Abstract
This study was performed to investigate the effectiveness of ultrasound-assisted osmotic dehydration on reducing foodborne pathogens (Escherichia coli, Enterococcus faecalis, Salmonella Typhi and Shigella sonnei), as well as its impact on moisture content and antioxidant properties of Saturn peach. Peach samples were inoculated and underwent ultrasonic waves (50 and 75% amplitudes). Afterwards, the osmotic dehydration was performed by immersing the samples in 70% sucrose solution (for 4, 8 and 12 h). Results indicated that ultrasound pre-treatment markedly diminished the microbial count and the reduction levels of pathogens enhanced with the increase of ultrasound amplitude. The greatest reduction in pathogens was observed at 75% amplitude after 12 h storage in osmotic solution, in which the mean number of E. coli, E. faecalis, S. Typhi and S. sonnei was reduced to 0.1, 4.2, 3.7 and 0.1 log CFU/g, respectively. Additionally, osmotic dehydration treatment significantly reduced the amount of moisture content, total phenolics and DPPH antiradical activity of samples. It should be added that increasing the ultrasound amplitude led to a further decrease in the mentioned parameters.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Saturn peach (Prunus persica var. platycarpa) is one of the varieties of peaches that is called by this name because of its appearance. This type of peach is flat and plate-shaped. Its flesh is white with yellow and red skin, and it is smaller and sweeter than traditional peaches [1]. Due to the high percentage of moisture, this product has a high metabolic activity, which is effective in its transportation and storage. Microbial growth, discoloration, bad taste, and reduced nutritional value are common factors in quality loss that occur in the storage of high-moisture fruit [2]. Therefore, moisture removal is widely used to increase the shelf life and quality of agricultural products after harvest. Meanwhile, traditional heat treatment for reducing the amount of water in fruit is recommended as a suitable method for moisture reduction and increasing the shelf life. This process increases the stability of the food by reducing water activity and the growth of microorganisms. But on the other hand, this method has disadvantages that can be attributed to the long duration of the process, the reduction in nutritional value and the quality of the food. On the other hand, processing at low temperatures has been shown to increase the prevalence of foodborne illness in recent decades [3]. Therefore, due to the tendency of consumers to healthy and nutritious foods, the use of non-thermal methods that can effectively eliminate pathogens has been considered.
Osmotic dehydration (OD) is the partial separation of water from the plants tissues by direct contact with a suitable concentrated solution. In this process, by immersing fruit or vegetables in a hyper-tonic solution, their cell wall acts as a semi-permeable membrane. Due to the presence of concentration gradient between the hyper-tonic solution and the intracellular fluids, the stimulus force for the water diffusion from the tissues into the osmotic solution is formed. As a result, water and small constituents of cells are leaked into the osmotic solution and simultaneously solutes are transported to the plant cells [4]. Although several studies have shown that high osmotic pressure affects the population of pathogens and reduces their survival [5, 6], this reduction may not be adequate with regard to food safety. Therefore, in order to increase the efficiency of microbial inactivation, other nonthermal methods must be utilized as a hurdle technology to ensure that the number of microorganisms is sufficiently diminished.
In recent years, the use of ultrasound (US) waves in the food industry as a method of non-destructive processing has received much attention [7, 8]. This technology is recommended by Food and Drug Administration (FDA), which can be used as an alternative to the thermal treatment of foods [9]. The effect of US on the inactivation of microorganisms is related to the cavitation phenomenon, which is caused by the process of formation, growth and disintegration of bubbles in the liquid medium. Since US alone cannot completely inactivate microorganisms, this method is used along with other technologies or as assisted-method. Although many studies have demonstrated the effects of US and osmosis on microbial inactivation and food quality [10, 11], very limited data have been reported about the impact of ultrasound-assisted osmotic dehydration (UAOD) on pathogens and antioxidant properties of food products. Hence, the objective of this study was to determine the impact of US treatment and OD on reducing numbers of four food-borne pathogenic bacteria (Escherichia coli, Enterococcus faecalis, Salmonella Typhi and Shigella sonnei) on Saturn peach. Moreover, antioxidant properties and moisture content of the treated samples were assessed.
Materials and methods
Bacterial strains
Escherichia coli PTCC 1533, Enterococcus faecalis PTCC 1393, Salmonella Typhi PTCC 1609 and Shigella sonnei PTCC 1777 were obtained from the culture collection at Iran Institute of Industrial and Scientific Research (Tehran, Iran). Each microorganism was cultured in 25 mL Mueller Hinton broth (Merck KGaA, Darmstadt, Germany) at 37 °C for 18 h, and centrifugation was done at 3500 × g for 20 min. The final pellets were resuspended in buffered peptone water to obtain 9.1–9.3 log CFU/mL.
