Abstract
The objective of this study was to determine the bioactive potential of hazelnut meal protein hydrolysates. Hazelnut meal protein isolate was hydrolyzed using Alcalase and Trypsin + Chymotrypsin to 23.5% and 13.7% degrees of hydrolysis, respectively. The peptide fractions (< 5 kDa and > 5 kDa) were screened for the in vitro inhibition of angiotensin I-converting enzyme (ACE), dipeptidyl peptidase-IV (DPP-IV), and α-glucosidase activities. Peptide fractions > 5 kDa showed a higher potency to inhibit ACE (IC50 = 0.10–0.13 mg/mL), whereas peptide fractions < 5 kDa were more effective in inhibiting DPP-IV (IC50 = 0.37–0.45 mg/mL) and α-glucosidase (IC50 = 3.62–3.89 mg/mL), with no significant difference in treatment with Alcalase and Trypsin + Chymotrypsin. The results of the study showed that hazelnut meal protein is a potential source of bioactive peptide delivery and that the hydrolysates obtained could be used as an alternative ingredient for the development of new functional foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioactive peptides, which are hidden in food proteins, can be released through hydrolysis via gastrointestinal digestion, fermentation, or in vitro enzymatic hydrolysis. They have been identified as potential therapeutic agents because of their wide range of preventive effects against chronic diseases. Some of these beneficial health effects include ACE-inhibitory, antioxidative, antimicrobial, immunomodulatory, antithrombotic, and antidiabetic effects [1].
Angiotensin I-converting enzyme is related to the renin angiotensin system. This enzyme regulates peripheral blood pressure by facilitating the conversion of angiotensin from an inactive decapeptide (angiotensin-I) to a powerful vasoconstrictor octapeptide (angiotensin-II), and it also inactivates the antihypertensive vasodilator bradykinin [2]. As the inhibition of ACE is considered an alternative approach to preventing and treating hypertension, some synthetic ACE inhibitors are widely used as antihypertensive drugs. Due to certain adverse effects reported, much attention has been focused recently on food-derived bioactive peptides as a natural alternative or complement to the synthetic drugs [3].
Diabetes mellitus (DM) is a major health problem worldwide, and it is expected to be acquired by 550 million people by 2030. Among all cases, 90% have been reported as Type II DM (T2DM). Dipeptidyl peptidase-IV is a metabolic serine protease that causes the degradation and inactivation of incretin hormones and is responsible for stimulating insulin secretion [4]. Alpha-amylase and α-glucosidase are key enzymes involved in starch digestion, and the rate and extent of starch digestion are important parameters for healthy and diabetic patients. Therefore, inhibiting both DPP-IV and digestion-related enzymes has become a major therapeutic target for the control of T2DM by contributing to a significant reduction in blood glucose levels [5, 6].
Studies have been conducted on animal and plant-derived proteins. Nevertheless, especially oil meals, have become popular in recent years as a sustainable protein source. Hazelnuts (Corylus avellana L.) are a major product of Turkey, which produced 515,000 and 776,000 tons of hazelnuts in 2018 and 2019, respectively. With approximately 70% of total global production, Turkey is the largest hazelnut producer in the world [7]. It is well known that 90% of hazelnuts are used in food industries as shelled nuts [8], and during hazelnut industrial production some by-products are produced (shell, green leafy cover, and skin). Hazelnuts are an effective source of health-promoting compounds and have a high protein content (10.86–16.30 g/100 g), with nine essential amino acids (except tryptophan in some studies) [9]. Hazelnut meal (oil cake), obtained after the extraction of hazelnuts for oil production, is generally used as a feed ingredient because of its high protein content of approximately 55% [3], making the hazelnut meal a potential low-cost source of bioactive peptides.
Amino acid sequences and the extent of hydrolysis are key parameters in the bioactive peptide potential of proteins. To date, hazelnut protein (intact) and protein hydrolysates have mainly been studied for their functional and rheological properties [10,11,12], in vitro ACE-inhibitory activity [3, 12,13,14,15,16], and antioxidant activity [17]. An in silico study showed a high probability of the release of bioactive peptides with DPP-IV and ACE-inhibitory activities following hydrolysis of the ribosomal hazelnut proteins with papain, thermolysin, and bromelain [18]. No reports have been made on the in vitro antidiabetic potential of hazelnut meal protein hydrolysates.
