Abstract
In the present investigation, effect of chitosan, Alyssum homolocarpum gum (AHG) and complex (1:1) of chitosan and AHG (CCA) on the properties of nanoencapsulated Mentha piperita phenolic extract (polydispersity index (PDI), particle size, ζ-potential, encapsulation efficiency, release of phenolic compounds and evaluating effect of nanoencapsulation process on the antioxidant activity of phenolic extract of M. piperita in the soybean oil) was studied. The use of a high-pressure homogenizer, at pressure of 11,000 psi, reduced the particle size of W/O/W nanoemulsions coated with chitosan, CCA, and AHG, with a diameter of 108.66, 65.18, and 70.81 nm, respectively. Moreover, the PDI of nanoemulsions in AHG and CCA in all conditions was lower than 0.5, indicating the uniform size distribution and thus the success of the nanoparticle production process. The Intensity curve also showed that the emulsion generated by CCA had better emulsion droplets than the others due to its lower curve width and therefore greater uniformity. Also, the amount of ζ-potential of droplets of emulsions coated with chitosan, CCA and AHG were 28.69, 20.16 and − 37.4 mV, respectively. The results of the peroxide and p-anisidine test revealed that CCA extract caused the best oxidative stability in soybean oil, followed by AHG and chitosan, respectively, which were consistent with the results of the phenolic release test. Gradual and more release of phenolic compounds over time in the CCA-coated sample was superior to other samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxidation reactions are the main cause of spoilage of oils and edible fats during storage or heat treatments such as frying or cooking. Spontaneous oxidation is the most common oxidation phenomenon that occurs through the reaction between oxygen and unsaturated fatty acids by a process involving the chain mechanism of free radicals [1, 2]. To prevent these reactions, antioxidant compounds are used in various foods. TBHQ is a very common synthetic antioxidant that is commercially added to lipids and lipid-rich foods to prevent the oxidation process and increases their shelf life [3, 4]. However, the problems with the use of synthetic antioxidants are well known today, and their anti-health effects have been confirmed. These chemical antioxidants actually increase the lifespan of food and reduce the lifespan of humans [5]. However, the use of natural antioxidants is expanding in the world. One of the ways to increase the oxidative stability of edible oils is to use antioxidant extracts of various plants, whose beneficial results have been reported in many researches. Extracts from plants are important sources of natural antioxidant compounds [6, 7].
The Lamiaceae family is one of the plants that grows in different parts of Iran. These plants are very important because of having essential oil and a variety of terpenoids. Mentha is one of the most important aromatic and perennial medicinal plants in the Lamiaceae family and is widely distributed in the world, with the exception of South America [8,9,10]. The leaves, stems and flowers of plants belonging to this genus are important and rich sources of essential oil, which are widely used in the pharmaceutical, food, cosmetics and health industries [8, 9, 11]. Mentha species include M. spicata, M. piperita and M. longifolia, which have also been reported in Iran. Among mint species, M. piperita species has the most commercial use [12, 13]. A study of the antioxidant properties of different species of Mentha found that their aqueous extracts had good antioxidant activity due to the high extraction of phenolic compounds [11]. In a study, Singh et al. examined the antioxidant activity of essential oil and the petroleum ether extract from M. piperita. The results showed that essential oil and extract of this plant had good antioxidant activity compared to BHT synthetic antioxidant [14].
Phenolic compounds are the main antioxidants in plant extracts. It should be noted that the addition and release of antioxidants at once in foods and oil is one of their practical problems. Because in the early stages of foods preservation, a small amount of antioxidants are needed to prevent oxidation. Also, The enter of phenolic compounds into food has its limitations, including rapid release, low solubility, low permeability, low access, and rapid degradation by environmental compounds. To overcome the above problems, phenolic compounds need to be coated using microencapsulation and nanocapsulation methods [15, 16]. In addition, herbal extracts, if coated, can be added to other formulations, making them easier to produce, use, store, and transfer to food [17]. Today, attention has been paid to biodegradable food polymers for microporous phenolic compounds. The reasons for the tendency to these compounds are due to their biocompatibility, ease of preparation, variety in structure and low cost [18].
Recently, a wide variety of polysaccharide-based nanoparticles have been used to coat natural compounds [19, 20]. Chitosan is a linear polysaccharide with N-acetyl glucosamine and D glucosamine units. Chitosan is widely used as a suitable polymer in pharmaceuticals and food additives. Optimal biological properties of chitosan (such as biodegradability, biocompatibility and low toxicity) have been used as wall materials in the development of transmission systems [21]. Moreover, the use of gums for micro-coating is increasing. Estakhr et al. reported in a study that the combination (1:1) of locust bean gum and chitosan improved the antioxidant activity of the ethanol–water extract of Ferula persica [20]. Other studies have also shown that the use of nano-encapsulation improves the antioxidant activity of various plant extracts in foods and edible oils [22,23,24,25,26]. Alyssum Homolocarpum seed gum is one of the native gums of Iran that has various uses as a stabilizer and thickener (1), which has not been used to cover plant extracts yet [24, 27, 28]. Therefore, according to the aforementioned cases, in the present study, it was decided to investigate the effect of nano-encapsulation process on the antioxidant properties of phenolic extract obtained from M. piperita. For this purpose, the creation of W/O/W nano-emulsions with the help of Alyssum homolocarpum gum (AHG) and chitosan alone and complex (1:1) of chitosan and AHG (CCA) were investigated. Finally, the effect of 300 ppm of the nano-encapsulated extract (based on total phenolic compounds) on oxidative stability of soybean oil compared to TBHQ was investigated.
