Abstract
The present study was performed to investigate the antioxidant and antimicrobial effects of hydro alcoholic nettle extract (NE) with sodium alginate (SA) and galbanum gum (GG) composite coating on the quality of rainbow trout fillet (Oncorhynchus mykiss) during 12-day refrigeration storage (4 ± 1 °C). Experiments were performed on 10 treatments (control, SA, GG separately and in combination with NE at concentrations of 0, 0.5 and 1%) in three replications. Results of the DPPH assay showed that the IC50 values for NE were 35.12 ± 0.95 μg/ mL. It also had an antimicrobial activity; MIC and MBC values for different bacteria were 12.00–25.75 μg/mL and 20.75–32.25 μg/ mL, respectively. The results about the fillet quality showed that the samples containing the coating and NE were able to decelerate the increase of chemical and microbial spoilage (p < 0.05). At the end of the storage period, the fillets treated with a composite coating (+ 1% NE) displayed the lowest amount of lipid oxidation and microbial deterioration. The sensory evaluation showed that the treatment maintained the fillet quality until the end of the storage. Overall, SA–GG composite coating incorporated with NE can delay the process of lipid oxidation and microbial spoilage, and improve the sensory properties of rainbow trout fillets kept in the refrigerator.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish is a valuable source of protein and contains large amounts of unsaturated fatty acids ω-3 (PUFAs), which plays a vital role in a healthy diet [1]. Rainbow trout (Oncorhynchus mykiss), a major member of the Salmonidae family as a cold-water fish, is a significant part of the diet in Iran and the world due to its aquaculture suitability, nutritional quality and delicious taste [2]. Consumption of rainbow trout faces some protective issues such as non-enzymatic lipid oxidation, microbial growth and high levels of unsaturated fatty acids, biogenic amine (BA) autolysis enzymes and a shorter shelf life than other meat products. Subsequently, they are considered very vulnerable foods [3, 4]. Maintaining the freshness and quality of fresh products is the main challenge in the food industry. Chemical preservatives are among the most universal and reliable ways to maintaining the quality of seafood and increasing its shelf life during storage [5]. Nowadays, the use of these chemicals is limited due to their destructive effects on DNA and their toxicity. Using plant extracts as natural additives in foods is a safe and acceptable way for protecting them against oxidation and preventing the growth and proliferation of microorganisms [4, 6]. Extracts of plants and their constituents are natural compounds that have been known for their antibacterial, antifungal, antiviral and antioxidant properties. Their numerous applications in controlling the growth of spoilage and foodborne pathogenic bacteria have led to their use as food preservatives [7, 8].
Urtica dioica L., commonly known as nettle, is a wild annual plant of the Urticaceae family. This plant is widely known for its extraordinary biological activity and has beneficial effects on human health. Various studies have confirmed the antioxidant, antimicrobial, anti-inflammatory, antibacterial and analgesic properties of nettle extract (NE) [9,10,11,12].
Although the use of plant extracts and essential oils in products is limited due to cost and other disadvantages such as severe perfume and, in some cases, potential toxicity, there is an approach to overcome these disadvantages including the combination of plant extracts in the formulation of edible coatings, which reduces their dose with the same protective effects [13, 14]. As the result of this method, tendency to using edible coatings enriched with essential oils and extracts with natural origins has increased [15]. Edible films and coatings are referred to the materials that can be digested by the human body or are biodegradable in the environment [16,17,18]. The most important advantage of these compounds is their ability to be consumed with food compounds [19, 20]. Among the food coatings used in foodstuffs, alginate has valuable colloidal properties that result in strong gels or low-solubility polymers that are capable of reacting with polyvalent metal ions, especially calcium ions [21, 22]. Sodium alginate (SA) is a nontoxic, biodegradable, biocompatible and cheap hydrocolloid [23]. Edible alginate and SA coating and films, separately or with various antioxidant additives, have been used by other researchers to preserve fish fillets [21, 23, 24]. Combining them with other polymers or gums can be a good way to improving the properties of SAs. Galbanum is an aromatic gum that is produced from traditional Iranian species with the common Iranian names “Barijeh” (Ferula gummosa, Ferula persica and Ferula tabasensis) and is widely found in the mountain slopes of northern Iran. The product of galbanum plant has a strong, fragrant smell and an astringent and bitter taste. Galbanum gum (GG) is traditionally used as an antiseptic, anti-epileptic, anticonvulsant, antispasmodic, analgesic, inflammation and memory-boosting tonic among Iranians and Egyptians [25, 26]. The antioxidant and antimicrobial properties of barium gum have been reported by other researchers [25, 27]. Since a dearth of research has been carried out on the antioxidant and antimicrobial activity of NE in a composite film and edible coating and checking their functionality in a real food system, it is necessary to study different methods to increase the shelf life of seafood due to the importance of fresh fish from the consumer’s point of view. Therefore, this study aimed to produce biodegradable composite edible coating based on SA and GG containing different concentrations of NE as well as investigate its effect on the quality and shelf life of rainbow trout fillets at 4 °C in the refrigerator.
Materials and methods
Materials
Galbanum plant (Ferula gummosa) was prepared from Alborz Mountains (51.47° E, 36.43° N), Iran. Nettle plant was prepared from Doehezar area of Tonekabon city (50.88° E, 36.82° N), located in Mazandaran Province, Iran, in 2020 and the leaves of the plant were washed and, then, dried in shade at the room temperature (25 °C) and ground for extraction. All the chemicals used were prepared by Merck Company (Darmstadt, Germany) and had a degree of decomposition.
Preparation and analysis of nettle extract (NE)
For extraction, 70% ethanol was used by the soaking method and 10 g of plant leaf powder was weighed separately and poured into the decanter; then, 100 mL ethanol was added step by step. The addition of ethanol was continued until the entire volume of the plant in the decanter was soaked and some ethanol was placed on the sample surface. The mixture of solvent and sample was shaken by an Erlene shaker (KS 501 digital, IKA, Germany). After 72 h of extraction, the solutions were filtered through Whatman filter paper no. 1 and the solvents were evaporated by vacuum evaporation at 50 °C [28]. One gram of the extract was injected into the GC/MS (Trace GC-ISQ mass spectrometer, Thermo scientific, USA). The relative percentage of each ingredient of extract was determined according to the area under the curve of each of the chromatogram peaks of the device and its comparison with the total area under the curve [29].
