Abstract
The present study investigated the impact of exposing strawberry fruits against UV-C intensity, besides basil and eucalyptus essential oils (EOs) with 40 µL on phytochemical content of the strawberry fruit. After treatment, fruits were transferred to 4 °C and the quality parameters comprising weight loss, firmness, antioxidant capacity, total phenolics, ascorbic acid, anthocyanins, titratable acidity, total soluble solids, and decay index were assessed after 1, 4, 8 and 12 days of cold storage. Results showed that all the factors were affected by the applied treatments, as well as the storage duration. The highest levels of firmness were found in BEO (2.47 N) and UV-C (2.44 N) treated fruits without significant difference. At the end of storage, the highest total phenol content (198.21 mg GAE L− 1) and antioxidant activity (3.51 mmol Fe II g− 1 FW) were found in fruits treated with UV-C radiation, as well as l-ascorbic acid content (33.0 mg ascorbic acid/100 g FW) compared to the control group. The highest and lowest decays were recorded in control fruits (80%) and those treated with basil essential oils (43.3%), respectively. Applying UV-C radiation and volatile oils in vapor phase could improve the quality properties of treated strawberry fruits by increasing antioxidant activity due to the preservation of phenolic compounds. Further investigations are required to introduce the most convenient, optimized, and effective methods for increasing the strawberry fruits storage time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Berry fruits consisting of strawberry (Fragaria × ananassa) are consumed as natural antioxidant source, due to their content of bioactive phytoconstituents. Strawberry (Fragaria × ananassa) fruit is the most produced berry worldwide, which is extensively used in human diet as well as in food industries [1]. Polyphenolic compounds are characterized as the most predominant strawberry fruit phytochemicals including ellagic acid, tannins (e.g. ellagitannins), anthocyanins (mainly aglycone and glycosylated cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin), and flavonols (majorly quercetin-rutinoside, quercetin-glucoside, quercetin-glucuronide, and kaempferol-glucuronide) [1, 2].
It has been proven that the above mentioned constituents along with minerals and vitamins are responsible for the radical scavenging power of strawberry fruit [3]. Post-harvesting is a crucial procedure in order to preserve food qualities by mainly reducing exposure risks against various contaminations. Many microorganisms such as bacteria, viruses, fungi, and nematodes are identified as strawberry fruit pathogens [4]. Botrytis cinerea, a fungal strain causing grey mould in strawberry fruit, is one of the major harmful agents, which economically damages strawberry fruit production [5]. In addition to B. cinerea harmful impact, high susceptibility to mechanical damages during the transportation is also a considerable challenge in strawberry fruit during storage period [5, 6].
In the recent years, increasing of consumers’ concerns for hazardous chemical compounds have led to development of low-risk antimicrobial strategies including utilizing of natural products, such as plant essential oils (EOs) [7], and alternative control methods with no residues, such as postharvest ultraviolet-C (UV-C) radiation (180–280 nm) [8]. Antimicrobial properties of EOs are usually reached at high concentrations of these products, which may change food tastes and affect qualitative properties [9]. To reduce these unpleasant impacts on food properties, application of EOs in vapor phase is an alternative option instead of the direct contact use [10].
Noteworthy, use of EOs in vapor-phase at a distance from the foods can control the pathogens without having any negative effects on the qualitative properties of food products [11]. In several studies, EOs (possessing antimicrobial properties) in vapor-phase were beneficially exploited to control postharvest fungal pathogens in various fruits [12,13,14].
In literature, EOs exhibited high antimicrobial effects against diverse range of Gram-positive, and Gram-negative bacteria, in addition to fungi strains [15,16,17,18,19], where these potencies can be employed to control those microorganisms. Activity of EOs and natural compounds is greatly related to phenolic compounds present as constituents in the EO, and to their antioxidant activity and antimicrobial properties [20,21,22,23,24]. Moreover, UV-C exposure has been experimented in different crops such as tomato [8], strawberry [25], sweet cherry [26], table grapes [27] and mango fruits [28], reporting the useful effects on shelf-life extension via reducing pathogen growth. In response to postharvest UV-C radiation, tomato fruit developed biochemical and physical barriers against B. cinerea growth by accumulating phenolic compounds, defense proteins, and by inducing fruit surface modifications [29]. Moreover, it has been reported that the postharvest UV-C radiation induces secondary metabolite production, which protects fruits against abiotic and biotic stresses [6].