In this study, hazelnut meal protein isolate was hydrolyzed using Alcalase, Protamex, and Trypsin + Chymotrypsin enzymatic processes. The objectives of the study were (i) to generate hazelnut meal protein hydrolysates and fractionate the hydrolysates into peptides of various molecular weights, (ii) to investigate the potential ACE inhibitory activity, and (iii) to investigate the potential DPP-IV and α-glucosidase inhibitory activities.
Materials and methods
Materials
Altaş Gıda (Ordu, Turkey) kindly provided hazelnut meal. Alcalase 2.5L and Protamex 2.5L were kindly provided by Novozymes (Bagsvaerd, Denmark). Trypsin from porcine pancreas (T0303), α-Chymotrypsin from bovine pancreas (C7762), ACE from rabbit lung (A6778), N-[3-(2-Furyl)acryloyl]-Phe-Gly-Gly (FAPGG) (F7131), intestinal acetone powders from rat (I1630), and 4-Nitrophenyl α-d-glucopyranoside (N1377) (PNPG) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Vivaspin 20 ultrafiltration membrane (5kDa) was procured from Sartorius Stedim Biotech (Goettingen, Germany), and the DPP-IV drug discovery kit (BML-AK499) was obtained from Enzo Life Sciences (Lausen, Switzerland). Pierce BCA protein assay kit was purchased from Thermo Fisher Scientific (Rockford, USA). Mini Protean® TGXTM gel (12%) was obtained from Bio-Rad Laboratories (Hercules, CA, USA). Other reagents used were of analytical grade.
Protein isolation from hazelnut meal
The hazelnut meal was first defatted by mixing with hexane (1:3 w/w) for 1 h and for three times. After filtration and following evaporation at room temperature overnight, the almost defatted meal was processed to acetone powder using a method previously described by Arcan and Yemenicioǧlu [19] with slight modification. The meal was mixed with cold acetone (70%, − 18 °C), and the mixture (1:6 w/w) was homogenized for 3 min in a Waring blender (8011Eb, USA). The slurry obtained was centrifuged (8000×g), and the solid residue was collected. This step was repeated twice. Finally, the collected residue was left overnight to evaporate the solvent and then stored at − 18 °C until the analysis was carried out.
For protein isolation, acetone washed meal was suspended in water (1:12.5 w/w, 0.15% Na2SO3), and then the pH of the slurry was adjusted to 9.5 with 2 M NaOH. This slurry was mixed using magnetic stirring for 30 min, and pH was readjusted if necessary. The slurry was then centrifuged at 10,000×g for 15 min. The protein in the supernatant was collected, and the residue was extracted once more. The combined supernatants were then precipitated at isoelectric point (pH 4.5) for 30 min. Precipitated proteins were collected by centrifugation, and suspended in water by adjusting pH to 7.0, lyophilized (Armfield, FT 33 Vacuum Freeze Drier, England), and stored at − 18 °C for analysis.
Preparation of hazelnut meal protein hydrolysates
Hazelnut meal protein isolate was hydrolyzed separately using different enzymes (Alcalase 2.5 L, Protamex 2.5 L, Trypsin + Chymotrypsin). Briefly, hazelnut protein was dispersed in water (1%), and the pH of the mixture was adjusted to 8.0 with 2 M NaOH for about 10 min. Hydrolysis was initiated by adding each enzyme at an enzyme/substrate ratio (10% w/w). Hydrolysis was carried out for 2.5 h, adjusting the pH 8.0 using 0.1 N NaOH solution by a pH–stat device (Kyoto Kem AT 510, Japan) at 50 ℃ for Alcalase, Protamex and 40 ℃ for Trypsin + Chymotrypsin. After the hydrolysis, the reaction was terminated by immersing the hydrolysate in boiling water for 10 min, cooled to room temperature, and then centrifuged at 10,000×g for 15 min. The hydrolysis degree (DH) of samples was determined using the amount of NaOH used according to the following equation given by Adler-Nissen [20]:
where B is the base consumption in liters; Nb is the normality of the base; α is the average degree of dissociation of α-NH2 groups; MP refers to a mass of protein (N × 6.25) in kg; htot is a total number of peptide bonds in the protein substrate (meqv/g protein) (htot = 8 meqv/g for proteins other than soybean, 1/α = 1.13 for pH 8.0, 50 °C).