Materials and methods
Materials
The M. piperita was obtained from Shiraz Agricultural Research Center (Shiraz, Fars, Iran, summer of 2019). After manually cleaning, it was dried in the shadow (20 °C) and afterward, it was milled (Mullinex Depose-Brevete S.G.C.G., France) to get the fine powder. The prepared powders were kept in 4 °C until the day of experiments. Seeds of Alyssum Homolocarpum was purchased from Tabibdaru Company in Shiraz city (summer of 2019). Soybean antioxidant-free oil was prepared from Narges Shiraz oil Company (Shiraz, Fars province, Iran). All the chemicals and solvents used were of analytical reagent grade and supplied by Merck and Sigma–Aldrich chemical companies (Darmstadt, Germany).
Extraction process
First, 50 g M. piperita powder sample was dispersed in 250 mL of ethanol/water (59.6: 40.4) solvent. Then the Erlenmeyer flask of the samples was placed in the ultrasonic bath (DT 102H; BANDELIN) (35 kHz, for 50 min at 65 °C) [20, 23].
Extraction of seed gums of Alyssum Homolocarpum
Seed gum of Alyssum Homolocarpum was extracted at explained condition (water to seed ratio of 40:1, pH 4, temperature = 36.3 °C and time = 60 min) by Koocheki et al. [27].
Biopolymer solutions preparation
Biopolymer (AHG, Chitosan and CCA) solutions was prepared using the explained method by Estakhr et al. [20] and Delfanian et al. [23].
Preparation of W/O/W double emulsions
W/O/W two-layer nano-emulsions are prepared using two emulsion-forming steps. First of all, W/O micro-emulsion was prepared by dropwise addition of 7% M. piperita extract in continuous phase containing 25% span 80 and 68% soybean oil without antioxidant. In the second phase of emulsification, W/O initial micro-emulsion was coated with biopolymers prepared to produce W/O/W double emulsions. As a result, 30% initial W/O emulsion was added to 70% prepared bipolymers and homogenized at 10 °C for 5 min at 12,000 rpm and then at 18,000 rpm for 8 min. Then the homogenizer was used at a pressure of 9500 to 12,500 psi in 3, 5 and 7 cycles (90 s each) to reduce the particle size and to better stabilize the emulsion [22, 29].
Particle size measurement
Average particle size (Z-average), Polydispersity Index (PDI) and particle distribution were measured using dynamic light scattering (DLS) instrument (Zetasizer Nano ZS, Malvern Instruments, Malvern, England) at 25 °C. The samples were diluted by 100 times using deionized water to prevent multiple scattering [20, 23].
ζ-Potential
ζ-Potential was measured by method described by Estakhr et al. [20] and Mohammadi et al. [22].
Freeze drying of nanoemulsions
The preparing nano-emulsions were frozen overnight at − 50 °C and lyophilized in a freeze dryer (Martin Christ, 8891, type 317, Germany) at P = 0.09 mbar and T = 0.01 °C for 48 h. The freeze-dried encapsulated samples were converted into powder with help of a pestle and mortar [23].
Encapsulation efficiency and total phenolic content
Encapsulation efficiency of nano-encapsulated powder was done using method described by Robert et al. [29]. In order to measure the total phenolic content of different samples, described method by Delfanian et al. [23].
Encapsulation efficiency was calculated using the following equation:
where P2 is the surface phenolic compounds and P1 is theoretical total polyphenols content.
Release kinetics
The stability of nano-coatings was measured based on the release of phenolic compounds from W/O/W nano-emulsions. About 12 g of nano-encapsulated samples were poured in sealed dark glass containers. Then they were kept for 6 weeks at 30 °C and at the end of each week, the content of superficial phenolic compounds was determined as described by Delfanian et al. [23]. Velocity constant (k) and half-life time (t1/2) were determined based on semi-logarithmic graph slope according to polyphenolic compounds remaining within the microcapsules during storage. Half-life time of polyphenolic compounds-expressed as a 50% reduction of their initial values in the capsule- was estimated based on curve slope and according to this formula: t1/2= 0.693/k [20, 30].
Peroxide value and p-anisidine value
The determination of peroxide value and p-anisidine value of the different oil samples was done based on the method ascribed by Tavakoli et al. [4] and Estakhr et al. [20], respectively.