Since the development of effective post-harvest strategies can assist foods’ shelf-life extension and quality maintenance, the present study aimed at evaluating the application of pad containing basil (Ocimum basilicum L.) and eucalyptus (Eucalyptus camaldulensis L.) EOs, and exposure to UV-C radiation on variations of bioactive phytonutrient contents and quality traits of strawberry fruit.
Materials and methods
Fruit sample preparation and treatments
Strawberry fruits (Fragaria ananassa Duch. cv. Camarosa) were collected at commercial maturity (level 3/4 red) from a local greenhouse in Dezful, Khuzestan Province, and transported to the laboratory. Several physical characteristics such as size, surface color, uniform ripening, and absence of defects were considered to select the studied fruits. Finally, 384 SFs were selected and divided randomly into four groups for treatments: control, basil essential oil (BEO), eucalyptus essential oil (EEO) and UV-C. Each treatment was carried out in triplicate and stored at 4 ± 1 °C and 90 ± 5% RH (relative humidity). Each replication consisted of eight fruits, which were analyzed in 1, 4, 8 and 12 days of storage life.
Preparation of infected fruits
The fruits were infected as previously described [30]. B. cinerea isolated from the infected strawberry fruits surface was grown on potato dextrose agar (PDA), thereafter the spores were removed after two weeks and suspended in 5 mL of a solution consisting sterile distilled water and Tween 80 (0.05% v/v). The concentration was adjusted to 1 × 106 spores mL− 1, after counting them with a hemocytometric counting chamber. Fruits were disinfected with 2.5% bleach solution, washed carefully with distilled water, and dipped in the respective solutions for 1 min. Then, strawberry fruit groups were inoculated with B. cinerea suspension and air dried for 1 h.
UV-C treatment
One group of the infected fruit was treated by the UV-C radiation produced by three germicidal bulbs, where they were placed 15 cm away from the bulbs. Irradiation was applied for 2 min and fruit received 0.5 kJ m− 2 [31].
Essential oil preparation and treatments
The EOs were extracted from the basil and eucalyptus leaves by hydro-distillation method (Clevenger apparatus) for three hours. In order to prepare pads, the gained EOs (40 µL) were injected into sterile gas with a porous plastic cover, then attached to the polyethylene clamshell lid, and two groups of the infected fruits were treated using these packages.
Fruit decay
The method described by [32] was employed to assess the fruit decay [33]. The observations were categorized using a scale, where no decay: 0%, traces: up to 5%, slight: 5–20%, moderate: 20‒50%, and severe ≥ 50% of the affected surfaces. At the end of experiment (after 12 days), the collected data were used to calculate following index:
Quality assessment
The weight loss was calculated by subtracting the final weight (W2) of the fruit from its initial weight (W1) using the following equation [33]:
A Lutron digital hardness tester (FG-5020, Taiwan) was used to measure fruit firmness. The determinations were carried out by penetrating (diameter of 5 mm) twice the fruit flesh, while the results were expressed in newton (N). Four fruits were considered for each replication [34].
The total soluble solids (TSS) of the strawberry fruit juice were measured with a portable refractometer (UV-Shimadzu, China), and reported as % Brix. The titratable acidity (TA) of the fruit extract was estimated using 0.01 N NaOH to the pH endpoint 8.1. Results are expressed as mg citric acid equivalents (CAE) per 100 g of fresh weight (FW) [34].
Phytonutrient contents and antioxidant effect assessment
The evaluation of ascorbic acid levels in fruit juice was carried out according to the 2,6-dichlorophenol indophenol method [35]. The results are expressed as mg ascorbic acid/100 g FW. The supernatant was obtained from mixture of fruit pulp homogenate (1 g) and 80% methanol, stirred at 200 rpm for 3 h and centrifuged at 6000 rpm for 15 min, which was utilized to analyze antioxidant capacity and total phenolic content. Antioxidant capacity was determined by FRAP (ferric reducing antioxidant power) assay with slight modifications [36].