Extracting gum from galbanum
Seeds in the ratio of distilled water: galbanum (1:40) was added. At 80 °C and constant pH below 8, stirring was performed for 2 h and, then, a centrifuge (SIGMA brand 154 K) was used with 2.600 g and 20 °C for 20 min. The supernatants (mucilage) were transferred to high-density polyethylene bags. It was then stored at 4 °C until use [30].
Determining chemical composition of gum
Moisture, ash, crude protein and fat of GG were determined based on AOAC [31]. The percentage of Barijeh gum monosaccharides was determined after hydrolysis of gum with sulfuric acid (0.5 mg sulfuric acid, 80 °C, 18 h) using an HPLC system by a detector (RI Knauer, Germany) equipped with Eurokat H carbohydrate analysis column (300 × 8 mm, Knauer, Germany) [25].
Measuring total phenolic compounds
Total phenolic content was measured using the Folin–Ciocalteu reagent. Gallic acid was used as a standard for drawing calibration curves. Total phenolic content was reported based on the equivalent of mg gallic acid/g extract [32].
Measuring antioxidant activity
DPPH free radical scavenging activity test was determined by adding antioxidant compounds and based on the percentage of DPPH free radical scavenging. Different concentrations from both samples, 15 mL, were added to 5 mL of 0.004% of DPPH in methanol. The reaction mixture was mixed in the vortex mixer and kept in the dark at the room temperature for 1 h. After 60 min, the absorbance of the mixture was read against a blank at 517 nm by a spectrophotometer. DPPH free radical scavenging activity was estimated as follows:
In the next step, a slim serial was prepared from each sample, 50% scavenging concentration of three separate samples was measured and its mean was calculated. Vitamin C with different concentrations was also considered as a positive control. All the experiments were performed with three replications [33].
Determining the MIC and MBC
The studied bacteria included Escherichia coli PTCC: 1399 and Staphylococcus aureus PTCC: 1112 and Listeria monocytogenes PTCC: 1163 and Bacillus cereus PTCC: 1015, prepared from Iran Scientific Research Center. The minimum inhibitory concentration (MIC) was determined using a broth microdilution test. Bacterial inoculums were adjusted to 106 CFU/mL and concentration range of 0.8–100 µg/mL for each sample in dimethyl sulfoxide (DMSO). Subsequently, 95 µl of Mueller–Hinton broth, 100 µl of each sample concentration and 5 µl of inoculums were added to each well of the 96-well plates. Then, the plates were incubated at 26 °C–37 °C for 18–24 h. The optical density of the wells was determined at 620 nm in a microplate reader. For determining minimum bactericide concentration (MBC), 100 μl of the bacterial suspensions from MIC tests was cultured in Mueller–Hinton agar and the lowest concentration with no growth was described as MBC [8, 34].
Preparing SA/GG/NE
To produce the coating, the mixture was prepared by dissolving SA in distilled water (20 g/l) and, then, stirring at 50 °C until the mixture became clear [21]. Also, 30 g of GG was dissolved in 1 lit of sterile distilled water at 70 °C to produce GG coating. After preparing the solutions, they were mixed for 60 min at 30 °C and, then, the viscous solutions were cooled down to 20 °C; 0.1 mL of glycerol monostearate, as a plasticizer, was added to increase the strength and flexibility of viscous solutions. For active edible coating, different concentrations of NE were mixed on a magnetic stirrer at 55 °C. The final solution was homogenized by a homogenizer at 7000 g for 2 min and the final coating was prepared [25].
To prepare a combined coating, GG (15 g) and SA (15 g) were mixed and the rest of the methods was prepared based on the mentioned methods. Also, different concentrations of NE (0.5% and 1%) were added to the formulations [25].
Preparing coatings of SA/ GG/ NE on fish fillets
First, 30 alive rainbow trout with an average weight of 400 ± 50 g were bought from a local aquaculture farm and transported to the food laboratory in less than 1 h using boxes containing ice. After cutting, emptying the viscera, skinning and removing the bones of the fish, they were washed with cold water. Then, about 60 fillets of 100 ± 2 g were prepared and kept in the refrigerator until the experiment. To create a coating on the surface of the fillets, the fillets were first immersed in the prepared solutions for 1 min. Afterwards, they were removed from the solution and, after about 2 min, they were placed in the coating solution again for another 1 min. The control was left uncovered. To dry the fillets, they were hung on sterile mesh plates for 5 h and subjected to a gentle stream of air (at 10 °C) to form a coating on the fillets. The samples were placed in sterile polyethylene bags and stored at 4 °C until testing. Microbiological and chemical evaluation of the fillets was performed continuously from the 3rd to 12th days and compared with the control samples prepared in water solutions without coating materials. In total, the present study included 10 treatments (Table 1):
Chemical analysis
The peroxide value (PV) in the samples was determined according to the Pearson method [35]. The amount of thiobarbituric acid (TBA) was determined by the colorimetric method according to Valipour et al. [14] and expressed as mg malondialdehyde/kg sample. Total volatile basic nitrogen (TVB-N) of the fish fillets was determined using the micro-diffusion method according to Valipour et al. [14].
Microbiological analysis
For the microbiological experiments, 10 g of the fillet meat sample was sterilized under sterile conditions with 90 mL of 0.1% sterile peptone water for 3 min in a laboratory mixer. Each sample was sampled three times separately [15]. Homogenized samples were used for culture in the following microbiological media:
Plate count agar [total bacterial count (TVC) and psychrotrophic bacteria (PTC)], de Man, Rogosa and Sharpe (MRS) agar for lactic acid bacteria (LAB), pseudomonas agar for Pseudomonas spp., violet red bile glucose (VRBG) agar for Enterobacteriaceae family, Baird Parker agar for Staphylococcus aureus and dichloran rose bengal chloramphenicol (DRBC) agar for molds and yeasts (MY). The inoculated plates were incubated at 37 °C for 2 days for TVC, Pseudomonas spp., Enterobacteriaceae and Staphylococcus aureus. The incubation condition was 7 °C for 10 days for PTC, 30 °C for 2 days for LAB and 25 °C for 5 days for MY [25, 36].
