Abstract
Paraplantaricin L-ZB1 was produced by Lactobacillus paraplantarum L-ZB1, which was isolated from the traditional China fermented sausage. In this work, paraplantaricin L-ZB1 was used to maintain quality of rainbow trout fillets at 4 °C. Rainbow trout fillets were left untreated (CK) or treated with 200 activity units (AU)/ml paraplantaricin L-ZB1 (P1), 400 AU/ml paraplantaricin L-ZB1 (P2) or 200 AU/ml Nisin (N). The treated samples were stored at 4 °C for up to 10 days, and the quality changes were determined by microbiological (total viable count [TVC], Enterobacteriaceae, Pseudomonas, spore-forming bacteria), sensory, chemical (pH, total volatile basic nitrogen [TVB-N]) and biochemical (biogenic amines, K value) methods. Results show that paraplantaricin L-ZB1 could inhibit the growth of microflora, especially Enterobacteriaceae, Pseudomonas and spore-forming bacteria during sample storage. Meanwhile, the increases of pH, TVB-N, K value and biogenic amine levels were significantly delayed in paraplantaricin L-ZB1-treated samples compared to the control group. Paraplantaricin L-ZB1 of 400 AU/ml extended the rainbow trout fillets’ shelf life to 4–6 days, and the sample showed good sensory characteristics. Therefore, paraplantaricin L-ZB1 could be used as a suitable biological preservative for chilled rainbow trout fillets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rainbow trout is farmed in China over the past few decades and has been developed for more than ten culture species. It can be sold as either whole fresh fish in retail markets or in fillet form in supermarkets. Additionally, raw rainbow trout fillets or smoked fillets are consumed in high club and hotel as ready-to-eat food in big cities in China.
The quality of fresh fish is a major concern to industry and consumers considering that they are usually more perishable than most other foodstuffs. The initial loss of freshness is due to enzymatic and chemical reactions, while the subsequent spoilage is due to microbial activity [1]. In order to prevent the spoilage of fresh fish, various food preservation techniques have been used to improve the microbial safety and increase the shelf life of fish, including chemical preservation, active packaging, freezing and natural antimicrobials [2–5]. The consumption of food that has been formulated with chemical preservatives has also increased consumer concern and created a demand for more “nature” and “minimally processed” food [6]. As a result, the use of natural antimicrobial agents is desirable for the development of food products.
Bacteriocins are ribosomally synthesized peptides with a bactericidal activity against closely related bacteria and other bacteria of health or spoilage significance [6, 7]. Lactic acid bacteria bacteriocin, which has been shown to be safe, can be used as effective natural food preservatives [6]. Nisin, produced by Lactococcus lactis subsp. Lactis, has many applications in foods and is approved for use in various foods throughout the world [8]. Nisin is active against Gram-positive organisms including bacterial spores, but when it is associated with chelators, the activity of nisin against Gram-negative bacteria can increase [9].
Paraplantaricin L-ZB1 is produced by Lactobacillus paraplantarum L-ZB1, isolated from the traditional China fermented sausage. Compared to nisin, paraplantaricin L-ZB1 showed a broader antimicrobial spectrum and could inhibit Gram-negative bacteria, including Enterobacteriaceae and Pseudomonas (data not shown).
The objective of this article was to evaluate the effect of L. paraplantarum L-ZB1 on microbiological, chemical and sensory attributes of rainbow trout fillets stored at 4 °C. In addition, nisin was used to compare the effect of preservative to L. paraplantarum L-ZB1 during storage.
Materials and Methods
Sample Preparation
Rainbow trout weighing approximately 1.5 kg was purchased from Beijing Hui Longguan wholesale market. The duration between catch and arrival of the fish at the laboratory was less than 4 h where they were always kept in ice. Upon arrival, the whole fish was washed under sterile water, headed, gutted, filleted (approximately each fillet of weight 50 g) and rinsed again. Then, the rainbow trout fillets were kept at 0–2 °C until use.