Furthermore, 80 µL of supernatant was combined with 3.6 mL of FRAP reagent (10 mM of 2,4,6-tripyridyltriazine solution in 40 mM of HCl, 20 mM of FeCl3·6 H2O, and 0.3 M of sodium acetate buffer), then incubated at 37 °C for 50 min. The absorbance was read at 593 nm and compared to a FRAP blank. The obtained data are expressed as mmol Fe II. g− 1 FW. For experimenting of total phenolic content, the Slinkard & Singleton method [37] was followed. Briefly, 200 µL of sample was added to 1.5 mL of 10% Folin–Ciocalteu reagent. Thereafter, 1.5 mL of 6% sodium carbonate solution was added and vortexed after 5 min. A UV-2100 spectrophotometer (UV-Shimadzu, China) was operated to read absorbance after incubation in dark for 90 min, and the results were presented as mg GAE (Gallic acid equivalents) g− 1 FW. Moreover, the pH differential method was applied to determine total anthocyanin concentration [38]. The absorbance was recorded at 520 nm and 700 nm in buffers at pH 1.0 and 4.5, while the findings are stated as mg of pelargonidin-3-glucoside (PG) g− 1 FW.
Statistical analysis
The treatment was analyzed using a completely randomized design (CRD). The two-way variance analysis using SAS (v. 9.1) was applied to examine the quality parameters. The mean differences were determined using LSD (least significant difference) test at p < 0.01.
Results and discussion
Decay index
The fruit decay was elaborated on 12th day of storage. Data analysis of decay index revealed that the treatment effect was considerable at p < 0.01. As demonstrated in Fig. 1, UV-C and EOs exposures effectively inhibited fungal growth, whilst the decay index was recorded as following order: BEO > EEO > UV-C > control groups with maximum difference of ~ 43.3% (Fig. 1). Our results revealed that the application of BEO and EEO in vapor phase was efficient to reduce fruit decay index, possibly due to prevention of the fungal growth on the fruit surfaces. It has been shown that the use of vapor-phase EO increased the resistance of strawberry fruit against B. cinerea through induction of H2O2 burst and several defense related enzymes in the first period of incubation [39].
UV-C treatment significantly inhibited gray mold decay development in strawberry fruits inoculated with B. cinerea. The enhanced activity of antioxidant enzymes could be part of the mechanism(s) of UV-C elicited disease resistance in strawberries [30]. In line with our results, Aloysia citriodora [40] and Pistacia atlantica [41] EOs exhibited a significant antimicrobial activity. In another study, UV-C treatment also resulted in remarkable effect on reducing gray mold decay caused by B. cinerea in strawberry fruits that was not only attributable to direct inhibition of pathogen, but also to enhanced disease resistance mechanisms of the host [39].
Weight loss and fruit firmness
The results showed that the pads containing EEO, BEO, and exposure to UV-C radiation had no significant effect on fruit weight loss, while storage time was significant (p ≤ 0.05). Additionally, the simple impact of treatment (p < 0.05), storage time (p ≤ 0.01), and their interaction (p ≤ 0.05) on firmness was remarkable (Table 1).
As illustrated in Fig. 2A, the group treated with UV-C decreased weight loss (1%), compared to the control group (1.95%); in addition, the EEO (1.62%) and BEO (1.57%) treatments also reduced weight loss. Weight loss mainly occurs due to the transpiration and respiration of fruits. On the other hand, as cell strength decreases and membrane permeability increases over time, the water vapor in the product is released into the surrounding atmosphere and weight of the fruit will be decreased [42]. Other fruits treated with natural compounds [13, 43] and UV-C radiation [26] had reduced WL. It is suggested that water vapor permeability of fruits and vegetables decreases due to the hydrophobic property of the EOs. Although the mechanism of reducing the weight loss by UV-C radiation is not clear, it has been proposed that UV-C radiation through the formation of a thin dried layer on the commodity surface may reduce water loss that may, in turn, restrict water vapor [26].