Hydrolysates were then ultrafiltered (Vivaspin 20, Germany), wherein fractions with higher and lower than 5 kDa were collected and lyophilized for further analysis.
Protein content
The protein content of hazelnut meal and hazelnut meal protein isolate was determined using a LECO FP-528 instrument (Leco Instruments, UK) in accordance with the AOAC 992.23 method [21]. The results were obtained as N (%), and the protein content (%) calculated by using conversion factor of 6.25. The soluble protein content of hydrolysates was determined by the BCA protein assay kit (Thermo Pierce, Rockford, USA) according to the kit instructions using bovine serum albumin as standard.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE)
The electrophoretic profile of hazelnut meal protein isolate and protein hydrolysates was determined using Bio-Rad Mini Protean TGX gel (12%). Samples were prepared with 1:5 sample buffer containing SDS, bromophenol blue, glycerol, Tris–HCl, and β-mercaptoethanol. Following incubation at 95 °C for 5 min, 15 µL samples were loaded onto the gel, and electrophoresis was applied at 100 V with Mini-Protean® Tetra Cell apparatus (Bio-Rad, China). The resolved protein bands were stained with Coomassie blue and then destained for clear visualization.
Antihypertensive activity of protein hydrolysates
The antihypertensive activity of protein hydrolysates was determined in vitro by measuring their inhibitory effects on the ACE with a modification of the method given by Shalaby et al. [22]. Briefly, 10 µL of ACE (0.125 units/mL) was mixed with 10 µL of sample at different concentrations. The enzyme-sample mixture was incubated for 10 min at 37 °C, and the enzymatic reaction was initiated by adding 150 µL 1 mM FAPGG substrate solution (prepared in 0.05 M Tris-HCl with 0.3 M NaCl, pH 7.5) pre-incubated at 37 °C. The assay was performed in 96 well microtiter plates (UV flat bottom) using the Thermo Scientific Varioskan Flash spectrophotometer (USA). The absorbance of the reaction mixture was monitored at 340 nm for 30 min at 37 °C, whereas the ACE inhibitory activity was determined from the slope of the initial linear portion of absorbance versus the time curve. The ACE inhibition (%) was expressed as the IC50 value, or peptide concentration needed to inhibit by 50% of the original ACE activity.
Antidiabetic activity of protein hydrolysates
DPP-IV inhibitory activity
The DPP-IV inhibitory activity of the samples was determined in vitro using the DPP-IV drug discovery analysis kit, and according to the kit instructions. Briefly, the DPP-IV enzyme was diluted to 17.3 µU/µL with buffer, and 15 µL enzyme was transferred into the microplate. Then 35 µL buffer (blank) or sample at different concentrations were added and incubated for 10 min at 37 °C. Following the addition of 50 µL chromogenic substrate of H-Gly-Pro-pNA, the absorbance of the reaction mixture was monitored at 405 nm for 20 min at 37 °C. The inhibitory activity of the samples was determined from the slope of the absorbance versus time curve and expressed as the IC50 value, or peptide concentration needed to inhibit by 50% of the original DPP-IV activity.
α-glucosidase inhibitory activity
The α-glucosidase inhibitory activity of protein hydrolysates was determined in vitro using the method described by Koh et al. [5] with some modifications. Briefly, 70 µL sample at different concentrations were added to 10 µL enzyme (15 mg/mL), and the mixture was incubated at 37 °C for 10 min. After the pre-incubation period, 20 µL PNPG (30 mM) was added as a substrate, and further 10 min incubation was applied. The reaction was terminated by the addition of 100 µL 1 M Na2CO3, and the absorbance was read at 400 nm using the Thermo Scientific Varioskan Flash spectrophotometer (USA). All analysis was performed by substituting the active enzyme with the inactive one denatured at 100 °C for 10 min. The inhibitory activity of the enzyme was calculated using the following formula:
where Acontrol, Acontrol blank, Asample, and Asample blank refer to the absorbance value of reaction vial containing active enzyme and buffer; inactive enzyme and buffer; active enzyme and sample; inactive enzyme and sample, respectively. The substrate was present in all of these vials.