Sample preparation and treatments
Saturn peaches and white sugar (sucrose) were purchased from a local distributor and a local market, respectively, in Shiraz city, Fars province, Iran. Approximately, 70 g of sugar was dissolved in 100 mL tap water to produce a solution with 70% sugar (w/v). Then, heat treatment of the solution was done at 80 °C for 10 min. The °Brix measured was 7.3–7.4. Approximately, 100 μL of each pathogen resulting to 9.1–9.3 log CFU/mL of each strain separately was spotted on the Saturn peach samples. Subsequently, the samples were air dried at 25 °C for 2 h before process to allow microorganism attachment. For osmotic dehydration and sonication treatment, 25 g of inoculated samples were transferred into 100 mL of sugar solution in a sterile 500 mL flask and sonication treatment (Hielscher ultrasonic device; UP100H, Germany; 100 W, 30 kHz) was performed at 0, 50 and 75% amplitudes for 15 min. Temperature of medium was kept at 40 °C using a water bath (ZENITH LAB, China) and osmotic dehydration process was carried out for 4, 8 and 12 h.
Bacterial enumeration
Each sample was transferred to a sterile stomacher bag containing 0.1% peptone water and homogenization was carried out for 2 min with a stomacher (BagMixer 400 W, Interscience Co., France). For enumeration of E. faecalis, Brain heart infusion agar was used and for other strains Nutrient agar was applied. After that, incubation was done at 37 °C for 24–48 h and colonies were counted.
Moisture content
Approximately 2 g of sample was placed in a vacuum oven (SH-VDO-08NG; SH-Scientific; South Korea) at 70 °C for 24 h and moisture content (MC) was calculated [12].
Total soluble phenolics
A 50 µL of sample extract was mixed with 800 µL of water and 25 µL of Folin–Ciocalteu’s reagent (0.25 N), and incubation was carried out for 5 min at 25 °C. After that, 100 µL sodium carbonate (1 N) was mixed with the solution and incubation was done for 2 h. The absorbance was read at 750 nm using a UV–VIS spectrophotometer [13, 14].
DPPH radical scavenging activity
Five grams of each sample was mixed with 20 mL of methanol and after shaking (30 s), centrifugation was performed at 5000 g for 15 min. Then, 150 µL of supernatant was mixed with 2850 µL of DPPH solution in methanol (0.047 g/L) and kept for 20 h at room temperature. Subsequently, absorbance of the solution at 517 nm was read [14, 15].
Statistical analysis
All tests were performed in triplicate (mean ± SD) and Statistical analyses were performed with ANOVA and significant differences at P < 0.05 were carried out by Duncan’s multiple range tests using SPSS package program (SPSS Inc., Chicago, IL, USA).
Results and discussion
Reduction levels of pathogenic bacteria in treated Saturn peach
The effects of US treatment and OD against pathogenic bacteria (E. coli, E. faecalis, S. Typhi and S. sonnei) artificially inoculated on Saturn peach are illustrated in Fig. 1a–d. The initial population of mentioned bacteria (9.1–9.3 log CFU/g) in Saturn peach were significantly decreased (P < 0.05) when subjected to US pre-treatment alone (Fig. 1a). In addition, reduction levels of the four pathogenic bacteria enhanced with the increase of US amplitudes from 50 to 75% compared to the control (unsonicated) sample. Treatment with 50% amplitude US reduced the numbers of E. coli, E. faecalis, S. Typhi and S. sonnei to 8.1, 8.2, 8.4 and 8.5 log CFU/g, respectively while the amplitude of 75%, decreased their population to 7.7, 7.7, 8 and 7.9 log CFU/g, respectively. The impact of US on the inactivation of pathogens is related to a physical phenomenon which is called cavitation [16]. Cavitation is the process by which a vapor bubble forms in an area of low-pressure liquid. US works by creating intense compressive waves in a liquid medium. These waves create flow in the liquid and cause the rapid formation of microbubbles. The growth and unification of these bubbles and reaching the maximum size eventually leads to bursting. The shear force resulting from the explosion of bubbles as well as the turbulent currents caused by the sound vibration lead to detachment and destruction of bacterial cells from the food product [17]. Similarly, Sagong et al. [18] demonstrated that treating lettuce by US significantly reduced the cell number of Salmonella Typhimurium, Listeria monocytogenes and Escherichia coli O157:H7 which was due to the physical separation of pathogens from the surface of lettuce. Moreover, Kordowska-Wiater and Stasiak [16] revealed that the US treatment led to effective reduction of microbial numbers on the surface of chicken skin. The transmission of microorganisms from the surface of food into the aqueous solution was recognized. As it was seen, with the increase in US amplitude, the number of pathogens notably decreased. The amplitude indicates the intensity and strength of the US. As the intensity of the US increases (high-power US), the zone of liquid medium undergoes cavitation increases during US treatment. As a result, cavitation is increased, leading to an increase in local pressure and the formation of free radicals, which has a greater effect on inactivating microorganisms [19]. Previous study has confirmed our findings about higher bacterial reduction levels at higher US amplitude [20].