Preparation of Paraplantaricin L-ZB1 and Nisin and Quantification of Bacteriocin Activity
The bacteriocin producer L. paraplantarum L-ZB1 was grown in MRS medium [10] and incubated at 37 °C. Lactobacillus plantarum pl-2 was used as the indicator strain in the bacteriocin assay. After 24 h cultivation of L. paraplantarum L-ZB1, supernatant was collected by centrifugation (9,000g, 30 min). The bacteriocin present in the supernatant fraction was concentrated by ammonium sulphate precipitation (70 % saturation). After the mixture had been stirred overnight at 4 °C, the precipitate was pelleted by centrifugation (12,000g, 30 min). The collected precipitate was then dissolved in 20 mM sodium phosphate buffer (pH 7.0) and dialysed using a 3.5 kDa cut-off membrane against the same buffer at 4 °C overnight. A stock solution of nisin (Sigma-Aldrich, St. Louis, MO) was dissolved in 20 mM sodium phosphate buffer (pH 7.0). The bacteriocin and nisin preparation was serially diluted, and 100 μl from each dilution was spotted onto a lawn of appropriate indicator bacteria. Activity units (AU) per millilitre were calculated as previously reported [11].
Treatment of Fish Samples
Rainbow trout samples were randomly divided into four treatment groups: (1) 200 AU/ml paraplantaricin L-ZB1 (P1), (2) 400 AU/ml paraplantaricin L-ZB1 (P2), (3) 200 AU/ml nisin (N) and (4) autoclaved distilled water (CK). The four groups were sprayed with 40 ml four different preservative solutions per kilogram of fish. After that, they were packaged in sterile plastic trays wrapped with polyvinyl chloride film (oxygen transmission rate = 3,000 cc/m2/24 h at 5 °C), respectively, and stored under refrigeration at 4 °C. Samples were analysed immediately, and the data collected was labelled as the data for day 0. The rest of the samples were then stored at 4 °C and analysed on days 2, 4, 6, 8 and 10. All samples were analysed in triplicate.
Microbiological Analyses
A sample (25 g) was transferred aseptically to 225 ml of sterile physiological saline (0.85 %, w/v) and homogenized for 60 s. For microbial enumeration, 1 ml samples of serial dilutions (1:10, diluent, 0.85 % physiological saline) of fish homogenates were placed in a Petri dish, and 15 ml of total count and selective agar was added. Bacteria were performed on pour plates. Total viable bacterial count (TVC) was determined using plate count agar (PCA, code HB0101, Qingdao Hope Biol-Technology Co., Ltd. China) and incubated at 30 °C for 2 days. Pseudomonas were enumerated on cetrimide fusidin cephaloridine (CFC) agar (code HB8689, supplemented with CFC additive code HB8689a) and incubated at 20 °C for 2 days. Enterobacteriaceae were investigated in violet red bile glucose agar (VRBGA, code HB0176) after incubation at 37 °C for 24 h. Spore-forming bacteria were investigated in Luria-Bertani (LB) agar (code HB0128) at 36 °C for 2 days after samples of serial dilution preliminary heat treatment (10 min at 80 °C) in water bath (DK-S28, Shanghai Yifen Scientific Co., Ltd. China). Microbiological data were transformed into logarithms of the number of colony-forming units (log10 cfu/g). All plates were examined visually for typical colony types and morphology characteristics associated with each growth medium.
Sensory Analyses
Sensory analyses were conducted by a taste panel consisting of six experienced judges, according to the method of quality index method shown in Table 1. The sensory scale is based on the freshness quality grading system for thawed cod developed by Warm et al. [12], with some modifications. Four levels (0–3) and four parameters (texture, odour, colour and gapping) were used to evaluate the sensory of rainbow trout fillets.
TVB-N and pH Analyses
Total volatile basic nitrogen (TVB-N) value was measured by semi-micro steam distillation [13]. The method was measured by distillation after adding MgO to the homogenized samples (10 g). TVB-N values were determined with Kjeldahl apparatus (KDY-9820, Beijing, China). The results were expressed as milligram TVB-N per 100 g rainbow trout.
The pH was measured in 5 g of minced sample mixed with 45 ml of distilled water. The mixture was homogenized for 3 min. After letting the mixture stand for 30 min, the pH of the sample was measured using a digital 320 pH metre (Mettler Toledo, Zurich, Switzerland).
