the amount of this coefficient in all months in the control sample was higher than the samples containing cobalt (II) oxalate. Among the samples containing cobalt (II) oxalate, a glass container with a volume of 50 mL had the lowest coefficient Κ232. In all storage months, K232 coefficient was significantly different in glass and PET containers (p < 0.05) and K232 coefficient in glass containers was less than PET containers. Abbadi et al. [21, 23] examined the amount of K232 in the glass, polyethylene terephthalate (PET), high density polyethylene (HDPE), cans containers in olive oil. They found that the lowest coefficient K232 belonged to a glass container. Table 3 shows the initial values of the K270 indices and their final values after 6 months of storage. Indices K270 increased during the storage period (p < 0.05). The amount of K270, which indicates the secondary oxidation in all samples up to the first 4 months has a decreasing trend and the amount of this coefficient in all months in the control sample was higher than the samples containing cobalt (II) oxalate. Among the samples containing cobalt (II) oxalate, a glass container with a volume of 50 mL had the lowest coefficient Κ270. In all storage months, K270 coefficient was significantly different in the glass and PET containers (p < 0.05) and K232 coefficient in glass containers was less than PET containers. Alvarruiz et al. [20, 22] said K270 depended on some important factors including the oil storage time, material of oil storage container and environmental conditions, such as temperature and light. Temperature and the presence of oxygen are both significant indicators for K270 changes.
Values quoted are mean values ± standard deviations of the results for three experiments. In the same row, different superscript letters (a, b, c, d) mean that values are significantly different (P ≤ 0.05) and also, different uppercase letters in each row indicate a significant difference in the PET and glass containers.
Conclusions
Concerns of olive oil producer, distributer and consumers about oil rancidity and shelf life have become an important issue in the food and related industries. In order to maintain quality and increase shelf life, which has become a problem for consumers at the home, the use of suitable package such as oxygen scavengers could have helped the industry to improve oil stability during consumption.
In this study, we used for the first time an oxygen adsorbent called cobalt (II) oxalate nanoparticles powder (filled in the plastic screw cap) in the two types of conventional packaging of olive oil, PET and glass. The utilization of screw-caps with bound oxygen scavenger in the sample with a volume of 50 mL resulted in approximately, 30% lower K232 and K270 compared to the control sample. It was shown that the use of cobalt (II) oxalate nanoparticles adsorbent, a non-toxic compound in olive oil, can have a positive and significant effect on reducing the acidity and peroxide of the oil. This screw-cap filled with oxygen scavenger showed a significant decrease in the peroxide values compared to PET bottles.
This new oxygen scavenger can be introduced to the food industry and with the continuation of the process of further analysis and more investigation on shelf-life parameters, more positive and effective results can be obtained in this industry.
References
F.N. Teumac. The history of oxygen scavenger bottle closures. Active food packaging(M.L. Rooney edt.). Springer, Boston, MA, pp. 193–202 (1995). https://doi.org/10.1007/978-1-4615-2175-4_8
C.E. REALINI, MARCOS, Begonya. Active and intelligent packaging systems for a modern society. Meat Sci. 98.3, 404–419 (2014). https://doi.org/10.1016/j.meatsci.2014.06.031
L. Jung-Soo et al., Ascorbic acid‐based oxygen scavenger in active food packaging system for raw meatloaf. J. Food Sci. 83.3, 682–688 (2018). https://doi.org/10.1111/1750-3841.14061
A. Mills et al., Demonstration of a novel, flexible, photocatalytic oxygen-scavenging polymer film. J. Photochem. Photobiol., A 177(2-3), 328–331 (2006). https://doi.org/10.1016/j.jphotochem.2005.06.001