Furthermore, according to Fig. 2B, a gradual decrease in the value of firmness in all treatments after the 4th day was detected. The highest level of firmness was observed in BEO (2.44 N) and UV-C (2.47 N) treated fruits without significant difference, and the lowest one was recorded in untreated fruits (2.15 N). Fruit firmness is one of the most important quality attributes for strawberry fruit. Fruit tissue becomes soft due to loss of cell wall structure through enzyme activity that reduces hardness of fruit during storage time [5]. Similarly, many studies demonstrated that UV-C radiation maintained firmness by reducing the rate of respiration in strawberry fruit [34, 44]. In addition, application of UV-C on strawberry fruit showed that the treated fruits were firmer and had lower pectate lyase transcript accumulation than the control [8]. It has been shown that UV radiation prevents ethylene synthesis and halts the effective enzymes in the softening of fruits and reduces the rate of respiration which all lead to a delay in the firmness loss [26]. Furthermore, the firmness of peach fruit [13] and tomato [45] treated with cinnamon EO and strawberry treated with basil EO [3] was maintained compared to the control fruits.
Effects of storage time on weight loss (A) and firmness changes (B) of strawberry fruits during cold storage at 5 °C and 90 ± 5% of relative humidity. Data are represented as means ± standard error of three replications. Amount of LSD indicates least significant difference at 5% level of probability using LSD test
Total soluble solids (TSS) and titratable acidity (TA)
The TSS analysis indicated that the simple effect of treatment, storage time and their interaction was significant, while only simple effect of storage time was significant on TA (p ≤ 0.01) (Table 1). As demonstrated in Fig. 3A, BEO showed the least change, with the highest value difference after 4 days of strawberry fruit storage, compared to the first time, whereas there was no considerable difference on 8th days. Finally, the highest and lowest TSS was obtained in control (12.3) and EEO-treated fruits (9.0) with 26.83% at the end of storage (Fig. 3A). The amount of TSS in ripe strawberries stored in cold storage decreases as a result of respiration [1]. The higher levels of TSS were recorded in the control groups than the treated fruits on 12th days.
Figure 3B also shows that TA decreased by increasing of storage period, probably due to increased respiration and degradation of organic acids content [26]. The EEO and UV-C treatment maintained TA better than the others treatment, which did not show significant difference. These findings were in alignment with other researches on strawberry [3, 41], and sweet cherry [26].
Effects of the treatments on total soluble solids (TSS) (A) and titratable acidity (B) of strawberry fruits during cold storage at 5 °C and 90 ± 5% relative humidity. Data are represented as means ± standard error of three replications. Amount of LSD indicates least significant difference at 5% level of probability using LSD test
Bioactive phytonutrients
Our results showed that the effect of storage time, treatment, and their interaction on total phenolic content was significant (p ≤ 0.01), while only simple effect of time was significant on ascorbic acid and anthocyanin contents (p ≤ 0.01). In addition, antioxidant capacity was affected by storage time and treatment at p < 0.01 (Table 2).
Our results furtherly showed that the ascorbic acid amounts were decreased during the storage period (Fig. 4A). Ascorbic acid (ascorbate and vitamin C) is well-identified as natural antioxidant and cofactor in redox reactions. It has been reported that the strawberry fruit ascorbate content varied among the cultivars from 10 to 80 mg/100 g FW [46]. It seems that the reduction of ascorbic acid content may be due to the oxidation of ascorbic acid by ascorbate oxidase to yield dehydroascorbic acid [47]. The EEO and UV-C treatment maintained ascorbic acid better than the other treatments, which did not show significant difference. Similarly, the ascorbic acid content in other fruits such as strawberry [25], sweet cherry [26] and pomegranate [43] was higher in UV-C and EOs treated ones.
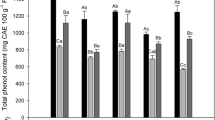
Moreover, as shown in Fig. 4B, the increasing trend of total phenolic content observed in all treatments after 4 days of storage. The highest total phenolic content was also detected in UV-C treated fruits with 45.66% difference compared to the initial day. Then, a decreasing trend was recorded for all the treatment groups up to 8 days of storage except BEO. Finally, all treated fruits showed a significant difference compared to the control, and the highest levels of total phenolic content were measured in fruits treated with UV-C (198.21 mg GAE g− 1 FW) and EEO (163.67 mg GAE g− 1 FW) with 47.75 and 36.47% difference in comparison with the control (103.97 mg GAE g− 1 FW) respectively. The results are consistent with other reports on sweet cherry [26] and peach [48]. Preservation of phenolic compounds was reported in carrot [49] and lettuce [50].