Statistical analysis
All experiments were conducted in three replications and the obtained results were analyzed by the aid of analysis of variance (ANOVA) (MStatC). Moreover, the Slide Write and Excel software were employed to design regression and graphs, respectively. Meanwhile the Duncan’s test was applied to compare the mean values.
Results and discussion
Evaluation of nano-emulsion
Distribution and size of emulsion droplets and ζ-potential
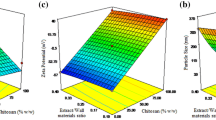
Drop size distribution and polydispersity index (PDI) of multiple emulsions with Chitosan, CCA and AHG were measured using DLS method under diluted conditions. One of the most important and stable parameters determined by DLS is z-average size [31]. In this study, Z-average was defined as the average harmonic diameter. To reduce the size of the droplets and homogenizing, a pressure of 9500 to 12,500 psi was used, and a pressure of 11,000 psi was chosen to create smaller droplets. At higher pressures, larger droplets were observed due to the phenomenon of droplet re-coagulation. To homogenize the emulsions at a pressure of 11,000 psi, time periods 3, 5, and 7 (90 round per second) were used. Previous research has confirmed that if the homogeneous input pressure is higher than the optimal value, the size of the emulsion’s droplets will increase [32, 33]. As shown in Table 1, the lowest z-average size of emulsion droplets generated by Chitosan, CCA, and AHG were 108.66, 65.18, and 70.81 nm, respectively, all observed in time cycle 5. Examination of the results showed that the combined use of Chitosan and AHG had a positive effect on the size of the emulsions. In a similar study using Locust bean gum and Chitosan to nano-emulsion of Ferula persica extract, it was found that similar to the present study, the smallest drop size was observed in the common use of these two compounds [20]. But in a study by Delfanian et al. the use of Hi-Cap 100 alone made it the best z-average size, and the use of different ingredients combined had a negative effect on the test [23]. Differences in emulsifying properties of various coatings (surface activity, surface adsorption rate at the droplet level, ductility and intramolecular interactions in the oil–water joint) can be the reason for differences in the size of the droplets of different emulsions.
Figure 1 shows the size distribution curve of the emulsion droplets prepared in this study based on the intensity distribution parameter. It should be noted that the results given by the z-average size for droplets emulsions population are only physical, and therefore the intensity distribution parameter is more accurate than the z-average size.
Examination of the intensity distribution curve obtained from the present study showed that all the emulsions created in this study were observed in time cycle 5 which was consistent with the results of particle size (Table 1). It was also found that each of the Chitosan, CCA, and AHG curves had a peak at 790, 570, and 490 nm, respectively, indicating the uniformity of droplet size distribution in all samples. Given that the lower width of the intensity distribution curve indicates an amplitude of smaller changes of emulsion particle, it can be concluded that the best and most uniform emulsion of the present study was related to the CCA sample which accords with the findings reported by Estakhr et al. [20]. In this study also found that the emulsion created with complex of Locust bean gum and Chitosan had the uniform droplet size compared to the other treatments. However, it should be noted that the emulsions prepared with the combined coating do not always have an average size of smaller droplets than the single coating. Delfanian et al. and Mohammadi et al. obtained different results from the present study [20, 23]. The reason for the difference in the results of the present study with other studies can be related to the different behavior of biopolymers in relation to the reduction of particle size.
PDI indicates the level of uniformity of the dispersions, which is in the range of zero and 1. If this index is close to zero, it indicates that the dispersion particles are homogeneous in size. If the PDI is greater than 0.5, it indicates the presence of particles of non-uniform size [34]. An examination of the results of Table 1 showed that the PDI of nanoemulsions in AHG and CCA was determined to be less than 0.5 in all conditions, indicating a uniform size distribution and thus the success of the nanoparticle production process. Also in chitosan, this index was close to 0.5 in time cycle 7 and slightly higher than 0.5 in time cycle 3 and 5. Overall, in terms of this test, nano-emulsions created by CCA were the most homogeneous among the various treatments, followed by those prepared by AHG and Chitosan, respectively. A similar study found that PDIs in nanoemulsions created with Locust bean gum and complex of Locust bean gum and Chitosan in three time cycles of 3, 5 and 7 were set between 0.378 to 0.439 and 0.256 to 0.344, respectively, which is less than 0.5 similar to the present study. It was also found that the combined use of Locust bean gum and chitosan also led to the best conditions [20]. In another study, Delfanian et al. reported that the PDI of multiple emulsions created with different natural materials (Hi-Cap 100, complex whey protein isolate-basil seed gum and (soy protein isolate-basil seed) was less than 0.3. In this study, the combined use of a gum and a protein combination (soy protein isolate-basil seed) resulted in the best result in the PDI assay [23]. In colloidal systems, if the functional groups of the constituent components are ionized or the ions in the environment are adsorbed on the surface of the colloidal particles, they cause a surface charge in these systems. The electrostatic repulsion from the surface charge of the nanoparticles prevents the accumulation of particles and thus stabilizes the system. One of the indicators used to investigate the stability of colloidal systems is ζ-potential, which is the surface load index that controls the possibility of interaction between droplets of emulsions [35]. The amount of z-potential drops of two-layer nano-emulsions prepared with different biopolymers is presented in Table 1. As it turns out, the amount of this factor was measured in droplets of emulsions coated with Chitosan, CCA and AHG at 28.69, 20.16 and − 37.4 mV, respectively. Experimental results from many studies have shown that emulsions with a zeta potential of − 30 to + 30 mV will have good stability in terms of electrostatic repulsive force [36]. Therefore, it can be predicted that the droplets of emulsions created by AHG will be more stable than emulsions with chitosan and CCA coatings. In a study, Estakhr et al. examined the properties of multilayer Ferula persica extract nanoemulsions created with different compounds. The results showed that the nanoemulsions created with complex of Locust bean gum and chitosan (ζ-potential = − 9.2) has the highest stability among different treatments [20]. Also, in another study, the amount of z-potential in the emulsions coated with composition of whey protein and basil gum and complex of isolated soy protein and basil seed gum were − 29.2 and − 30.3 mV, respectively [23], which differed from the results of the present study.