Statistical analysis
All analysis was performed as two replicates and two parallels. IC50 values were calculated using the Graphpad 8.0 program. All of the data were subjected to IBM SPSS 25.0 to evaluate the differences in mean values using one-way analysis of variance (ANOVA) and Tukey test. Values of p less than 0.05 were considered statistically significant.
Results and discussion
Isolation of proteins
In this study, the protein content of hazelnut meal was 47.9%. The total protein content of the meal was lower than the 54.4% determined by Aydemir et al. [12] and similar to the 46.8% determined by Emre et al. [23]. Aydemir et al. [12] showed that hazelnut meal is a promising source of protein extraction, as it has a similar protein content to soybean meal and a higher protein content than sunflower and rapeseed meals (32–35%). Before the protein was extracted, the meal was treated with hexane for defatting, and acetone powder was prepared to remove the residual oxidized lipids and soluble phenolics. During alkaline extraction, a solution containing sodium sulfite was used to prevent the oxidation of the remaining polyphenols. Following alkaline extraction, isoelectric precipitation, and lyophilization, a light brown protein isolate was obtained. The protein content of the isolate was 94.2%, consistent with the 93.3% found for acetone washed and extracted meal in Aydemir et al.’s study [12].
Degree of hydrolysis (DH)
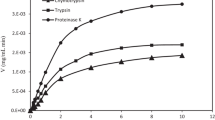
The hydrolysis profile was used to follow the extent to which peptide bonds were broken down to release shorter peptides. Figure 1 shows the kinetics of the hydrolysis of hazelnut meal protein by Alcalase, Protamex, and Trypsin + Chymotrypsin under different experimental conditions. For the first 20 min, the progress of hydrolysis was characterized by a high rate of all enzymatic treatments, and then the rate subsequently decreased. This initial high-rate period was related to the maximum cleavage of peptide bonds [24]. Hydrolysis was continued at a slower rate for Alcalase and Protamex after 20 min and almost stopped for Trypsin + Chymotrypsin after 20 min. At the end of the hydrolysis reaction (150 min), the maximum degree of hydrolysis obtained was 23.5%, 18.8%, and 13.7% for the Alcalase, Protamex, and Trypsin + Chymotrypsin treatments, respectively. No progressive increase was observed after 150 min (data not shown).
Alcalase, a serine endopeptidase, is usually limited to a maximum of 25% in hydrolysis due to its specificity [25]. Trypsin and Chymotrypsin are serine proteases. Chymotrypsin selectively hydrolyzes peptide bonds with aromatic or large hydrophobic side chains (Tyr, Trp, Phe, Met, Leu) at the carboxyl end of the bond, and Trypsin is selective for peptides on the C-terminal side of lysine and arginine residues. The lower hydrolysis degree of the combination of Trypsin + Chymotrypsin could be related to this specific selectivity. The results of the present analysis are consistent with those reports. The results obtained in the present study are lower than those obtained in the study of Cucu et al. [26], in which the hydrolysis of hazelnut proteins by pepsin for 3 h yielded hydrolysates with 35% DH.
SDS-PAGE
Hazelnut proteins can be classified as cupin superfamily (salt-soluble globulins), prolamin superfamily (water-soluble albumins), pollen-related proteins (part of the defense mechanism of the plant), and regulatory proteins (accumulated in seed plants in response to high-temperature stress) [27].
SDS-PAGE analysis under reduced conditions was performed to identify the protein composition of native hazelnut meal protein isolates and their enzyme-treated hydrolysates to investigate the effect of hydrolysis and the formation of smaller peptides. Figure 2 shows the SDS-PAGE profiles of hazelnut meal protein isolate and its hydrolysates. The molecular weight distributions of hazelnut meal protein were similarly observed by Prieto et al. [28] and Saricaoglu et al. [29] for hazelnut protein. In the hazelnut meal protein, all enzymatic processes hydrolyzed the protein into smaller peptides at varying levels and intensified low molecular fragments (lower than 10 kDa). For both the Alcalase and Trypsin + Chymotrypsin treatments, all the hydrolyzed fractions were lower than 20 kDa, whereas a band higher than 20 kDa remained in the Protamex hydrolysate. Thus, Alcalase- and Trypsin + Chymotrypsin-treated samples were selected for further analysis.