Sensory analysis
First, 12-member semi-trained panel (6 men and 6 woman, aged 23–26 years old) was selected. Uncoated and coated fillets were examined after cooking in an oven at 185 °C for 60 min in terms of taste, odor, color, texture and general acceptance characteristics, and the results were expressed on a 5-point hedonic scale (7).
Statistical analysis
Data analysis was performed using analysis of variance (ANOVA) according to the normality of the data and the homogeneity of variance. Duncan’s test at the 5% level was used to compare the mean of the data. All the data were reported as mean standard deviation and the evaluations were performed in three replications. The parametric bread method of the Chi-squared test was used for the statistical analysis of the sensory test. Software (SPSS version 18) was used for data analysis and Excel for drawing the graphs.
Results and discussion
Investigating chemical compounds of NE
According to the results related to the chemical compounds of NE (Table 2), 18 compounds (99.61%) were identified. The most common components of the extract were Neophytadiene (26.69%), Phtaleic acid (13.12%) and Dibutylphtaleate (9.39%). Habibi Lahigi et al. [37] stated that the most constituents of NE were Neophytadiene (25.21%), Phtaleic acid (8.15%), 1,2‐benzenocli carboxylic acid (7.62%) and Dibutylphtaleate (7.37%), respectively. Moradi et al. [11] stated that the most constituents of NE were Neophytadiene (25.21%), Phtaleic acid, (8.15%), Dibutyl phtaleate, (7.37%) Bis (2ethyl hexyl) maleate (6.32%) and 1.2‐benzenocli carboxylic acid (7.62%). The chemical compounds in the mentioned studies were consistent with the present study.
Investigating chemical analysis of GG
According to the results related to the chemical compounds of GG, moisture was 6.95%, ash 1.24%, protein 0.83%, ash 0.1% and carbohydrates 25.11%; monosaccharide compounds of GG included galactose was equal to 67.22% and arabinose was equal to 25.92%. These results were consistent with the results of Hamedi et al. [25], stating that the chemical compounds of GG were as follows: moisture 6.54%, ash 1.12%, protein 0.79%, ash 0.12% and carbohydrates 24.91%. The monosaccharide compounds of GG were galactose 67.30%, arabinose 18.20% and uranic acid 14.5%. Jalali et al. [38] reported the results of barium gum sugar analysis for galactose, arabinose and uronic acid 69, 17, and 14, respectively. Slight differences in the results can be justified by different plant growth conditions and extraction processes.
Evaluating phenolic compounds of NE and GG
The phenolic compounds of NE and GG were 91.11 ± 2.03 mg gallic acid/g and 39.25 ± 0.69 mg/g, respectively (Table 3). Bourgeois et al. [39] reported the phenolic compounds of NE as 482.34–86.90 mg/g gallic acid/g. Mzid et al. [40] reported the phenolic compounds of NE extracted with aqueous and ethanol solvents were 29.56 and 31.41 mg gallic acid/g, respectively. According to the report by Hamedi et al. [25], the value of phenolic compounds in GG was 44.13 mg/g. Differences in the amount of phenolic compounds in the extract can be observed in various studies; the content of phenolic compounds in plants may vary during the processing stages such as growth, harvesting, storage and technological methods used [40, 41].
Investigating DPPH free radical activity
A factor called IC50 is usually used to compare the anti-radical activity of different essential oils. Based on the definition, it is the concentration of the extract, in which 50% of the free radical DPPH is inhibited in the reaction medium. Therefore, the lower the concentration, the more the anti-radical activity would be. The IC50 values in the present study for NE, GG and vitamin C were 35.12 ± 0.95 μg/ mL, 562.49 ± 1.43 μg/ mL and 5.05 ± 0.11 μg/ mL, respectively (Table 3). Therefore, the antioxidant activity of the extract was higher than that of the gum. As can be seen, the antioxidant activity of the plant extracts containing polyphenol components was due to their capacity to donate hydrogen atoms or electrons and absorb free radicals [42]. In fact, the higher the phenolic compounds, the greater the antioxidant properties would be. Nettle has a high reducing/antioxidant capacity due to its very high polyphenol and phenolic acids content (rutin, quercetin, isoquercetin, caffeic acid, chlorogenic acid, ferulic acid) [43]. The antioxidant properties of NE have also been reported by Kőszegi et al. [43] and Repajić et al. [44]. Mzid et al. [40] reported that the IC50 values of extract of NE with aqueous and ethanol solvents were 142.94 and 245.65 μg/ mL, respectively. According to the report by Hamedi et al. [25], the IC50 value of GG was 542.32 μg /mL. As mentioned, phenolic compounds are directly related to antioxidant properties. The reason for the difference in IC50 values in the present study from other studies may be due to differences in phenolic compounds.
Investigating MIC and MBC
The results showed (Table 4) that NE had an antimicrobial activity against all the four bacteria. There was no single mechanism for its antibacterial activity due to the number of chemical compounds in NE. One of the important properties of NE and its components is their hydrophobicity, which causes the penetration of these substances into the lipids of bacterial cell membranes and mitochondria, disrupts their structures and creates greater permeability. This causes ions and other cell contents to leak out. Although the release of limited amounts of these substances is tolerable for the bacterium, it affects its bioavailability and release of large amounts of cellular contents or the release of vital ions and molecules will cause cell death [40, 45]. GG also has an antimicrobial activity against all the four bacteria. Similar results about the point that GG has antimicrobial properties against pathogenic bacteria have been reported by Hamedi et al. [25], Jalali et al. [38] and Afshar et al. [46]. The antimicrobial properties of NE were higher than GG, which is due to the higher levels of phenolic compounds in the extract. Neophytadiene has an antibacterial activity according to reports [47]. Nettle can be considered as effective agents in inhibition of microbial infections because it is rich in phytochemicals such as phenolic compounds and minerals [12, 48].