Encapsulation efficiency and release properties
Normally, antioxidant compounds are added to foods at once. However, at the beginning of food storage, all of these compounds do not need to be present for antioxidant activity. Over time, during storage, the need for antioxidants to prevent oxidation increases [20]. Encapsulation of antioxidants between two-layer W/O/W nanoemulsions increase their strength. So that the controlled and gradual release of antioxidant compounds from nano-encapsulation has a great impact on the continuation of their antioxidant power in the food environment [37]. In the current study, the encapsulation efficiency and release of polyphenols from the inner phase of double-layered emulsions were determined within 24 days of storage at 30 °C by measuring the total polyphenols. It was revealed that encapsulation efficiency is affected by coating type.
Thus, CCA-coated powder had a higher encapsulation efficiency than other coatings with a lower level of phenols attached to the surface. In general, coating produced with Chitosan, CCA, and AHG had an initial efficiency of 80.5, 90.8, and 88.7%, respectively (Table 2). In a similar study, the encapsulation efficiency of F. persica extract coated with chitosan and locust bean gum was determined between 85.3 to 93.3%, which was different from the results of the present study. It was also found that, as in the present study, the combined use of the two coatings resulted in the best efficiency. In a study conducted by Delfanian et al., the initial encapsulation efficiency by combining basil seed gum with whey and soy proteins was determined 90.9% and 92.88%, respectively, which was close to CCA efficiency [23]. Mohammadi et al. reported that the encapsulation efficiency of W/O/W emulsions covered with whey protein–pectin as an external aqueous phase was 96.64% [38]. Robert et al. also reported an encapsulation efficiency of about 84% for complex of soy proteins and maltodextrin [29]. Also, the results of 24 days of storage at 30 °C showed that the lowest reduction in encapsulation efficiency was observed in CCA-encapsulated powder (19.9%), followed by AHG-encapsulated powder (23%) and Chitosan (24.3%), respectively (Table 2). Previous research has shown that the smaller the emulsion droplets, the greater the encapsulation efficiency of encapsulated powders [39, 40], which was consistent with the results of the present study. The highest efficiency was observed in CCA-encapsulated powder, which had the lowest Z-average size. Estakhr et al. reported similar results in another study [20]. Mohammadi et al. encapsulated the polyphenolic compounds of olive leaf extract among W/O/W nanoemulsions coated with the whey protein–pectin. Their findings showed that a system based on protein–polysaccharide interaction was very effective in reducing the release of polyphenols. So that during 20 days of storage at 30 °C, only 7.7% of the release was created [38]. Benichou et al. also reported that the interaction between whey protein and xanthan gum increased the efficiency and reduced the release of vitamin B1 during 20 days of storage at 25 °C [41]. Hammer et al. also considered the effect of W/O/W nanoemulsions covered with soy protein–sodium caseinate to be very effective in reducing the release process of Resveratrol antioxidant compounds, so that only 10 percent release was observed during storage at 25 °C for 21 days [42]. Sadeghi et al. [43] and Esfanjani et al. [44] also considered the coatings of soy protein isolate and whey protein–pectin to be very effective on the control release of palm fruit extract and saffron extract, respectively, so that the release rate of kernel compounds is less than 12%. In Table 2 and Fig. 2, rate constant (k) and half-life period (t1/2) of encapsulated powders are reported. These two factors were determined by the slope of the semi-logarithmic graph of the values of the remaining phenolic compounds in the micro-coating against the storage conditions. Compared to other coatings, nano-encapsulated powders containing CCA had the lowest constant rate with the longest half-life period (0.011 and 63 days, respectively) during 6 weeks of storage at 30C, followed by AHG. (0.0127 and 54.6 days) and Chitosan (0.0143 and 48.5 days). Another study reported that the amount of constant rate and half-life periods of nano-encapsulated F. persica extract with complex of chitosan and locust bean gum was 0.0092 and 75.3 days [20], respectively, which was different from the results of the present study. Delfanian et al. in a similar study determined the amount of these two parameters mentioned in the nanoencapsulated phenolic extract with combinations of basil seed gum-whey protein and basil seed gum-soy protein 0.0563 and 0.0745 and 12.3 and 9.3 days [23], respectively, which is very different from the results of our finding which may be attributed to the difference in the type of coating material.