The strawberry fruit samples affected with storage duration possessed remarkable total anthocyanin concentration (Fig. 4C). Total anthocyanin content decreased in both control and treated fruits during storage; however, higher anthocyanin content was recorded in control products compared to the treated ones, which may be due to the water loss of tissue in this treatment. Predictably, a direct correlation between concentration of anthocyanins and water loss can be supposed [51]. Correspondingly, increasing of the anthocyanin content after 4 days of storage has been associated to loss of the fruit moisture. Anthocyanins are a flavonoid class possessing flavylium cation (AH+) structure acting as acidic pigments and responsible for the reddish color in strawberry fruit. Pelargonidin-3-glycoside and cyanidin-3-glycoside are documented as the most important anthocyanins in strawberry fruit [52]. These compounds are mainly known for their ability in free radical scavenging activity. Numerous investigations have proved remarkable antioxidant activity of anthocyanins, which is predominantly due to their chemical structures [53].
According to Fig. 4D, antioxidant capacity increased in treated and untreated fruits during storage time. UV-C radiation (3.51 mmol Fe II g−1 FW) and EEO (3.40 mmol Fe II g−1 FW) was the most effective treatment with 29.91 and 27.64% difference compared to the control (2.46 mmol Fe II g− 1 FW) respectively (Fig. 4D). The beneficial effects of UV radiation have been attributed to the increased levels of phenolic compounds including flavonoids as well as the increased phenylalanine ammonia lyase activity [26, 54]. Our results agreed with the results on strawberry fruits [34]. Taking into note that the UV radiation triggers stress in plant tissues, the synthesis of secondary metabolites possessing antioxidant activity may represent a functional response to the irradiation. These compounds support to maintain the quality, increase the shelf life of fruits and vegetables, besides improving the nutritional value of the product [54]. Likewise, an increased antioxidant activity was described which may be due to the increased phenolic content [20, 43, 49].
Effects of the treatments on ascorbic acid (A), phenolic contents (B), anthocyanin content (C), and antioxidant activity (D) of strawberry fruits during cold storage at 5 °C and 90 ± 5% relative humidity. Data are represented as means ± standard error of three replications. Amount of LSD indicates least significant difference at 5% level of probability using LSD test
Conclusions
Due to the short life of strawberry fruits, finding out effective methods to maintain their freshness and inhibit the spoilage is crucial. The strawberry fruit shelf-life was positively influenced by vapor-phase eucalyptus and basil EOs, and UV-C treatment, which induced higher phytochemical contents compared to the corresponding untreated fruit samples. It can be concluded that the use of EOs in vapor phase and UV-C exposure are potential techniques for shelf-life extension and improvement of both strawberry fruits’ quality characteristics and biochemical parameters during the storage period. Probably, the observed increase of antioxidant activity in fruits treated with EOs and UV is attributed to the preservation of phenolic and ascorbic acid contents. Evaluation of the effects of other plants’ EOs can also be of interest, specifically those possessing potent antimicrobial effects; besides the study of the volatile phytoconstituents in pure forms (e.g. monoterpenes and sesquiterpenes) due to their antimicrobial activities may be considered as a possible option. The future techniques need to be safe, inexpensive, convenient, and effective. Moreover, synergic effects of the UV-C exposure and EOs can be a promising approach.