ACE inhibitory activity
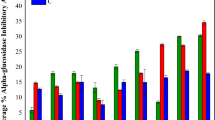
The possible antihypertensive activities of protein hydrolysates were determined based on ACE inhibitory capacities. Fractions obtained by ultrafiltration have been analyzed for their ability to inhibit ACE. The fractions of hydrolysates produced from the two enzymes exhibited different levels of inhibition (Table 1). No significant difference (p > 0.05) was observed for Trypsin + Chymotrypsin (IC50 = 0.10 ± 0.01 mg/mL) and Alcalase (IC50 = 0.13 ± 0.01 mg/mL) treatments for fractions > 5 kDa, and the lowest activity (highest IC50) was reported for the < 5 kDa fraction treated with Alcalase (IC50 = 0.18 ± 0.05 mg/mL). The lowest ACE inhibitory activity for the Alcalase-treated hydrolysate of the < 5 kDa fraction could be related to the further hydrolysis of ACE inhibitory peptides into less potent forms. Such a decrease in ACE inhibitory activity with an increase in the degree of hydrolysis was also reported for whey protein using a crude protease from Lactobacillus helveticus LB13 [30], Jatropha curcas protein using Alcalase [25], and sunflower protein using Alcalase and Flavourzyme [31]. Segura-Campos et al. [25] reported the highest ACE inhibitory activity for 5–10 kDa fractions of the Alcalase-treated hydrolysate of Jatropha curcas protein. The higher number of hydrophobic and aromatic amino acids in the C-terminal content in the > 5 kDa fractions could be responsible for the higher ACE inhibitory activity [32].
Several ACE inhibitory activities for hazelnut proteins and their hydrolysates have been reported in the literature. Aydemir et al. [12] reported the ACE inhibitory activities of hazelnut meal protein obtained under different extraction conditions and an IC50 value of 0.57–1.0 mg/mL. The lower IC50 values in the present study could be attributed to enzymatic hydrolysis, which led to the production of bioactive peptides. Liu et al. [3] reported three peptides (AVKVL, YLVR, and TLVGR) from Alcalase-treated hazelnut protein, with an IC50 value of 73.06, 15.42, and 249.3 µM, respectively, without any information related to the total hydrolysate. Gülseren et al. [13] reported that Trypsin-hydrolyzed hazelnut meal protein showed approximately 40% ACE inhibition after 240 min hydrolysis. Eroğlu and Aksay [33] examined the hydrolysis of hazelnut protein with pepsin and obtained ACE inhibition with an IC50 value of 0.22 mg/mL after 60 min of hydrolysis. Çağlar et al. [15] reported ACE inhibitory activity with IC50 values ranging between 0.13 and 4.83 mg protein/mL for Fast protein liquid chromatography (FPLC) fractions of thermolysin, trypsin, or chymotrypsin hydrolysates of hazelnut cake protein. Liu et al. [14] reported the highest ACE inhibitory activity as 72.31% for an Alcalase-treated hazelnut peptide fraction following gel filtration chromatography and RP-HPLC. Using an in silico approach, Gülseren [18] reported potential bioactive peptides with ACE inhibitory activity in ribosomal hazelnut proteins hydrolyzed with gastrointestinal and non-gastrointestinal enzymes. Write et al. [34] and Li et al. [35] reported higher IC50 values for peanut (0.295 mg/mL) and pistachio (0.87 mg/mL), respectively.