Effect of nano-encapsulated extracts on oxidative stability of soybean oil
Checking the oxidative stability of edible oils is one of the most important factors in identifying the strength of antioxidant compounds that are commonly used in aggravated oxidative conditions [45]. In this study, the effect of free and finely nano-encapsulated phenolic extracts of M. piperita on oxidative stability of soybean oil during 24 days of storage at 60 °C was evaluated and peroxide value and p-anisidine value were used to interpret the results.
Peroxide value
The peroxide index determines the amount of hydroperoxides formed during the initial oxidation stage. These compounds are unstable to heat and decompose into alcohols, aldehydes, and ketones [4, 45, 46]. The peroxide value levels of various samples of soybean oil treated by the free and nano-encapsulated extracts of M. piperita and TBHQ during the storage period are shown in Table 3. The initial values of peroxide in different samples of soybean oil were not statistically significant. A study of changes in peroxide values after the incubation period showed that the oil sample containing the nano-encapsulated extract with CCA showed the lowest increase in peroxide value, followed by the samples of oils containing the nano-encapsulated extract with AHG, Chitosan, free extract, and TBHQ and pure soybean oil whose peroxide value was increased by 38.4, 49.5, 77.5, 114.5, 142.3, and 166%, respectively. Therefore, the study of these results showed that the addition of various extracts improved the oxidative stability of soybean oil. It was also found that the nanoencapsulation process increased the antioxidant power of M. piperita phenolic extract. Among the coatings used, CCA coatings had the best effect on antioxidant activity of extract, followed by AHG and Chitosan coatings. The antioxidant activity of chitosan-coated extract in soybean oil was lower due to its very low release. It seems that chitosan has created a very strong wall covering around the inner layer and has blocked the release of phenols. Similar results from other studies, including Najafi et al. were in agreement with the findings of the present study [30]. In a study on the effect of nano-encapsulation process on the antioxidant activity of phenolic extract of Pistacia atlantica skin, it was found that the antioxidant effect encapsulated extract was superior to its free extract. Also, the antioxidant effect of these extracts was weaker than TBHQ [23]. The reason for the difference in results can be attributed to the type and amount of phenolic compounds in the extracts. In a similar study, Estakhr et al. investigated the effect of F. persica nano-encapsulated phenolic extract on the oxidative stability of soybean oil, which found that the combined use of chitosan and locust bean gum caused the best antioxidant effect [20].
p-Anisidine value
The peroxide value is the primary oxidation index and does not alone determine the oxidative stability of edible oils. Therefore, to investigate secondary oxidation, it is necessary to perform a test such as Anisidine value, which is an indicator of the rate of oxidation development and production of secondary products of this reaction [4, 9, 12]. Table 4 shows the p-Anisidine value of different oil treatments during 24 days of storage at 60 °C.
The rate of this index at the moment of zero was between 1.33 and 1.41, and there was no significant difference between them. Moreover, the study of changes in the p-anisidine value over time showed that after incubation, the amount of this factor in different samples reached between 1.76 and 2.12. These results indicated that unlike peroxid value, there was not considerable difference between different samples in changing the anisidine index. In other words, the use of different extracts, unlike the peroxide value test, could not have a strong effect on the secondary oxidation of soybean oil compared to the oil sample containing TBHQ and pure oil. In a study conducted by Delfanian et al., it was found that unlike the present study, nanoencapsulation of phenolic extract of P. atlantica skin extract using a combination of whey protein and basil seed gum and a combination of soy protein isolate and basil seed gum prevented oxidation better than free extract [23].
In many scientific papers, it has been reported that natural antioxidants extracted from various plants have the ability to compete with TBHQ and even perform better than it, only at concentrations above 5 times [47,48,49]. However, in the present study, a high level of antioxidant activity was achieved at lower concentrations of phenolic compounds by nano-encapsulation mechanism by multilayer nanoemulsion. These results were in agreement with other published scientific results, including the studies of Estakhr et al. [20], Mohammadi et al. [22], Esfanjani et al. [43], and Carneiro et al. [50]. They also reported that encapsulation of bioactive compounds among the two-layer W/O/W nanoemulsions coated with protein–polysaccharide compounds led to the gradual release and control of phenolic compounds and the improvement of their antioxidant activity.