References
P. Hernández-Muñoz, E. Almenar, V. Del Valle, D. Velez, R. Gavara, Food Chem. 110, 428–435 (2008)
A. Menevseoglu, S. Dıblan, M. Türkyılmaz, M. Özkan, Food Meas. 14(5), 2611–2622 (2020)
L. Mohammadi, A. Ramezanian, F. Tanaka, Food Meas. 15, 353–362 (2021)
A. Maryam, R. Anwar, A.U. Malik et al., Food Meas. 15, 1437–1451 (2021)
S. Petrasch, S.J. Knapp, Mol. Plant Pathol. 20, 877–892 (2019)
M.A. Pombo, H.G. Rosli, G.A. Martínez, P.M. Civello, Postharvest Biol. Technol. 59, 94–102 (2011)
A. Boveiri Dehsheikh, M. Mahmoodi Sourestani, P. Boveiri Dehsheikh, J. Mottaghipisheh, S. Vitalini, M. Iriti, Mini Rev. Med. Chem. 20, 958–974 (2020)
J. Severo, I.R. de Oliveira, A. Tiecher, F.C. Chaves, C.V. Rombaldi, LWT - Food Sci. Technol. 64, 685–692 (2015)
K. Tyagi, A. Malik, Food Chem. 126, 228–235 (2011)
A. Amiri, J. Mottaghipisheh, F. Jamshidi-Kia, K. Saeidi, S. Vitalini, M. Iriti, Appl. Sci. 10, 8103 (2020)
M.J. Velázquez-Nuñez, R. Avila-Sosa, E. Palou, A. López-Malo, Food Control 31, 1–4 (2013)
G. Peretto, W.X. Du, R.J. Avena-Bustillos, S.B.L. Sarreal, S.S.T. Hua, P. Sambo, T.H. McHugh, Postharvest Biol. Technol. 89, 11–18 (2014)
P. Montero-Prado, A. Rodriguez-Lafuente, C. Nerin, Postharvest Biol. Technol. 60, 211–219 (2011)
K.N. Khumalo, P. Tinyane, P. Soundy, G. Romanazzi, M. Glowacz, D. Sivakumar, Sci. Hortic. 214, 195–199 (2017)
V.A. Sabo, P. Knezevic, Ind. Crops Prod. 132, 413–429 (2019)
M. Daneshzadeh, H. Abbaspour, L. Amjad, A. Mohammadi Nafchi, Food Meas. 14, 708–715 (2020)
E. Ostad Asiaei, E. Moghimipour, M.H. Fakoor, Jundishapur J. Nat. Pharm. Prod. 13, e65050 (2018)
A. Semeniuc, C.R. Pop, A.M. Rotar, J. Food Drug Anal. 25, 403–408 (2017)
U. Złotek, K. Rybczyńska-Tkaczyk, M. Michalak-Majewska, M. Sikora, A. Jakubczyk, Appl. Sci. 10, 4315 (2020)
S. Roshanpour, J. Tavakoli, F. Beigmohammadi, S. Alaei, Food Meas. 15, 23–32 (2021)
P. Estakhr, J. Tavakoli, F. Beigmohammadi, S. Alaei, A. Mousavi Khaneghah, Food Sci. Nutr. 8, 2817–2826 (2020)
J. Tavakoli, H. Abbasi, A. Zarei Jelyani, A. Mousavi Khaneghah, J. Food Qual. 5519857 (2021). https://doi.org/10.1155/2021/5519857
R. Afkhami, M. Goli, J. Keramat, Int. J. Food Sci. Technol. 53, 634–643 (2018)
J. Tavakoli, M.J. Rashidi, S.M.B. Hashemi, Curr. Res. Nutr. Food Sci. 13, 319–322 (2017)
R. de Oliveira, G.R. Crizel, J. Severo, C.M.G.C. Renard, F.C. Chaves, C.V. Rombaldi, Plant Physiol. Biochem. 108, 391–399 (2016)
M. Abdipour, P. Sadat Malekhossini, M. Hosseinifarahi, M. Radi, Sci. Hortic. 264, 109197 (2020)
F.K. Sabir, A. Sabir, S. Unal, Erwerbs-Obstbau (2020), doi:https://doi.org/10.1007/s10341-020-00494-x
P. Pristijono, J.B. Golding, M.C. Bowyer, Horticulturae 5, 1 (2019)
M.T. Charles, J. Mercier, J. Makhlouf, J. Arul, Postharvest Biol. Technol. 47, 10–20 (2008)
P. Jin, H. Wang, Y. Zhang, Y. Huang, L. Wang, Y. Zheng, Sci. Hortic. 225, 106–111 (2017)