According to the MIC and MBC results, Gram-positive bacterium Staphylococcus aureus was the most sensitive and Gram-negative bacterium Escherichia coli was the most resistant bacterium (p < 0.05). The reason for the difference in sensitivity susceptibility between Gram-positive and -negative bacteria can be attributed to the difference in morphological structures between these microorganisms. Gram-negative bacteria have an outer phospholipid membrane that carries the structural components of lipopolysaccharide. This makes the cell wall impermeable to antimicrobial chemicals. On the other hand, Gram-positive bacteria are more sensitive and have only one outer layer of peptidoglycan, which does not prevent effective permeability. The antimicrobial properties of NE were also reported by Mzid et al. [40], who stated that the antimicrobial properties of NE against Gram-positive bacteria were higher than that of Gram-negative bacteria. Similar results about the antibacterial properties of NE have been reported by Moradi et al. [11] and Mirtaghi et al. [12]. The difference in the effects of plant extracts on bacteria depends on various factors including ecological, climatic and geographic factors, plant’s age, methods of drying and extraction of active components, type of solvent, concentration of the extract and type of culture medium [12].
Chemical evaluation of coated fish fillets
By increasing time, the values of PV (Fig. 1a) significantly increased in all the treatments and these changes were more in the control treatment (p < 0.05). The use of edible coatings slowed the upward trend of PV (p < 0.05). In general, biodegradable films and coatings have very low permeability to oxygen and carbon dioxide. Therefore, the coating formed on the surface of the fillets significantly reduces the rate of contact of the product with oxygen, which decreases the rate of initial oxidation of fats and subsequent formation of hydroperoxides [14]. Also, the combination of SA and GG could more effectively slow down the process of PV increase (p < 0.05), indicating the synergistic property of these two coatings in combination with each other and, thus, increasing its antioxidant properties. Adding the extract to the coating also slowed down the PV increase process (p < 0.05). Lower PV was due to the phenolic compounds in the extract because phenolic compounds prevented oxidation by inactivating fat-free radicals and peroxyl radicals [36]. Increasing the concentration of the extract had a positive effect on this process, so that on the 12th day of storage, the lowest amounts of PV were observed in the treatment of SA + GG + NE 1% (2.97 mEq/kg fat) and the highest values in the control treatment (4.65 mEq/kg fat) (p < 0.05). Numerous studies have reported that the antioxidant effect of plant extracts is dependent on the concentration used and, with increasing concentration; their antioxidant properties also increase [36, 42, 49]. Zhang et al. [5] reported that the combined coating of SA-agar with ginger essential oil slowed down the process of increasing PV of beef meat.
TBA values significantly increased in all the treatments with increasing time and these changes were more in the control treatment (p < 0.05). Increased TBA levels in rainbow trout fillets have also been reported by other researchers [13, 21, 50, 51]. The use of SA coating slowed the upward trend of TBA (Fig. 1b) (p < 0.05). Some researchers have found the effect of edible SA coating on fish fillets to control lipid oxidation [21, 50]. They believed that SA coatings, which are placed on the surface of food, are also resistant to oxygen release. Lipid oxidation can be initiated and accelerated through various mechanisms such as the production of single oxygen, enzymatic and non-enzymatic free radicals and reactive oxygen species [50]. Also, the use of GG and NE slowed down the upward trend of TBA and had a positive effect on this process by increasing the concentration of the extract (p < 0.05). Better results were observed in most of the storage times in the combined treatment of edible coatings and extract, so that on the 12th day of storage, the lowest value of TBA was in the treatment of SA + GG + NE 1% (0.46 mg malondialdehyde/kg fat) and the highest values were in the control treatment (1.49 mg malondialdehyde/kg fat) (p < 0.05). It is possible to use dried plants and their extracts effectively to reduce the oxidation of fats in meat products. The compounds in the extracts are good donors of electrons and protons, and their intermediate radicals are very stable due to the phenomenon of electron movement in the benzene ring and the lack of a sensitive site to oxygen attack. Compounds in NE, including Neophytadiene, have the property of neutralizing free radicals and are able to inhibit metal ions such as Fe2+, thus reducing the rate of formation of reactive oxygen molecules [40, 52].
TVB-N values (Fig. 1c) in all the treatments significantly increased with increasing time (p < 0.05). The increase in TVB-N in fish might be due to various enzymatic processes such as deamination of free amino acids, decomposition of nucleotides and oxidation of amines [14]. At the end of the storage period, the highest amount of TVB-N was observed in control (38.08 mg/100 g) (p < 0.05). The use of edible coatings significantly slowed the increase in TVB-N (p < 0.05). Edible coatings can reduce the water loss of the fillets or act as a barrier to the entry of oxygen; thus, it may also affect the TVB-N content of the fillets and the results showed that the composite coating more effectively slowed the process of reducing TVB-N and ATP degradation than single coatings. Differences in antibacterial activity in each coating may be the main reason that in combination they increased antibacterial activity [53]. Adding the extract slowed down the increasing process of TVB-N and increasing the concentration of the extract had a positive effect on this process, so that on the 12th day of storage, the lowest values of TVB-N were observed in the treatment of SA + GG + NE 1% (25.10 mg/100 g) (p < 0.05), which can be due to the reduced bacterial population of these treatments or the reduced oxidative ability of bacteria to separate amines from TVB-N compounds or both due to the effect of the extract on the bacteria in the fillet. With increasing the concentration of the extract due to the increase of phenolic compounds, its antibacterial effect also increased. Therefore, in the treatment containing higher concentrations of essential oil, the amount of TVB-N was lower [4].