Biogenic Amine Analyses
Putrescine, cadaverine, tryptamine, tyramine, histamine, phenethylamine, spermine and spermidine were extracted as described by Shi et al. [14] and stored at −20 °C prior to analysis. Biogenic amines were analysed by using a high-performance liquid chromatographic (HPLC) method according to Shi et al. [14]. Results are expressed as milligram per kilogram rainbow trout. Total level of biogenic amine (BA) was calculated as the sum of putrescine, cadaverine, tryptamine, tyramine, histamine, phenethylamine, spermine and spermidine. Biogenic amine index (BAI) was calculated as the sum of putrescine, cadaverine, tyramine and histamine [15].
K Value Analyses
Nucleotide extracts were prepared according to Ryder [16] and stored at −20 °C prior to analysis. Nucleotide analysis was performed by HPLC as described by Aubourg et al. [17]. The K value was calculated as the percent rate of HxR and Hx to the sum of ATP and degradation products as follows:
where HxR is the inosine, Hx is the hypoxanthine, ATP is the adenosine triphosphate, ADP is the adenosine diphosphate, AMP is the adenosine monophosphate and IMP is the inosine monophosphate.
Statistical Analyses
Analyses were performed with the SPSS 13.0 statistics software (SPSS, Chicago, IL, USA). Descriptive statistics (mean and standard deviation), one-way ANOVA and Pearson correlation analysis were applied. Significance level was set at 5 %.
Results and Discussion
Microbiological Analyses
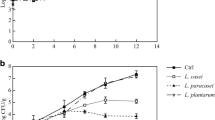
The changes in microflora of rainbow trout fillets as a function of treatment and storage time are shown in Fig. 1a–d. All sample started with low microbial counts of near 1.9 log cfu/g which indicated that the rainbow trout studied was of good quality. TVC significantly increased (P < 0.05) to 7 log cfu/g on days 2–4 for the CK and P1, days 4–6 for the P2 and N. P2 and N resulted in a shelf life extension of about 2 days while P1 had little effect on shelf life extension compared to CK. These indicated that paraplantaricin L-ZB1 had inhibitory effect on microbial growth in chilled rainbow trout fillets and the antibacterial effective of 400 AU/ml paraplantaricin L-ZB1 was close to that of 200 AU/ml nisin.
Changes in total viable counts (TVC) (a), Pseudomonas (b), Enterobacteriaceae (c) and spore-forming bacteria (d) of rainbow trout fillets stored at 4 °C, (×) control; (white square) P1; (black up-pointing triangle) P2; (black square) N. Each point presented is the mean value of three determinations for each sampling. Bars represent the standard deviation
Pseudomonas was reported to be the specific spoilage bacteria in fish from temperate and tropical water [18], and it was dominant in filleted rainbow trout stored at 4 °C [2]. Initial counts of Pseudomonas for all rainbow trout samples were below the sensitivity limit of method (1 log cfu/g). They significantly increased from day 0 to day 6 (P < 0.05), and thereafter, they declined during the remaining storage period (Fig. 1b). The decrease of Pseudomonas may be attributed to its sensitivity to the change of ambient environment, and thus, it lost the competitive advantage during the later stage [19]. As noted previously for TVC, Pseudomonas population was significantly (P < 0.05) lower for P2 and N compared to P1 and CK between day 0 and day 6 of storage. During the entire storage period, the counts of Pseudomonas in P2 and N were keeping nearly the same value (P < 0.05). It indicated that paraplantaricin L-ZB1 significantly inhibited the growth of Pseudomonas and 400 AU/ml paraplantaricin L-ZB1 achieved the same effect as 200 AU/ml nisin.