H. Ebrahimi, S.M.H. Mortazavi, M.E. Khorasani Ferdavani, M. Mehrabi Koushki, J. Food Process. Preserv. (2019). https://doi.org/10.1111/jfpp.14128
M. Shafiee, T.S. Taghavi, M. Babalar, Sci. Hortic. 124, 40–45 (2010)
K. Razzaq, A.S. Khan, A.U. Malik, M. Shahid, S. Ullah, Postharvest Biol. Technol. 96, 23–32 (2014)
S.M.H. Mortazavi, B. Siruie, N. Moalemi, S. Eshghi, Acta Hortic. 1049, 749–754 (2014)
F. Habibi, A. Ramezanian, Food Chem. 227, 1–8 (2017)
F.F. Benzie, J.J. Strain, Anal. Biochem. 239, 70–76 (1996)
K. Slinkard, V.L. Singleton, Am. J. Enol. Vitic. 28, 49 (1977)
J. Lee, R.W. Durst, R.E. Wrolstad, J. AOAC Int. 88, 1269–1278 (2005)
X. Shao, H. Wang, F. Xu, S. Cheng, Postharvest Biol. Technol. 77, 94–101 (2013)
S.M.B. Hashemi, A. Mousavi Khaneghah, M. Koubaa, F.J. Barba, E. Abedi, M. Niakousari, J. Tavakoli, Process Biochem. 65, 197–204 (2018)
M.B. Najafi, R. Habibi, F. Hajimohamadi, J. Tavakoli, S. Madayeni, Chem. Nat. Compd. 50, 376–378 (2014)
H. Lu, L. Li, J. Limwachiranon, J. Xie, Z. Luo, Sci. Hortic. 213, 104–109 (2016)
A. Amiri, A. Ramezanian, S.M.H. Mortazavi, S.M.H. Hosseini, E. Yahia, J. Sci. Food Agric. 101, 778–3786 (2021)
R. Vicente, C.H. Crisosto, G.O. Sozzi, G.A. Manganaris, Postharvest Handling: A Systems Approach, pp. 57–106 (2009). https://doi.org/10.1016/B978-0-12-374112-7.00005-6
H. Aloui, K. Khwaldia, Int. J. Biol. Macromol. Compr. Rev. Food Sci. Food Saf. 15, 1080–1103 (2016)
M. Fenech, I. Amaya, V. Valpuesta, M.A. Botella, Front. Plant Sci. 9, 2006 (2019)
P. Li, X. Yu, B. Xu, J. Food Qual. 8785121 (2017). https://doi.org/10.1155/2017/8785121
M. Abdipour, M. Hosseinifarahi, N. Naseri, Sci. Hortic. 256, 108564 (2019)
Z. Ghorbani, N. Zamindar, S. Baghersad, S. Paidari, S.M. Jafari, L. Khazdooz, Food Meas. 4, 1–12 (2021)
L. Akhbariye, N. Zamindar, S. Nasiri, S. Paidari, G. Mohammad, H. Abbasi, Food Meas. 1–8 (2021). https://doi.org/10.1007/s11694-021-00991-x
D. Zhou, Q. Liu, J. Peng, S. Tu, L. Pan, K. Tu, Postharvest Biol. Technol. 167, 111227 (2020)
H. Liu, D. Zabaras, L.E. Bennett, P. Aguas, B.W. Woonton, Food Chem. 115, 495–500 (2009)
N. Tena, J. Martín, A.G. Asuero, Antioxidants 9, 451 (2020)
N. Mariz-Ponte, S. Martins, A. Gonçalves, C.M. Correia, C. Ribeiro, M.C. Dias, C. Santos, Sci. Hortic. 246, 777–784 (2019)
Acknowledgements
Authors acknowledge the staff of Shahid Chamran University of Ahvaz for comprehensively supporting this study.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, AA; methodology, AA, AR, SV; writing—original draft preparation, AA; review and editing, JM, SV, AR, MI; supervision, MI, MMS. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amiri, A., Mortazavi, S.M.H., Ramezanian, A. et al. Prevention of decay and maintenance of bioactive compounds in strawberry by application of UV-C and essential oils. Food Measure 15, 5310–5317 (2021). https://doi.org/10.1007/s11694-021-01095-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-01095-2