Microbial evaluation of coated fish fillets
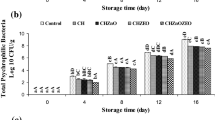
TVC values (Fig. 2a) significantly increased in all the treatments with increasing time and these changes were higher in the control treatment (p < 0.05). At the end of the storage period, it was equal to 8.28 log CFU/g in the control treatment. The use of SA edible coatings slowed the increase in TVC (p < 0.05). SA had unique colloidal properties through cross-linking with calcium by becoming treated with calcium chloride solution and was capable of producing insoluble polymers or forming strong gels. These types of biopolymer-based films can maintain good quality and increase food shelf life by preventing microbial contamination, preserving flavor, reducing wrinkles and decreasing fat oxidation. SA also acts as a barrier to oxygen transport and inhibits the growth of aerobic bacteria [50, 54]. Use of GG and extract slowed down the increasing process of TVC and increasing the concentration of the extract had a positive effect on this process; better results were observed in most of the storage times in the combined treatment of food coatings and extract. The lowest value of the TVC was in SA + GG + NE 1% treatment (6.96 log CFU/g) (p < 0.05). GG also had antimicrobial properties due to its compounds such as phenolic and flavonoid [25]. The reason for the lower amount of TVC in extract-containing treatments can be due to the existence of phenolic compounds. Phenolic compounds in plant extracts degrade the outer membrane of microorganisms, cause the release of liposaccharides and increase the permeability of the cytoplasmic membrane to ATP. ATP release leads to depletion of cell energy storage and cell death [55]. A permissible TVC of 7 log CFU/g has been suggested for fish [56]. According to the results at the end of the storage period, the TVC of SA + GG + NE 1% had a permitted range. PTC values (Fig. 2b) significantly increased in all the treatments with increasing time and these changes were higher in the control treatment (p < 0.05). At the end of the storage period, it was equal to 8.04 log CFU/g in the control treatment. The use of edible coatings and extracts slowed down the increasing process of PTC value and increasing the concentration of extract had a positive effect on this process (p < 0.05). In most of the storage times, better results were observed in the combined treatment of edible coatings and extracts, so that on the 12th day, the minimum amount of PTC was observed in the treatment of SA + GG + NE 1% (6.81 log CFU/g).
It should be stated that 7 log CFU/g is the allowable PTC that has been suggested for fish [56]. According to the results at the end of the storage period, the PTC of SA + GG + NE 1% had a permitted range.
LAB values (Fig. 2c) significantly increased in all the treatments with increasing time and these changes were more in the control treatment (p < 0.05). At the end of the storage period, it was equal to 7.69 log CFU/g in the control treatment. The use of edible coatings and extracts slowed down the increasing process of LAB and increasing the concentration of extract had a positive effect on this process (p < 0.05). In most of the storage times, better results were observed in combined treatment of edible coatings and extracts, so that on the 12th day, the lowest LAB value was in SA + GG + NE 1% treatment (5.98 log CFU/g) (p < 0.05). LAB is reported to be the most resistant Gram-positive bacterium against antimicrobial agents. GG contains antimicrobial peptides that show antimicrobial properties against many microorganisms and SA coatings act as oxygen barriers and prevent the growth of lactic acid bacteria. Neophytadiene has also been reported to have an antibacterial activity, which is effective in the process of reducing lactic acid bacteria [1, 47].
It is now well-defined that Pseudomonas spp. can be a significant part of the microbial flora and cause the spoilage of fish meat during storage in the refrigerator. Pseudomonas spp. (Fig. 3a) significantly increased in all the treatments with increasing time and these changes were higher in the control treatment (p < 0.05), so that at the end of the storage period, it was equal to 8.28 log CFU/g in the control treatment. The use of edible coatings and extracts slowed down the increasing process of Pseudomonas spp. bacteria and increasing the concentration of the extract had a positive effect on this process. On the 12th day of storage, the lowest value of Pseudomonas spp. was in SA + GG + NE 1% (6.57 log CFU/g) (p < 0.05). This inhibition of bacterial growth is probably due to the low oxygen permeability in food films and the antimicrobial properties of plant extracts. The results of the present study were consistent with the results of the study carried out by Hamedi et al. [25] concerning the edible coating of SA with GG and the combined coating of SA-gum and Iranian Ziziphora essential oil on Pseudomonas spp. on chicken fillet as well as the results of the study carried out by Bazargani-Gilani [50] concerning the effect of SA and dietary supplement on the amounts of Pseudomonas spp., so that the use of these coatings slowed the growth of Pseudomonas aeruginosa.
Enterobacteriaceae have been reported as an important part of the microbial flora of rainbow trout spoilage during storage at 4 °C [57]. The amount of Enterobacteriaceae (Fig. 3b) in all the treatments significantly increased with increasing time and these changes were higher in the control treatment (p < 0.05). At the end of the storage period, it was equal 8.28 log CFU/g in the control treatment. The use of edible coatings and extracts slowed down the increasing process of Enterobacteriaceae bacteria and increasing the concentration of the extract had a positive effect, so that on the 12th day of storage, the lowest value of Enterobacteriaceae was in the treatment of SA + GG + NE 1% (6.89 log CFU/g) (p < 0.05). This indicated the synergistic property of SA–GG coating and NE. The results of the present study were consistent with the results of the study carried out by Hamedi et al. [25] about edible coating of SA + GG + Iranian Ziziphora essential oil on chicken fillet, the results of the study carried out by Bazargani-Gilani [50] about the effect of SA and dietary supplement on the levels of Pseudomonas aeruginosa of trout and the study of Nagarajan et al. [1] about the effect of edible coating of chitosan–gelatin with longkong pericarp extract on Enterobacteriaceae values in black tiger shrimp, so that the use of these coatings slowed down the growth of Enterobacteriaceae.
Staphylococcus aureus is one of the most disturbing bacteria among food pathogens [58]. The number of Staphylococcus aureus (Fig. 4a) in all the treatments significantly increased with time and these changes were higher in the control treatment (p < 0.05). At the end of the storage period, it was equal to 5.41 log CFU/g in the control treatment. The use of edible coatings and extracts slowed down the increasing process of Staphylococcus aureus and increasing the concentration of the extract had a positive effect on this process, so that on the 12th day of storage, the lowest value of Staphylococcus aureus bacteria was in SA + GG + NE 1% (3.99 log CFU/g) (p < 0.05). This indicated the antimicrobial properties of NE against Staphylococcus aureus. Antimicrobial properties of NE against Staphylococcus aureus have also been reported in studies by other researchers [11, 40].