Enterobacteriaceae were also found to be significant part of the spoilage microflora of rainbow trout fillets stored at 4 °C [2, 20]. Treatments P1, P2 and N resulted in significantly lower (P < 0.05) Enterobacteriaceae counts than CK between day 0 and day 8 (Fig. 1c). On a given sampling day (day 4), Enterobacteriaceae counts reached the value of 7.38 log cfu/g for CK. On the same day, Enterobacteriaceae counts were reduced by 0.46 log cfu/g for P1, 2.53 log cfu/g for P2 and 3.53 log cfu/g for N. Obviously, 200 AU/ml nisin is the most effective, followed by 400 AU/ml paraplantaricin L-ZB1 and 200 AU/ml paraplantaricin L-ZB1.
Spore-forming bacteria (Clostridium or Bacillus) are undesirable bacteria in food and may grow in fish products that are subjected to a mild heat treatment, particularly if unsalted [21]. The spore-forming bacteria were small part of the microflora of the rainbow trout fillets over the entire storage period (below 3 log cfu/g), and they were not detected during the initial 2 days for all samples (Fig. 1d). On the final day 10 of storage, P1 and P2 reduced the spore-forming bacteria counts by 0.25 and 0.79 log cfu/g, respectively, compared to CK. It is noteworthy that spore-forming bacteria counts for N were not detected during the whole storage, indicating that spore-forming bacteria were more sensitive to the action of nisin than that of paraplantaricin L-ZB1 in rainbow trout.
Most bacteriocins produced by lactobacilli inhibit other lactobacilli or closely related Gram-positive bacteria [22–24], whereas others are active against a wide spectrum of Gram-positive or Gram-negative bacteria [25, 26]. The inhibitory effect of paraplantaricin L-ZB1 was observed against Gram-negative bacteria Enterobacteriaceae and Pseudomonas by using the agar-well diffusion method (data not shown). In this research, the effect of paraplantaricin L-ZB1 against Enterobacteriaceae and Pseudomonas was observed in rainbow trout fillets stored at 4 °C, suggesting that paraplantaricin L-ZB1 could be used as an effective antimicrobial agent for chilled fish.
Nisin is active against Gram-positive organisms including bacterial spores, but it is not generally active against Gram-negative bacteria [27]. Treatment with chelators can alter the outer membrane permeability of Gram-negative bacteria [8]. In our study, lower counts of Gram-negative bacteria, Pseudomonas and Enterobacteriaceae were detected in the nisin-treated fish between day 0 and day 6 of storage. Nisin being effective against Gram-negative organisms in the experiment may be due to the synergistic action of nisin with phosphate (nisin solutions were prepared by solubilizing purified nisin in sodium phosphate buffer). It is reported that trisodium phosphate increases sensitivity of Escherichia coli and Pseudomonas fluorescens to nisin [2, 28].
Sensory Analyses
The changes in the sensory score of rainbow trout in different treated groups during storage are shown in Fig. 2. Zero presented absolutely fresh, and six was considered to be the lowest limit of acceptability. The scores of the sensory assessment of all samples showed similar increasing trend. P1, P2 and N gave lower sensory scores and showed better characteristics of texture, odour, colour and gapping than those of CK. The observed shelf life of rainbow trout fillets was about 2–4 days for CK and P1 and 4–6 days for P2 and N. Correlation between sensory and microbiological (TVC) attributes was generally good for CK, P1, P2 and N (r 2, 0.928, 0.913, 0.958, 0955, respectively). Based on sensory analyses, P2 extended shelf life by approximately 2 days compared to CK. Interestingly, P2 showed lower sensory scores (P < 0.05) than N at the end of the storage (day 10), indicating that 400 AU/ml paraplantaricin L-ZB1 could better retain good quality characteristics of rainbow trout than 200 AU/ml nisin.