Phenolic compounds release
In order to better identify the performance of nano-encapsulated extracts, the release of phenolic compounds from them into soybean oil during storage at 60 °C for 24 days was investigated (Fig. 3). As seen, the highest release rate of phenolic compounds was observed in soybean oil containing CCA-coated extract, followed by samples with micronutrient M. piperita extract coated with AHG and chitosan coatings. The results of the peroxide value revealed that CCA provided the best oxidative stability in soybean oil, followed by AHG and chitosan, respectively, which were consistent with the results of the phenolic compounds release test. The addition of more phenolic compounds from CCA-coated phenolic extract to soybean oil was the reason for the superiority of this coating. Similar to the results presented in this study, Estakhr et al. [20], Esfanjani et al. [44], Carneiro et al. [50], Kaimainen et al. [51] reported the use of phenolic compounds coated with W/O/W nanoemulsions, compared to non-encapsulated types, can increase the stability and biological activity of phenolic compounds. Belščak-Cvitanović et al. reported that encapsulation of phenolic compounds from some medicinal plants using the alginate-chitosan system compared to the free extract could increase the antioxidant power of these compounds [47]. In contrast to the present study, Delfanian et al. reported that the nano-encapsulation of phenolic extract of P. atlantica skin extract could not delay the oxidation of soybean oil with the help of a combination of basil seed gum with whey protein and soy protein, respectively [23]. Differences in the type of coatings used and the way they are coated have led to different results in various studies.
Conclusion
In the present study, M. piperita phenolic extract was coated in two-layer W/O/W nano-emulsions encapsulaed with Chitosan, AHG and complex (1:1) of Chitosan and AHG (CCA) as external phase. Examination of the properties of the resulting emulsions using various tests showed that the use of CCA resulted in the best W/O/W nano-emulsions, followed by AHG and Chitosan, respectively. The results of oxidative stability tests also showed that CCA caused the highest resistance to oxidation in soybean oil, followed by AHG and chitosan, respectively, which were consistent with the results of the release test of phenolic compounds. The release rate of phenolic compounds in soybean oil environment in incubation conditions was a good indication of the controlled release of these compounds using nano-encapsulation prepared by CCA. Overall, the findings show that nanoencapsulation of natural antioxidant compounds is a good way for increasing their antioxidant activity in foods over time and making optimal use of them.
Change history
06 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11694-020-00631-w
References
N.T. Dunford, in Oxidative Stability of Sunflower Seed, ed. by E. Martnez-Force, N.T. Dunford, J.J. Salas (AOCS Press, Urbana, 2015), pp. 465–489
J. Tavakoli, T. Emadi, S.M.B. Hashemi, A. Mousavi Khaneghah, P.E.S. Munekata, J.M. Lorenzo, M. Brnčić, F.J. Barba, Chemical properties and oxidative stability of Arjan (Amygdalus reuteri) kernel oil as emerging edible oil. Food Res. Int. 107, 378–384 (2018)
J.A. Michiels, C. Kevers, J. Pincemail, J.O. Defraigne, J. Dommes, Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 130, 986–993 (2012)
J. Tavakoli, K.H. Hajpour Soq, A.R. Yousefi, P. Estakhr, M. Dalvi, A. Mousavi Khaneghah, Antioxidant activity of Pistacia atlantica var mutica kernel oil and it’s unsaponifiable matters. J. Food Sci. Technol. 56, 5336–5345 (2019)
J.M. Lorenzo, A. Mousavi Khaneghah, M. Gavahian, K. Marszałek, I. Eş, P.E. Munekata, I.C.F.R. Ferreira, F.J. Barba, Understanding the potential benefits of thyme and its derived products for food industry and consumer health: from extraction of value-added compounds to the evaluation of bioaccessibility, bioavailability, anti-inflammatory, and antimicrobial activities. Crit. Rev. Food Sci. Nutr. 59, 2879–2895 (2018)
A. Szydłowska-Czerniak, A. Tułodziecka, Antioxidant capacity of rapeseed extracts obtained by conventional and ultrasound-assisted extraction. J. Am. Oil Chem. Soc. 91, 2011–2019 (2014)
D.B. Muñiz-Márquez, G.C. Martínez-Ávila, J.E. Wong-Paz, R. Belmares-Cerda, R. Rodríguez-Herrera, C.N. Aguilar, Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L. and their antioxidant activity. Ultrason. Sonochem. 20, 1149–1154 (2013)