Mold and yeast are effective in spoiling fish fillets while refrigerated. The values of mold and yeast (Fig. 4b) in all the treatments significantly increased with increasing time and these changes were higher in the control treatment (p < 0.05). At the end of the storage period, it was equal to 4.71 log CFU/g in the control treatment (p < 0.05). The use of edible coatings and extracts slowed down the increasing process of mold and yeast and increasing the concentration of the extract had a positive effect on this process, so that on the 12th day of storage, the lowest values of mold and yeast bacteria were in the treatment of SA + GG + NE 1% (3.26 log CFU/g) (p < 0.05). In general, food coatings are an important factor in increasing the shelf life and preventing microbial spoilage of meat products, and their good inhibition of oxygen has led them to be an anti-lipid oxidation agent that delays the growth of mold. NE improves the antimicrobial effects of this coating due to its active terpenoid and phenolic groups [59].
Sensory evaluation of coated fish fillet
Food spoilage is determined based on product quality evaluations by various sensory, chemical and microbiological methods. Sensory evaluation is a suitable method for evaluating the quality and freshness of fish during the storage period and is used as a simple and fast method. It should be noted this method alone cannot be accepted as a fixed standard in the laboratory. Over time, the results of sensory properties (Fig. 5) were significantly reduced in all the treatments (p < 0.05). The use of compound coatings and extracts had a minor, but adverse, effect on fish fillet samples. Also, in the control group, unpleasant sensory properties could be inhaled after 6 days of storage at the refrigerator temperature and the control samples were completely unusable after 6 days. Changes in sensory properties were consistent with chemical and microbial results, so that on the 12th day of storage, the highest values of sensory points were observed in SA + GG + NE 1% (p < 0.05), which can be because fat oxidation leads to degradation and loss of sensory quality, and reduces the number of nutrients, including PUFA and the production of toxic oxidation products [14]. On the other hand, increasing fat hydrolysis and accumulation of free fatty acids leads to a decrease in some product acceptance indicators because free fatty acids have been proven to affect the stability of proteins and cause tissue destruction by reacting with proteins. Oxidation of proteins in this state occurs faster than fats that are high molecular weight fats (such as triglycerides and phospholipids) due to increased protein access to oxygen and other peroxide molecules. Also, the coherence between changes in the process of bacterial spoilage and sensory evaluation was already proven by Jalali et al., [60] which may be related to the activity of microorganisms responsible for food spoilage.
Conclusion
According to the results, SA–GG coating with NE slowed down the increasing trend of oxidative spoilage indices compared to the control treatment. Results of the microbial analysis indicated that, in all the treatments, there was an increase in microbial load over time, but this increase occurred slower in the treatments containing extracts; better results were observed with increasing concentration. SA + GG + NE 1% had an acceptable condition in terms of both indicators until the 12th day of storage. The results of the sensory evaluation were consistent with chemical and microbial results. However, further studies are needed to assess the impact of SA + GG + NE 1% on other meat products with other kinds of packaging methods. This study proposed that SA–GG composite coating containing NE at 1% was the best treatment with the highest inhibitory impact, which can be used as an edible coating to create long shelf life for food products such as rainbow trout fillets.
References
M. Nagarajan, B. Rajasekaran, S. Benjakul, K. Venkatachalam, Influence of chitosan–gelatin edible coating incorporated with longkong pericarp extract on refrigerated black tiger Shrimp (Penaeus monodon). Curr. Res. Food Sci. 4, 345–353 (2021)
P. Khadem, A. Motalebi, N. Rokni, V. Razavilar, Effects of Capparis spinosa root extract and modified atmosphere packaging on the shelf life of rainbow trout (Oncorhynchus mykiss) fillets by measuring of antioxidant and antimicrobial parameters. IJFS 19(1), 272–285 (2020)
M. Mozaffarzogh, A. Misaghi, Y. Shahbazi, A. Kamkar, Evaluation of probiotic carboxymethyl cellulose-sodium caseinate films and their application in extending shelf life quality of fresh trout fillets. LWT 126, 109305 (2020)
N. Shakour, Z. Khoshkhoo, A. Akhondzadeh Basti, A. Khanjari, S.P. Mahasti, Investigating the properties of PLA-nanochitosan composite films containing Ziziphora Clinopodioides essential oil and their impacts on oxidative spoilage of Oncorhynchus mykiss fillets. Food Sci Nutr. 9, 1299–1311 (2021)
Z. Zhang, G. Xia, Q. Yang, X. Fan, S. Lyu, Effects of chitosan-based coatings on storage quality of Chinese shrimp. Food Sci. Nutr. 7(12), 4085–4094 (2019)
F. Afshar Mehrabi, A. Sharifi, M. Ahvazi, Effect of chitosan coating containing Nepeta pogonosperma extract on shelf life of chicken fillets during chilled storage. Food Sci. Nutr. 9, 4518–4529 (2021)
S.M.B. Hashemi, D. Jafarpour, The efficacy of edible film from Konjac glucomannan and saffron petal extract to improve shelf life of fresh-cut cucumber. Food Sci Nutr. 8, 3128–3137 (2020)
S.M.B. Hashemi, D. Jafarpour, Synergistic properties of Eucalyptus caesia and Dracocephalum multicaule Montbr & Auch essential oils: antimicrobial activity against food borne pathogens and antioxidant activity in pear slices. J. Food Process. Preserv. 44(9), e14651 (2020)