DPP-IV inhibitory activity
The control of DPP-IV activity is an important strategy for regulating insulin metabolism in the treatment of T2DM. All hazelnut meal protein hydrolysates inhibited DPP-IV activity in a dose-dependent manner (data not shown). In general, for each enzymatic process, DPP-IV inhibitory activity was significantly higher (p < 0.05) for < 5 kDa fractions, which were almost 2.9-fold lower IC50 on average, than that for > 5 kDa fractions (Table 1). This result is consistent with previous studies that reported peptide fractions of a relatively small size, showing better DPP-IV inhibitory activity [36]. No significant difference (p > 0.05) was found between Alcalase (IC50 = 0.37 ± 0.00 mg/mL) and Trypsin + Chymotrypsin (0.45 ± 0.11 mg/mL) hydrolysates for < 5 kDa fractions. Conversely, for > 5 kDa fractions, Alcalase-treated hydrolysates had a significantly higher (p < 0.05) inhibitory activity (IC50 = 1.06 ± 0.14 mg/mL) than Trypsin + Chymotrypsin-treated hydrolysates (IC50 = 1.28 ± 0.01 mg/mL). Several studies have examined in vitro DPP-IV inhibitory activity, especially for milk proteins, but the number of studies on plant protein hydrolysates is limited. The highest DPP-IV inhibitory activity (IC50 = 0.37 mg/mL) reported in the present study was higher than those reported by Nongonierma and Fitzgerald [6] (0.73–3.54 mg/mL) for hemp, pea, rice, and soy hydrolysates and by Nongonierma et al. [37] (0.88 mg/mL) for quinoa protein hydrolysate prepared with papain hydrolysis for 3 h and lower than that for navy bean hydrolysates (0.093 mg/mL) following digestion with pepsin/pancreatin [38]. To date, this is the first time that the in vitro DPP-IV inhibitory activity of hazelnut protein hydrolysates has been reported. The results showed that both ultrafiltration fractions, especially the < 5 kDa fractions, had potential DPP-IV inhibitory activity. This result is also supported by Gülseren [18], who reported in an in silico analysis that ribosomal hazelnut proteins had a higher frequency of DPP-IV inhibitory peptides than egg, soy, and milk proteins.
α-glucosidase inhibitory activity
One of the strategies for diabetic patients is to inhibit carbohydrate-hydrolyzing enzymes to control glucose homeostasis. Alpha-glucosidase inhibitors are commonly used as oral hypoglycemic drugs that help maintain normal blood sugar levels and prevent hyperglycemia. These peptides generally have 3–6 amino acid residues, particularly OH-containing amino acid residues or basic amino acid residues at the N-terminal. Methionine and Alanine at the C-terminal and Proline close to the C-terminal have been reported to be involved in the inhibitory activity of α-glucosidase [39].
The fractions obtained by ultrafiltration were analyzed for their ability to inhibit α-glucosidase. Fractions of < 5 kDa were found to have α-glucosidase inhibitory activity with IC50 value of 3.62 ± 0.72 mg/mL and 3.89 ± 0.12 mg/mL for Alcalase and Trypsin + Chymotrypsin hydrolysates, respectively (Table 1). However, > 5 kDa fractions had a significantly lower (p < 0.05) inhibitory potential for Alcalase hydrolysate (IC50 = 4.76 ± 0.12 mg/mL), and a lower than 50% inhibition was observed for Trypsin + Chymotrypsin hydrolysate within the studied concentrations. The results obtained in the present study are lower than those obtained by Vilcacundo et al. [40] (IC50 = 1.64 mg/mL) after the intestinal digestion of quinoa for < 5 kDa fractions. Similarly, > 5 kDa fractions did not show any α-glucosidase inhibition. Gu et al. [41] reported that almond oil manufacture residue protein hydrolyzed with ProteAx and Protease M showed glucosidase inhibition with IC50 value of 2.08 mg/mL for < 5 kDa fractions. The results obtained in the present study are comparable with those in the study of González-Montoya et al. [42], in which digested germinated soybean by pepsin and pancreatin yielded hydrolysates with an IC50 value of 3.73 mg/mL. The hazelnut meal protein hydrolysate was found to be more potent than that determined by Alcalase-treated hemp seed hydrolysate with IC50 value of 5 mg/mL [43] and soybean protein hydrolyzed with alkaline protease with IC50 of 4.94 mg/mL [44]. As the hydrolysates obtained by Alcalase and Trypsin + Chymotrypsin were crude peptide mixtures, the possible purified fractions could have the potential for a higher α-glucosidase inhibitory activity.