Sh Khakzad, F. Rahmani, M. Hojjati, M.R. Tabandeh, Anti-carcinogenic effects of Satureja khuzistanica and Zataria multiflora essential oils on K562 cell line proliferation. J. Food Bioprocess. Eng. 2, 127–132 (2019)
Y. Salmaki, Sh Zarre, R. Govaerts, Ch. Bräuchler, A taxonomic revision of the genus S tachys (Lamiaceae: Lamioideae) in Iran. Bot. J. Linn. Soc. 170, 573–617 (2012)
M.S. Bazrafshani, B. Kalantari Khandani, A. Pardakhty, H. Tajadini, R. Malek Pour Afshar, V. Moazed, A. Nemati, N. Nasiri, H. Sharifi, The prevalence and predictors of using herbal medicines among Iranian cancer patients. Complement Ther. Clin. Pract. 35, 368–373 (2019)
H.J. Dorman, M. Koşar, K. Kahlos, Y. Holm, R. Hiltunen, Antioxidant properties and composition of aqueous extracts from mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 51, 4563–4569 (2003)
M. Abootalebian, J. Keramat, M. Kadivar, F. Ahmadi, M. Abdinia, Comparison of total phenolic and antioxidant activity of different Mentha spicata and M. longifolia accessions. Ann. Agric. Sci. 61, 175–179 (2016)
A. Benabdallah, Ch. Rahmoune, M. Boumendjel, O. Aissi, Ch. Messaoud, Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of Algeria. Asian Pac. J. Trop. Biomed. 6, 760–766 (2016)
R. Singh, M.A.M. Shushni, A. Belkheir, Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 8, 322–328 (2011)
A. Munin, F. Edwards-Lévy, Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 3, 793–829 (2011)
Z. Fang, B. Bhandari, Encapsulation of polyphenols—a review. Trends Food Sci. Technol. 21, 510–523 (2010)
L. Sagalowicz, M.E. Leser, Delivery systems for liquid food products. Curr. Opin. Colloid Interface Sci. 15, 61–72 (2010)
A.F. Esfanjani, S.M. Jafari, Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 146, 532–543 (2016)
F. Sadegh Hassani, A. Mohammadi Nafchi, Preparation and characterization of bionanocomposite films based on potato starch/halloysite nanoclay. Int. J. Biol. Macromol. 67, 458–462 (2014)
P. Estakhr, J. Tavakoli, F. Beigmohammadi, Sh Alaei, A. Mousavi Khaneghah, Incorporation of the nanoencapsulated polyphenolic extract of Ferula persica into soybean oil: assessment of oil oxidative stability. Food Sci. Nutr. 8, 2817–2826 (2020)
N.A. Negm, H.H.H. Hefni, A.A. Abd-Elaal, E.A. Badr, M.T.H. Abou Kana, Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 152, 681–702 (2020)
A. Mohammadi, S.M. Jafari, E. Assadpour, A.F. Esfanjani, Nano-encapsulation of olive leaf phenolic compounds through WPC–pectin complexes and evaluating their release rate. Int. J. Biol. Macromol. 82, 816–822 (2016)
M. Delfanian, S.M.A. Razavi, M.H. Haddad Khodaparast, R. Esmaeilzadeh Kenari, S.H. Golmohammadzadeh, Influence of main emulsion components on the physicochemical and functional properties of W/O/W nano-emulsion: effect of polyphenols, Hi-Cap, basil seed gum, soy and whey protein isolates. Food Res. Int. 108, 136–143 (2018)
R. Esmaeilzadeh Kenari, Z. Raftani Amiri, A. Motamedzadegan, J. Mohammadzadeh Milani, J. Farmani, R. Farahmandfar, Optimization of Iranian golpar (Heracleum persicum) extract encapsulation using sage (Salvia macrosiphon) seed gum: chitosan as a wall materials and its effect on the shelf life of soybean oil during storage. J. Food. Meas. Charact. (2020). https://doi.org/10.1007/s11694-020-00528-8
E.M. Mekawi, A.M. Sharoba, M.F. Ramadan, Reduction of acrylamide formation in potato chips during deep-frying in sunflower oil using pomegranate peel nanoparticles extract. J. Food. Meas. Charact. 13, 3298–3306 (2019)
A. Seyed Yagoubi, F. Shahidi, M. Mohebbi, M. Varidi, Sh Golmohammadzadeh, Extraction and encapsulation of Laurus nobilis leaf extract with nano-liposome and its effect on oxidative, microbial, bacterial and sensory properties of minced beef. J. Food Meas. Charact. 12, 378–385 (2018)
A. Koocheki, S.A. Mortazavi, F. Shahidi, S.M.A. Razavi, R. Kadkhodaee, J.M. Milani, Optimization of mucilage extraction from Qudume Shirazi seed (Allyssum homolocarpum) using response surface methodology. J. Food Process Eng. 33, 861–882 (2010)
A. Mohammadi Nafchi, A. Olfat, M. Bagheri, L. Nouri, A.A. Karim, F. Ariffin, Preparation and characterization of a novel edible film based on Alyssum homolocarpum seed gum. J. Food Sci. Technol. 54, 1703–1710 (2017)
P. Robert, T. Gorena, N. Romero, E. Sepulveda, J. Chavez, C. Saenz, Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 45, 1386–1394 (2010)
M.N. Najafi, R. Kadkhodaee, S.A. Mortazavi, Effect of drying process and wall material on the properties of encapsulated cardamom oil. Food Biophys. 6, 68–76 (2011)