K.K. Ghaima, N.M. Hashim, S.A. Ali, Antibacterial and antioxidant activities of ethyl acetate extract of nettle (Urtica dioica) and dandelion (Taraxacum officinale). J. Appl. Pharm. Sci. 3(5), 96–99 (2013)
T.A. Johnson, J. Sohn, W.D. Inman, L.F. Bjeldanes, K. Rayburn, Lipophilic stinging nettle extracts possess potent anti-inflammatory activity, are not cytotoxic and may be superior to traditional tinctures for treating inflammatory disorders. Phytomedicine 20(2), 143–147 (2013)
P. Moradi, K. Amiri, Extraction and identification of Urtica dioica L. extract and its antibacterial and antifungal properties. J. Mazandaran Univ. Med. Sci. 22(151), 74–85 (2017)
S.M. Mirtaghi, P. Torbati Nejad, M. Mazandarani, F. Livani, H. Bagheri, Evaluation of antibacterial activity of Urtica dioica L. leaf ethanolic extract using agar well diffusion and disc diffusion methods. Med. Lab. J. 10(5), 15–21 (2016)
S. Shokri, K. Parastouei, M. Taghdir, S. Abbaszadeh, Application an edible active coating based on chitosan—Ferulago angulata essential oil nanoemulsion to shelf life extension of Rainbow trout fillets stored at 4 °C. Int. J. Biol. Macromol. 153, 846–854 (2020)
F. Valipour Kootenaie, P. Ariaii, D. Khademi Shurmasti, M. Nemati, Effect of chitosan edible coating enriched with eucalyptus essential oil and α-tocopherol on silver carp fillets quality during refrigerated storage. J. Food Saf. 37(1), e12295 (2017)
M. Sayadi, A. Mojaddar Langroodi, D. Jafarpour, Impact of zein coating impregnated with ginger extract and Pimpinella anisum essential oil on the shelf life of bovine meat packaged in modified atmosphere. J. Food Meas. Charact. 15, 5231–5244 (2021)
S.M.B. Hashemi, D. Jafarpour, Bioactive edible film based on Konjac glucomannan and probiotic Lactobacillus plantarum strains: physicochemical properties and shelf life of fresh-cut kiwis. J. Food Sci. 86(2), 513–522 (2021)
S. Paidari, N. Zamindar, R. Tahergorabi, M. Kargar, S. Ezzati, N. Shirani, S.H. Musavi, Edible coating and films as promising packaging: a mini review. J. Food Meas. Charact. 15, 4205–4214 (2021)
S. Paidari, R. Tahergorabi, E.S. Anari, A.M. Nafchi, N. Zamindar, M. Goli, Migration of various nanoparticles into food samples: a review. Foods. 10(9), 2114 (2021)
P. Cazon, G. Velazquez, J.A. Ramirez, M. Vazquez, Polysaccharide-based films and coatings for food packaging: a review. Food Hydrocolloids 68, 136–148 (2017)
N. Oladzadabbasabadi, A.M. Nafchi, F. Ariffin, M.J.O. Wijekoon, A.A. Al-Hassan, M.A. Dheyab, M. GhasemLou, Recent advances in extraction, modification, and application of chitosan in packaging industry. Carbohydr. Polym. 227, 118876 (2022)
M.I. Sáez, M.D. Suárez, T.F. Martínez, Effects of alginate coating enriched with tannins on shelf life of cultured rainbow trout (Oncorhynchus mykiss) fillets. LWT 118, 108767 (2020)
E. Tavassoli-Kafrani, H. Shekarchizadeh, M. Masoudpour-Behbadi, Development of edible films and coatings from alginates and carrageenans. Carbohyd. Polym. 137, 360–374 (2016)
R. Heydari, S. Bavandi, S.H. Javadian, Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4°C. Food Sci. Nutr. 3, 188–194 (2014)
S.M. Kazemi, M. Rezaei, Antimicrobial effectiveness of gelatin–alginate film containing oregano essential oil for fish preservation. J. Food Saf. 35, 482–490 (2015)
H. Hamedi, M. Kargozari, P. Mahasti-shotorbani, N.B. Moghadam, M. Fahidmanesh, A novel bioactive edible coating based on sodium alginate and galbanum gum incorporated with essential oil of Ziziphora persica: the antioxidant and antimicrobial activity, and application in food model. Food Hydrocolloids 72, 35–46 (2017)
M. Mahboubi, Ferula gummosa, a traditional medicine with novel applications. J. Diet. Suppl. 13, 700–718 (2016)
A. Abbaszadegan, A. Gholami, H. Mirhadi, M. Saliminasab, A. Kazemi, M.R. Moein, Antimicrobial and cytotoxic activity of Ferula gummosa plant essential oil compared to NaOCl and CHX: a preliminary in vitro study. Restor. Dent. Endod. 40(1), 50e57 (2015)
M. Maleki, P. Ariaii, H. Fallah, Effects of celery extracts on the oxidative stability of canola oil under thermal condition. J. Food Process. Preserv. 40(3), 531–540 (2016)
S. Mousavi, A. Dastpak, Subcritical water extraction of fennel seeds essential oil and comparison with hydrodistillation. J. Food Sci. Technol. (Iran) 16(91), 119–128 (2019)
L. Munoz, Mucillage from chia seeds (Salvia hispanica L.); Microstructure, physicochemical characterization and applications in food industry (Pontificia Universidad catolica de chile escuela ingenieria santiego de chile, Macul, 2012), pp. 1–146
AOAC, Official method of analysis, 17th edn. (Association of Official Analytical chemists, Washington, 2005)
A.A.L. Ordoñez, J.D. Gomez, M.A. Vattuone, M.I. Lsla, Antioxidant activities of sechiumedule (Jacq) Swartz extracts. Food Chem 97, 452–458 (2006)
M. Soheili, M.A. Khandan, M. Salam, Evaluation of anti-oxidant activity of Lavandula angustifolia using DPPH method. Arak Med. Univ. J. 19(117), 70–77 (2017)
N.M. Asl, H. Ahari, A.A.M. Moghanjoghi, S. Paidari, Assessment of nanochitosan packaging containing silver NPs on improving the shelf life of caviar (Acipenser persicus) and evaluation of nanoparticles migration. J. Food Meas. Charact. 15, 5078–5086 (2021)
R. Bagheri, R. Izadi Amoli, N. Tabari Shahndash, S.R. Shahosseini, Comparing the effect of encapsulated and unencapsulated fennel extracts on the shelf life of minced common kilka (Clupeonella cultriventris caspia) and Pseudomonas aeruginosa inoculated in the mince. Food Sci. Nutr. 4(2), 216–222 (2016)