TVB-N and pH Analyses
Changes in the TVB-N content of rainbow trout fillet over storage time are shown in Fig. 3a. Initial (day 0) average TVB-N value in rainbow trout fillets was 11.17 mg N2/100 g similar to the values reported by Mexis et al. [2]. At the time of sensory rejection, TVB-N values were ca. 30 mg N2/100 g for CK, 15.62 mg N2/100 g for P1, 36.13 mg N2/100 g for P2 and ca. 35 mg N2/100 g for N. Such values are not considered very reliable for measuring the deterioration of certain fish species [2, 29]. It was also observed that different TVB-N values of acceptable level for different treated rainbow trout fillets stored at 4 °C [30]. The TVB-N levels for P1, P2 and N were significantly lower than those for CK (P < 0.05) between day 2 and day 8 of storage. Since TVB-N is produced mainly by bacterial decomposition of fish [31], the inhibition of TVB-N production in rainbow trout samples may be attributed to the slower growth of bacterial populations due to the effect of paraplantaricin L-ZB1 and nisin. It is noteworthy that P1 produced significantly lower (P < 0.05) TVB-N values as compared to P2 and N after day 4 and until end of storage period. This phenomenon probably resulted from proteolysis and deamination of paraplantaricin L-ZB1 and nisin. Therefore, lower bacteriocin resulted in lower TVB-N. The hypothesis needs validation in the future.
Changes in total volatile basic nitrogen (TVB-N) (a) and pH (b) of rainbow trout fillets stored at 4 °C, (×) control; (white square) P1; (black up-pointing triangle) P2; (black square) N. Each point presented is the mean value of three determinations for each sampling. Bars represent the standard deviation
pH values are depicted in Fig. 3b. The initial pH of the fish samples of CK, P1, P2 and N was 6.37, 6.37, 6.34 and 6.36, respectively. During the storage, the pH values increased sharply in CK and N, whereas in P1 and P2, the values were fairly close. P1, P2 and N showed lower (P < 0.05) pH compared to CK between day 0 and day 8, and P1 showed the lowest pH during the whole storage. It is reported that decomposition of nitrogenous compounds leads to an increase in pH in the rainbow trout, which may be partially correlated to the production of alkaline compounds [31]. This was in agreement with our results, where the increase of the TVB-N was followed by increase in the pH (Fig. 3a, b).
K Value Analyses
Changes in the K value of rainbow trout fillet during storage are shown in Fig. 4. Previous study suggested that fish products with K value lower than 20 % are very fresh ones, with less than 50 % are moderately fresh, while greater than 70 % are not fresh [32]. All rainbow trout fillets started with K values of 13.00–17.38 %. The CK showed a rapid increase of K value, reaching number over 50 % on day 2, when P1, P2 and N were less than it (P < 0.05). On day 4, P2 showed the lowest K value (44.18 %), followed by N, P1 and CK (55.18, 62.25 and 70.24 %, respectively). On day 10, K value resulted in 72.04 % for P2, significantly lower than CK, P1 and N (P < 0.05). Therefore, P2 effectively reduced the degradation of ATP and extended the shelf life of rainbow trout fillets. K value showed good correlation values (r 2, 0.913–0.977) with TVC and sensory scores of CK, P1, P2 and N, indicating that it can be good quality index in rainbow trout stored at 4 °C. K value has been used as a freshness measure in many species [33, 34].
Biogenic Amine Analyses
BAs are nonvolatile compounds formed by microbial decarboxylation of amino acids, and their presence is related to spoilage [35, 36]. Veciana-Nogués proposed BAI to evaluate the quality of tuna stored at different temperatures [15]. The concentration of BA and BAI of rainbow trout during storage is shown in Fig. 5a, b. The concentration of BA and BA1 showed similar change trend during the whole storage. On day 10, BAI accounted for more than 95 % of BA in all cases (CK 96.0 %, P1 97.4 %, P2 98.6 % and N 97.7 %), indicating that the sum of putrescine, cadaverine, tyramine and histamine could be a suitable BAI for rainbow trout stored at 4 °C. BA and BAI increased steeply in CK on day 2 when TVC reached 6.38 log CFU/g, while P1, P2 and N showed sharp increase on day 6 when TVC reached approximately 8 log CFU/g. BAs were evident when TVC was around 7 log cfu/g, which agree with that obtained for sea bass [37]. Since 7 log CFU/g was the acceptability limit of fish during storage period (ICMSF 1986), it indicated that BA accumulation may be related to bacterial spoilage [38]. On day 4 and day 6, BAs and BAI of P2 and P1 were significantly lower (P < 0.05) than those of CK. On day 8 and day 10, P1 showed the lowest BAs and BAI among all examined samples (P < 0.05). The results suggested that paraplantaricin L-ZB1 could suppress the increase of BAs in rainbow trout and the inhibit effect of 200 AU/ml paraplantaricin L-ZB1 was better than that of 400 AU/ml paraplantaricin L-ZB1. Bodmer et al. [39] claimed that formation of BAs depends on the presence of decarboxylase-active microorganisms and the availability of free amino acids. Thus, we may infer that paraplantaricin L-ZB1 as protein complexes was degraded by endogenous enzymes and increase the availability of free amino acids of fish, which agree with the previous hypothesis that accounts for the effect of paraplantaricin L-ZB1 on TVB-N formation. So, that higher bacteriocin resulted in higher BAs. Interestingly, N resulted in significantly higher (P < 0.05) BA and BAI than P1, P2 and CK during the end of the storage (from day 8 to day 10); this proved that BA formation in rainbow trout may be caused from bacteria growth; its actual development may be affected by the bacteriocin-endogenous enzyme interactions. Kurt also reported that nisin increased some BAs in Turkish dry fermented sausage [40].