A. Bryła, G. Lewandowicz, W. Juzwa, Encapsulation of elderberry extract into phospholipid nanoparticles. J. Food Eng. 167, 189–195 (2015)
G. Kolb, K. Viardot, G. Wagner, J. Ulrich, Evaluation of a new high-pressure dispersion unit (HPN) for emulsification. Chem. Eng. Technol. 24, 293–296 (2001)
P. Marie, J. Perrier-Cornet, P. Gervais, Influence of major parameters in emulsification mechanisms using a high-pressure jet. J. Food Eng. 53, 43–51 (2002)
R. Lutz, A. Aserin, L. Wicker, N. Garti, Release of electrolytes from W/O/W double emulsions stabilized by a soluble complex of modified pectin and whey protein isolate. Colloids Surf. B. Biointerfaces 74, 178–185 (2009)
J. Rao, D.J. McClements, Food-grade microemulsions and nanoemulsions: role of oil phase composition on formation and stability. Food Hydrocoll. 29, 326–334 (2012)
A. Laouini, C. Jaafar-Maalej, S. Sfar, C. Charcosset, H. Fessi, Liposome preparation using a hollow fiber membrane contactor—application to spironolactone encapsulation. Int. J. Pharm. 415, 53–61 (2011)
R. Bagheri, R. Izadi Amoli, N. Tabari Shahndasht, S.R. Shahosseini, Comparing the effect of encapsulated and unencapsulated fennel extracts on the shelf life of minced common kilka (C lupeonella cultriventris caspia) and P seudomonas aeruginosa inoculated in the mince. Food Sci. Nutr. 4, 216–222 (2016)
A. Mohammadi, S.M. Jafari, A.F. Esfanjani, S. Akhavan, Application of nanoencapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 190, 513–519 (2015)
A. Soottitantawata, F. Bigeardb, H. Yoshiia, T. Furutaa, M. Ohkawarac, P. Linkod, Microencapsulation of l-menthol by spray drying and its release characteristics. Innov. Food Sci. Emerg. Technol. 6, 107–114 (2005)
L. Salvia-Trujillo, E.A. Decker, D.J. McClements, Influence of an anionic polysaccharide on the physical and oxidative stability of omega-3 nanoemulsions: antioxidant effects of alginate. Food Hydrocoll. 52, 690–698 (2016)
A. Benichou, A. Aserin, N. Garti, W/O/W double emulsions stabilized with WPI–polysaccharide complexes. Colloid Surf. A-Physicochem. Eng. Asp. 294, 20–32 (2007)
Y. Hemar, L.J. Cheng, C.M. Oliver, L. Sanguansri, M. Augustin, Encapsulation of resveratrol using water-in-oil-in-water double emulsions. Food Biophys. 5, 120–127 (2010)
S. Sadeghi, A. Madadlou, M. Yarmand, Microemulsification–cold gelation of whey proteins for nanoencapsulation of date palm pit extract. Food. Hydrocoll. 35, 590–596 (2014)
A.F. Esfanjani, S.M. Jafari, E. Assadpoor, A. Mohammadi, Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. J. Food Eng. 165, 149–155 (2015)
Y. Zhang, L. Yang, Y. Zu, X. Chen, F. Wang, F. Liu, Oxidative stability of sunflower oil supplemented with carnosic acid compared with synthetic antioxidants during accelerated storage. Food Chem. 118, 656–662 (2010)
S.R. Javadian, S.R. Shahosseini, P. Ariaii, The effects of liposomal encapsulated thyme extract on the quality of fish mince and Escherichia coli O157: H7 inhibition during refrigerated storage. J. Aquat. Food Prod. Technol. (2017). https://doi.org/10.1080/10498850.2015.1101629
A. Belščak-Cvitanović, R. Stojanović, V. Manojlović, D. Komes, I.J. Cindrić, V. Nedović, B. Bugarski, Encapsulation of polyphenolic antioxidants from medicinal plant extracts in alginate–chitosan system enhanced with ascorbic acid by electrostatic extrusion. Food Res. Int. 44, 1094–1101 (2011)
M. Taghvaei, S.M. Jafari, A.S. Mahoonak, A.M. Nikoo, N. Rahmanian, J. Hajitabar, N. Meshginfar, The effect of natural antioxidants extracted from plant and animal resources on the oxidative stability of soybean oil. LWT-Food. Sci. Technol. 56, 124–130 (2014)
A.A.A. Mohdaly, M.A. Sarhan, A. Mahmoud, M.F. Ramadan, I. Smetanska, Antioxidant efficacy of potato peels and sugar beet pulp extracts in vegetable oils protection. Food Chem. 123, 1019–1026 (2010)
H.C. Carneiro, R.V. Tonon, C.R. Grosso, M.D. Hubinger, Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 115, 443–451 (2013)
M. Kaimainen, S. Marze, E. Järvenpää, M. Anton, R. Huopalahti, Encapsulation of betalain into w/o/w double emulsion and release during in vitro intestinal lipid digestion. LWT-Food. Sci. Technol. 60, 899–904 (2015)
Acknowledgements
Authors would like to express their sincere gratitude to Islamic Azad University, Kermanshah Branch.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roshanpour, S., Tavakoli, J., Beigmohammadi, F. et al. Improving antioxidant effect of phenolic extract of Mentha piperita using nanoencapsulation process. Food Measure 15, 23–32 (2021). https://doi.org/10.1007/s11694-020-00606-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00606-x