M. Eslamian, M. Ahmady, P. Ariaii, L. Golestan, A.G. Ghorbani-HasanSaraei, Use composite coating of chitosan-chia seed gum enriched with microliposomes of Bay laurel essential oil to increase the shelf life of quail fillets. Food Sci. Nutr. 00, 1–14 (2021)
S. Habibi Lahigi, K. Amini, P. Moradi, K. Asaadi, Investigating the chemical composition of different parts extracts of bipod nettle Urtica dioica L. in Tonekabon region. Iran. J. Plant Physiol. 2(1), 339–342 (2011)
H.T. Jalali, Z.J. Ebrahimianb, D.V. Evtuguinb, C.P. Neto, Chemical composition of oleo-gum-resin from Ferula gummosa. Indus. Crops Prod. 33(2), 549–553 (2011)
C. Bourgeois, É.A. Leclerc, C. Corbin, J. Doussot, V. Serrano, J.R. Vanier, J.M. Seigneuret, D. Auguin, C. Pichon, É. Lainé et al., Nettle (Urtica dioica L.) as a source of antioxidant and anti-aging phytochemicals for cosmetic applications. C. R. Chim. 19, 1090–1100 (2016)
M. Mzid, S. Ben Khedir, M. Ben Salem, W. Regaieg, T. Rebai, Antioxidant and antimicrobial activities of ethanol and aqueous extracts from Urtica urens. Pharm. Biol. 55(1), 775–781 (2017)
I.M. Abu-Reidah, D. Arráez-Román, A. Segura-Carretero, A. Fernández-Gutiérrez, Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC-DAD-ESI-QTOF-MS. Food Chem. 141, 2269–2277 (2013)
S.S. Tometri, M. Ahmady, P. Ariaii et al., Extraction and encapsulation of Laurus nobilis leaf extract with nano-liposome and its effect on oxidative, microbial, bacterial and sensory properties of minced beef. Food Meas. 14, 3333–3344 (2020)
K. Kőszegi, E. Békássy-Molnár, N. Koczka, T. Kerner, É. Stefanovits-Bányai, Changes in total polyphenol content and antioxidant capacity of stinging nettle (Urtica dioica L.) from spring to autumn. Period. Polytech. Chem. Eng. 64(4), 548–554 (2020)
M. Repajić, E. Cegledi, Z. Zorić, S. Pedisić, I. Elez Garofulić, S. Radman, I. Palčić, V. Dragović-Uzelac, Bioactive compounds in wild nettle (Urtica dioica L.) leaves and stalks: polyphenols and pigments upon seasonal and habitat variations. Foods 10, 190 (2021)
S. Burt, Essential oils: their antibacterial propertied and potential application in foods-a review. Int. Food Mashinicrobiol. 94(3), 223–253 (2004)
F.F. Afshar, P. Saffarian, H.M. Hosseini, F. Sattarian, M. Amin, A.A.I. Fooladi, Antimicrobial effects of Ferula gummosa Boiss gum against extendedspectrum b-lactamase producing Acinetobacter clinical isolates. Iran. J. Microbiol. 8(4), 263e273 (2016)
S. Lalitharani, V.R. Mohan, G.S. Regini, GC–MS analysis of ethanolic extract of Zanthoxylum rhetsa (roxb.) dc spines. J. Herb. Med. Toxicol. 4(1), 191–192 (2010)
S.A. Dar, A.R. Yousuf, F.A. Ganai, P. Sharma, N. Kumar, R. Singh, Bioassay guided isolation and identification of antiinflammatory and anti-microbial compounds from Urtica dioica L. (Uriticaceae) leaves. Afr. J. Biotechnol. 11(65), 12410–12420 (2012)
S.S. Rashidaie Abandansarie, P. Ariaii, M. Charmchian Langerodi, Effects of encapsulated rosemary extract on oxidative and microbiological stability of beef meat during refrigerated storage. Food Sci. Nutr. 7, 3969–3978 (2019)
B. Bazargani-Gilani, Activating sodium alginate-based edible coating using a dietary supplement for increasing the shelf life of rainbow trout fillet during refrigerated storage (4± 1 °C). J. Food Saf. 38(1), e12395 (2018)
E.E. Fadıloğlu, Ö. Emir Çoban, Effects of chitosan edible coatings enriched with sumac on the quality and the shelf life of rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) fillets. J. Food Saf. 38, e12545 (2018)
H.M. Mohamed, H.A. Mansour, Incorporating essential oils of marjoram and rosemary in the formulation of beef patties manufactured with mechanically deboned poultry meat to improve the lipid stability and sensory attributes. LWT-Food Sci. Technol. 45(1), 79–87 (2012)
Q. Li, J. Zhang, D. Zhang, T. Sun, Effect of a chitosan-alginate bilayer coating incorporated with lysozyme on quality of refrigerated turbot fillets. IOP Conf. Ser. Earth Environ. Sci. 792, 012016 (2021)
Y. Song, L. Liu, H. Shen, J. You, Y. Luo, Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 22, 608–615 (2011)
K.N. Jan, Z. Khan, S. Sukhcharn, Stinging nettle (Urtica dioica L.): a reservoir of nutrition and bioactive components with great functional. J. Food Meas. 11, 423–433 (2017)
ICMSF, Microorganisms in foods 6: microbial ecology of food commodities, 2nd edn (1st edn published 1998) (Kluwer Academic/Plenum Publishers, New York, 2005)
M. Gui, B. Zhao, J. Song, Z. Zhang, Z. Peng, P. Li, Paraplantaricin L-ZB1, a novel bacteriocin and its application as a biopreservative agent on quality and shelf life of rainbow trout fillets stored at 4 °C. Appl. Biochem. Biotechnol. 174, 2295–2306 (2014)
S. Paidari, H. Ahari, The effects of nanosilver and nanoclay nanocomposites on shrimp (Penaeus semisulcatus) samples inoculated to food pathogens. J. Food Meas. Charact. 15(4), 3195–3206 (2021)
M. Rezaloo, Z. Mashak, A. Shakerian, Study of the effect of gelatin and cumin essential oil on microbial and organoleptic properties of chicken meat under refrigerator conditions. New Find. Vet. Microbiol. 1(1), 51–61 (2018)
N. Jalali, P. Ariiai, E. Fattahi, Effect of alginate/carboxyl methyl cellulose composite coating incorporated with clove essential oil on the quality of silver carp fillet and Escherichia coli O157:H7 inhibition during refrigerated storage. J. Food Sci. Technol. 53(1), 757–765 (2016)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zarandi, M., Hasani, M., Shotorbani, P.M. et al. Assessing edible composite coating of sodium alginate–galbanum gum impregnated with nettle extract on improving the shelf life of rainbow trout fillet. Food Measure 16, 2556–2570 (2022). https://doi.org/10.1007/s11694-022-01357-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01357-7