Changes in total biogenic amine (BA) value (a) and biogenic amine index (BAI) (b) of rainbow trout fillets stored at 4 °C, (×) control; (white square) P1; (black up-pointing triangle) P2; (black square) N. Each point presented is the mean value of three determinations for each sampling. Bars represent the standard deviation
Conclusions
This study showed that 400 AU/ml paraplantaricin L-ZB1-treated samples could effectively inhibit microbial growth, including TVC, Pseudomonas, Enterobacteriaceae and spore-forming bacteria. Meanwhile, the increases of pH, TVB-N, K value and BA levels were significantly delayed in paraplantaricin L-ZB1-treated samples compared to the nisin treated and the control during 4 °C storage period. In addition, it could extend the shelf life of rainbow trout fillets for about 2 days compared with the control. Owing to good protective features exhibited by paraplantaricin L-ZB1, it could be used as a suitable biological fish preservative.
References
Mohan, C. O., Ravishankar, C. N., & Srinivasagopal, T. K. (2008). Journal of the Science of Food and Agriculture, 88, 442–448.
Mexis, S. F., Chouliara, E., & Kontominas, M. G. (2009). Food Microbiology, 26, 598–605.
Rahimabadi, E. Z., Rigi, M., Rahnama, M., Safari, R., Arshadi, A., & Barani, M. (2012). Probiotics and Antimicrobial Proteins, 4, 116–121.
Lu, F., Ding, Y., Ye, X., & Liu, D. (2010). LWT Food Science and Technology, 43, 1331–1335.
Oraei, M., Motalebi, A. A., Hoseini, E., & Javan, S. (2011). Iranian Journal of Fisheries Sciences, 10, 75–84.
Cleveland, J., Montville, T. J., Nes, I. F., & Chikindas, M. L. (2001). International Journal of Food Microbiology, 71, 1–20.
Jack, R. W., Tagg, J. R., & Ray, B. (1995). Microbiological Reviews, 59, 171–200.
Delves-Broughton, J., Blackburn, P., Evans, R. J., & Hugenholtz, J. (1996). Antonie Van Leeuwenhoek, 69, 193–202.
Calo-Mata, P., Arlindo, S., Boehme, K., de Miguel, T., Pascoal, A., & Barros-Velazquez, J. (2008). Food and Bioprocess Technology, 1, 43–63.
De Man, J. C., Rogosa, D., & Sharpe, M. E. (1960). Journal of Applied Microbiology, 23, 130–135.
Reenen, V. (1998). Journal of Applied Microbiology, 84, 1131–1137.
Warm, K., Boknass, N., & Nielsen, J. (1998). Journal of Aquatic Food Product Technology, 7, 45–59.
Özoğul, F., & Özoğul, Y. (2000). Turkish Journal of Zoology , 24, 113–120.
Shi, C., Cui, J., Lu, H., Shen, H., & Luo, Y. (2012). Journal of the Science of Food and Agriculture, 92, 3079–3084.
Veciana-Nogués, M. T., Mariné-Font, A., & Vidal-Carou, M. C. (1997). Journal of Agricultural and Food Chemistry, 45, 2036–2041.