L. Meng. Combined effects of sunlight and titanium dioxide nanoparticles on dietary antioxidants and food colors. (2014). Ph.D. Thesis. https://doi.org/10.13016/M29C88
Y. SHIN, J. SHIN; LEE, Youn Suk. Preparation and characterization of multilayer film incorporating oxygen scavenger. Macromolecular Research, 19.9: 869 (2011). https://doi.org/10.1007/s13233-011-0912-y
J. Durec, et al., Effect of oxygen scavenger screwcaps on quality of pineapple juices.https://doi.org/10.1007/s11696-020-01226-x
Darex Technical Information. Darex Active packaging technology brochure. (2002)
A. Dey, N. Sudarsan, Oxygen scavengers for food packaging applications: A review. Trends Food Sci. Technol. 90, 26–34 (2019). https://doi.org/10.1016/j.tifs.2019.05.013
J. Xiaomin Liu, Jiang, L. Ai, Non-precious cobalt oxalate microstructures as highly efficient electrocatalysts for, oxygen evolution reaction. J. Mater. Chem. A 3, 9707–9713 (2015)
W.-W. Wang, Y.-J. Zhu, Microwave-assisted synthesis of cobalt oxalate nanorods and their thermal conversion to Co3O4 rods. Mater. Res. Bull. 40, 1929–1935 (2005)
R. Sadanandam, M.F. Fonseca, K. Srikant, A.K. Sharma, S.K. Tangri, A.K.Suri, Production of high purity cobalt oxalate from spent ammonia cracker catalyst. Hydrometallurgy 91, 28–34 (2008)
L. Chen, X. Tang, Y. Zhang, L. Li, Z. Zeng, Y.Z. Chen, X. Tang, Y. Zhang, L. Li, Z. Zeng, Y. Zhang, Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 108, 80–86 (2011)
B. Gomes et al., Zinc nanostructures for oxygen scavenging. Nanoscale 9, 16: 5254–5262 (2017). https://doi.org/10.1039/C7NR01367A
E.N. Hidayah, R.R. Triastuti, A.A. Abdillah. The effect of iron powder as oxygen absorber active packaging on fish oil total oxidation value. E&ES, 441.1: 012009.) 2020( https://doi.org/10.1088/1755-1315/441/1/012009
E.C.C. Regulation “2568/91 on the characteristics of olive oil and olive-pomace oils and their analytical methods.“ Official Journal of the European Communities: Legislation, European Union, Brussels (1991)
R. Amutha et al., Template-free synthesis of self-assembled Co3O4 micro/nanocrystals. J. Nanosci. Nanotechnol. 11.4, 3171–3179 (2011). https://doi.org/10.1166/jnn.2011.3848
V. Baco-Carles et al., Correlation between the morphology of cobalt oxalate precursors and the microstructure of metal cobalt powders and compacts. Powder Technol. 185.3, 231–238 (2008). https://doi.org/10.1016/j.powtec.2007.10.016
R. Sacchi, M. Savarese, A. Del Regno, A. Paduano, R. Terminiello, M.L. Ambrosino, “Shelf life of vegetable oils bottled in different scavenging polyethylene terephthalate (PET) containers. Packaging Technol. Science: Int. J. 21(5), 269–277 (2008)
K. Andersson, L., Hans, Influence of oxygen concentration and light on the oxidative stability of cream powder. LWT-Food Sci. Technol. 31(2), 169–176 (1998). https://doi.org/10.1006/fstl.1997.0330
Y. Michiels, P. Van Puyvelde, and Bert Sels. “Barriers and chemistry in a bottle: mechanisms in today’s oxygen barriers for tomorrow’s materials.“ Applied Sciences 7, no. 7 665. (2017) https://doi.org/10.3390/app7070665
A. Alvarruiz, José Emilio Pardo, Maria Elena Copete, Concepción de Miguel, Adrián Rabadán, Eulogio López, and Manuel Álvarez-Ortí. “Evolution of virgin olive oil during long-term storage. J. Oleo Sci. 69(8), 809–814 (2020)
J. Abbadi, Z. Ayyad, F. Al-Rimawi, and Wadie Sultan. Evaluation of the effect of packaging materials and storage temperatures on quality degradation of extra virgin olive oil from olives grown in Palestine. American Journal of Food Science and Technology 2(5), 162–174 (2014)https://doi.org/10.12691/ajfst-2-5-5
Acknowledgements
Thanks to Danzeh Co. and Dr. Alizadeh for providing olive oil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
z,1 Corresponding author: javanmard@irost.ir.
Rights and permissions
About this article
Cite this article
Pourshahbazi, H., Javanmard dakheli, M., Salehirad, A. et al. Novel oxygen scavenger screw-cap for shelf-life improvement in virgin olive oil packaging during storage. Food Measure 16, 2831–2837 (2022). https://doi.org/10.1007/s11694-022-01358-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01358-6