Conclusion
The bioactive potential of hazelnut meal protein hydrolysates was demonstrated in this study. Commercially available proteases were used to hydrolyze hazelnut meal protein isolates. The high hydrolysate yields and gel electrophoretic profiles of the hydrolysates indicated the efficient hydrolysis of hazelnut meal protein into low molecular weight peptides. Hydrolysates prepared from hazelnut meal protein exhibited significant in vitro inhibitory activities against ACE, DPP-IV, and α-glucosidase. The inhibitory activities did not differ significantly between the types of enzymes used for hydrolysis. However, peptides > 5 kDa showed a higher potency to inhibit ACE, whereas peptides < 5 kDa were more effective in inhibiting DPP-IV and α-glucosidase. This study is the first to report the production of hazelnut meal protein hydrolysates with strong in vitro antidiabetic potential. The results obtained for ACE inhibitory activity were also promising with the action of Alcalase and Trypsin + Chymotrypsin hydrolysis. Hazelnut meal protein hydrolysates could be a potential food ingredient in formulations, especially for people with diabetes and hypertension. The study also showed the valorization of an agro-industrial byproduct into a value-added food ingredient for sustainable production. Future research are required to purify and identify the sequence of peptides responsible for antidiabetic and antihypertensive activities and determine their resistance to gastrointestinal digestion conditions.
References
H. Korhonen, A. Pihlanto, Int. Dairy J. 16, 945–960 (2006)
P. Mudgil, B. Jobe, H. Kamal, M. Alameri, N. Al Ahbabi, S. Maqsood, LWT-Food Sci. Technol. 101, 251–258 (2019)
C. Liu, L. Fang, W. Min, J. Liu, H. Li, Food Chem. 245, 471–480 (2018)
A. Lambeir, C. Durinx, S. Scharpé, I. De. Meester, Crit. Rev. Cl. Lab. Sci. 40(3), 209–294 (2003)
L.W. Koh, L.L. Wong, Y.Y. Loo, S. Kasapis, D. Huang, J. Agric. Food Chem. 58(1), 148–154 (2010)
A.B. Nongonierma, R.J. Fitzgerald, Food Diges. 6, 19–29 (2015)
Tarım Ürünleri Piyasaları: Fındık (2020) https://arastirma.tarimorman.gov.tr/tepge. Accessed 8 Aug 2020
T. Esposito, F. Sansone, S. Franceschelli, P. Del Gaudio, P. Picerno, R.P. Aquino, T. Mencherini, Int. J. Mol. Sci. 18, 392–404 (2017)
C. Alasalvar, F. Shahidi, J.S. Amaral, B.P.P. Oliveira, in Tree Nuts: Composition, Phytochemicals, and Health Effects. ed. by C. Alasalvar, F. Shahidi (CRC Press, Boca Raton, 2008), p. 185
G.M. Sharma, M. Su, A.U. Joshi, K.H. Roux, S.K. Sathe, J. Agric. Food Chem. 58, 5457–5464 (2010)
F. Tatar, M.T. Tunç, T. Kahyaoglu, J. Food Sci. Technol. 52(2), 1024–1031 (2013)
L.Y. Aydemir, A.A. Gökbulut, Y. Baran, A. Yemenicioǧlu, Food Hydrocolloid 36, 130–142 (2014)
İ Gülseren, B. Çakır, A.F. Çağlar, J. Food 44(2), 309–317 (2019)
C. Liu, Y. Yu, F. Liu, L. You, Food Nutr. Sci. 10(11), 1374–1387 (2019)
A.F. Çağlar, B. Çakır, İ Gülseren, Eur. Food Res. Technol. 247, 1189–1198 (2021)
E.Ç. Eroğlu, K. Oztop, S. Aksay, J. Microbiol. Biotechnol. Food Sci. 10(1), 78–82 (2020)
C. Liu, D. Ren, J. Li, L. Fang, J. Wang, J. Liu, W. Min, J. Funct. Foods 42, 203–215 (2018)