Ryder, J. M. (1985). Journal of Agricultural and Food Chemistry, 33, 678–680.
Aubourg, S. P., Piñeiro, C., Gallardo, J. M., & Barros-Velazquez, J. (2005). Food Chemistry, 90, 445–452.
Gram, L., & Huss, H. H. (1996). International Journal of Food Microbiology, 33, 121–137.
Ward, D. R., & Baj, N. J. (1988). Food Technology, 42, 85–89.
Frangos, L., Pyrgotou, N., Giatrakou, V., Ntzimani, A., & Savvaidis, I. N. (2010). Food Microbiology, 27, 115–121.
Gram, L., & Dalgaard, P. (2002). Current Opinion in Biotechnology, 13, 262–266.
Delgado, A., Brito, D., Fevereiro, P., Peres, C., & Marques, J. F. (2001). Le Lait, 81, 203–215.
Yamato, M., Ozaki, K., & Ota, F. (2003). Microbiological Research, 158, 169–172.
Chumchalova, J., Stiles, J., Josephsen, J., & Plockova, M. (2004). Journal of Applied Microbiology, 96, 1082–1089.
Chin, H. S., Shim, J. S., Kim, J. M., Yang, R., & Yoon, S. (2001). Food Science and Biotechnology, 10, 461–467.
Hernandez, D., Cardell, E., & Zarate, V. (2005). Journal of Applied Microbiology, 99, 77–84.
Hampikyan, H., & Ugur, M. (2007). Meat Science, 76, 327–332.
Carneiro, De Melo, A., Cassar, C. A., & Miles, R. J. (1998). Journal of Food Protection, ® 61, 839–843.
Castro, P., Padrón, J. C. P., Cansino, M. J. C., Velázquez, E. S., & Larriva, R. M. D. (2006). Food Control, 17, 245–248.
Chytiri, S., Chouliara, I., Savvaidis, I. N., & Kontominas, M. G. (2004). Food Microbiology, 21, 157–165.
Sallam, K. I., Ahmed, A. M., Elgazzar, M. M., & Eldaly, E. A. (2007). Food Chemistry, 102, 1061–1070.
Saito, T., Arai, K., & Matsuyoshi, M. (1959). Nippon Suisan Gakkaishi, 24, 749–750.
Ocaño-Higuera, V. M., Maeda-Martínez, A. N., Marquez-Ríos, E., Canizales-Rodríguez, D. F., Castillo-Yáñez, F. J., Ruíz-Bustos, E., Graciano-Verdugo, A. Z., & Plascencia-Jatomea, M. (2011). Food Chemistry, 125, 49–54.
Özogul, F., Özden, Ö., Özoğul, Y., & Erkan, N. (2010). Food Chemistry, 122, 789–794.
Huss, H. H. (1995). Quality and quality changes in fresh fish. Rome: FAO fisheries technical paper.
Rodríguez, C. J., Besteiro, I., & Pascual, C. (1999). Journal of the Science of Food and Agriculture, 79, 1473–1480.
Paleologos, E. K., Savvaidis, I. N., & Kontominas, M. G. (2004). Food Microbiology, 21, 549–557.
Fernández Salguero, J., & Mackie, I. M. (1987). International Journal of Food Science and Technology, 22, 385–390.
Bodmer, S., Imark, C., & Kneubühl, M. (1999). Inflammation Research, 48, 296–300.
Kurt, Ş., & Zorba, Ö. (2010). Journal of the Science of Food and Agriculture, 90, 2669–2674.
Acknowledgments
This work was supported by Beijing Innovation Team of China Agriculture Research Center System (SCGWZJ 20131105-2) and China National Science Foundation (31071591).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gui, M., Zhao, B., Song, J. et al. Paraplantaricin L-ZB1, a Novel Bacteriocin and Its Application as a Biopreservative Agent on Quality and Shelf Life of Rainbow Trout Fillets Stored at 4 °C. Appl Biochem Biotechnol 174, 2295–2306 (2014). https://doi.org/10.1007/s12010-014-1160-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1160-3