İ Gülseren, J. Food Meas. Charact. 12(4), 2607–2614 (2018)
I. Arcan, A. Yemenicioglu, Food Chem. 103, 301–312 (2007)
J. Adler-Nissen, J. Chem. Technol. Biotechnol. 34, 215–222 (1984)
AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th edn. (AOAC International, Maryland, USA, 2000)
S.M. Shalaby, M. Zakora, J. Otte, J. Dairy Res. 73, 178–186 (2006)
Y. Emre, H. Sevgili, M. Şanlı, Aquac. Res. 39(3), 324–328 (2008)
F. Shahidi, X.Q. Han, J. Synowiecki, Food Chem. 53, 285–293 (1995)
M.R. Segura-Campos, F. Peralta-González, A. Castellanos-Ruelas, L.A. Chel-Guerrero, D.A. Betancur-Ancona, Biomed Res. Int. (2013). https://doi.org/10.1155/2013/541947
T. Cucu, C. Platteau, I. Taverniers, B. Devreese, M. De. Loose, B. De. Meulenaer, Food Control 30, 497–503 (2013)
C. Nitride, G. Picariello, G. Mamone, P. Ferranti, in Proteomics in Food Science: From Farm to Fork, ed. M. L. Colgrave, (Elsevier Academic Press, 2017), pp. 107–122. https://doi.org/10.1016/B978-0-12-804007-2.00007-2
N. Prieto, C. Burbano, E. Iniesto, J. Rodríguez, B. Cabanillas, J.F. Crespo, M.M. Pedrosa, M. Muzquiz, J.C. del Pozo, R. Linacero, C. Cuadrado, Foods 3(2), 279–289 (2014)
F.T. Saricaoglu, O. Gul, A. Besir, I. Atalar, J. Food Eng. 233, 98–108 (2018)
Y. Guo, D. Pan, M. Tanokura, Food Chem. 114, 328–333 (2009)
C. Megias, J. Pedroche, M. del Mar Yust, M. Alaiz, J. Giron-Calle, F. Millan, J. Vioque, LWT-Food Sci. Technol. 42, 228–232 (2009)
H.G. Akıllıoğlu, S. Karakaya, Eur. Food Res. Technol. 229, 915–921 (2009)
E.C. Eroglu, S. Aksay, Ind. J. Pharm. Educ. 51(3), 417–420 (2017)
B.L. White, T.H. Sanders, J.P. Davis, LWT-Food Sci. Technol. 56(2), 537–542 (2014)
P. Li, J. Jia, M. Fang, L. Zhang, M. Guo, J. Xie, Y. Xia, L. Zhou, D. Wei, Process Biochem. 49(5), 898–904 (2014)
R. Liu, J. Cheng, H. Wu, Int. J. Mol. Sci. 20, 2–22 (2019)
A.B. Nongonierma, S. Le. Maux, C. Dubrulle, C. Barre, R.J. FitzGerald, J. Cereal Sci. 65, 112–118 (2015)
L. Mojica, K. Chen, E.G. de Mejia, J. Food Sci. 80, 188–198 (2015)
E.D. Stefano, T. Oliviero, C.C. Udenigwe, Curr. Opin. Food Sci. 20, 7–12 (2018)
R. Vilcacundo, C. Martínez-Villaluenga, B. Hernández-Ledesma, J Funct. Foods 35, 531–539 (2017)
X. Gu, T. Gao, Y. Hou, D. Li, L. Fu, LWT-Food Sci. Technol. 134, 110215 (2020)
M. González-Montoya, B. Hernández-Ledesma, R. Mora-Escobedo, C. Martínez-Villaluenga, Int. J. Mol. Sci. 19, 2–14 (2018)
Y. Ren, K. Liang, Y. Jin, M. Zhang, Y. Chen, H. Wu, F. Lai, J. Funct. Foods 26, 439–450 (2016)
R. Wang, H. Zhao, X. Pan, C. Orfila, W. Lu, Y. Ma, Food Sci. Nutr. 7, 1848–1856 (2019)
Funding
I would like to thank Ege University Scientific Research Council for their financial support (Project number: 15-MUH-065).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no any conflict of interest/competing interest related to the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Simsek, S. Angiotensin I-converting enzyme, dipeptidyl peptidase-IV, and α-glucosidase inhibitory potential of hazelnut meal protein hydrolysates. Food Measure 15, 4490–4496 (2021). https://doi.org/10.1007/s11694-021-00994-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00994-8