Effect of ultrasound-assisted osmotic dehydration treatment against pathogenic bacteria (E. coli, E. faecalis, S. Typhi and S. sonnei) artificially inoculated on Saturn peach. Error bars indicate the standard deviation of microbial population in treated samples. a Ultrasound pretreatment at 50 and 100% amplitude, b osmotic dehydration for 4 h, c osmotic dehydration for 8 h, d osmotic dehydration for 12 h. The same lowercase letters are not significantly different between various bacteria strains within the same study treatment at P > 0.05
After the US pre-treatment, the treated peaches were subjected to the OD for 4, 8 and 12 h (Fig. 1b–d). Based on the data, it was found that the population of pathogenic bacteria significantly reduced with the osmotic process duration (P < 0.05). The greatest impact of treatments on bacterial reduction was observed for E. coli and S. sonnei, followed by S. Typhi and E. faecalis. In addition, the results showed that the microbial reduction in the sonicated samples was markedly higher than the control sample (unsonicated) under OD condition. The highest decrease in the population of studied pathogens was observed in 75% amplitude US, so that after 12 h of OD, the mean number of E. coli, E. faecalis, S. Typhi and S. sonnei was reduced to 0.1, 4.2, 3.7 and 0.1 log CFU/g, respectively. During the OD, when bacterial cells are exposed to a concentrated solution with higher osmotic pressure than intracellular pressure, cellular water is diffused into the osmotic solution via the plasma membrane. This leads to a phenomenon called plasmolysis, which prevents microbial growth [21]. Studies have shown that osmotic treatment effectively destabilizes the cell membrane and thus causes cell death. In fact, two mechanisms have been reported for the lethal impact of OD, one is the phase transition and structural modification of membrane phospholipids and the other is the cell volume variation [5]. Filipović et al. [10] demonstrated that osmotic dehydration markedly reduced the number of Listeria monocytogenes, Escherichia coli and Salmonella spp. inoculated on chicken meat. According to the data, higher pathogen reduction was observed for sonicated samples compared to the unsonicated ones. When US is applied, the cells become more sensitive to any stress, and by being in an osmotic environment, the cell's integrity is severely affected, leading to cell death. Our results are in synchronization with the reports of Wong et al. [22, 23]. Furthermore, we observed that among studied pathogens, E. faecalis was more resistant to the treatments. It was revealed that gram-positive bacteria are more resistant to US and desiccation due to the adherent and thick peptidoglycan layer of their cell wall [5].
Moisture content of Saturn peaches
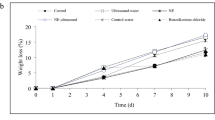
Figure 2 shows the moisture content (MC) data for US-assisted osmotic dehydrated Saturn peach samples immersed in sugar solution. As can be seen, US pre-treatment alone did not significantly alter the MC of the samples (P > 0.05). Our findings were consistent with the reports of other researchers. MC of Physalis [24] and kiwifruit [25] was not influenced by US application prior to OD. In addition, an increase in the duration of OD resulted in a notable decrease in the MC of peaches. This could be because of the concentration gradient between the Saturn peach and the surrounding hyper-tonic solution [26]. Similar results were achieved by Rahaman et al. [27]. It was also found that the MC of Saturn peaches exposed to US pre-treatment was lower than the control sample during the OD period. Several studies have indicated the same phenomenon on pumpkin [28] and pulm [27]. This process can be related to the cavitation caused by US waves, which along with osmotic pressure create microscopic channels and disrupt tissue cells, resulting in increased water transfer from the fruit tissue into the osmotic solution [29]. It has been stated that numerous other factors can affect the reduction of MC, including: US treatment time, osmotic solution concentration, type of solute and structure of fruit [30]. Furthermore, the increase in US amplitude markedly decreased the MC of the samples (P > 0.05). After OD period, the initial moisture content of Saturn peaches (88%) decreased to 64, 59 and 53% in the control sample, 50% and 75% US amplitude treatments, respectively. Similarly, Bermúdez Aguirre et al. [31] demonstrated that increasing the amplitude and power of US will generate high pressure in the surrounding medium, which cause an increase in the cavitation and also sponge impact of ultrasonic waves, thus enhance the water loss from the fruit.
Changes in moisture content (%) of Saturn peach samples. Error bars indicate the standard deviation of moisture content in sonicated (at 50 and 75% amplitude) and unsonicated (control) Saturn peach samples during 12 h of osmotic dehydration. The same lowercase letters are not significantly different between various treatments within the same study hour at P > 0.05
Impact of UAOD on total soluble phenolics and antioxidant capacity
The total soluble phenolic (TSP) contents of Saturn peaches undergoing UAOD are illustrated in Fig. 3. According to the figure, there was no significant difference in TSP content in the US pretreatment samples (P > 0.05). Our results are in agreement with Alighourchi et al. [32], who reported insignificant differences for TSP in US treated pomegranate juices at different amplitude levels (50, 75 and 100%). Whereas, Bhat et al. [33] have expressed an increase in total phenol content at some amplitude levels due to the sonochemical effect of acoustic cavitation and addition of hydroxyl radicals to the phenolic compounds. On the other hand, when the sonicated samples were subjected to the OD, the TSP content decreased markedly over time (P < 0.05). In addition, with increasing US amplitude, the amount of TSP decreased significantly during OD, so that the highest decrease in TSP was observed in treatment of 75% amplitude followed by 50% as compared to the control sample. The initial amount of TSP in the sonicated samples prior to OD was 321 mg/100 g, which finally reached to 203, 178 and 151 mg/100 g in the control sample, 50% and 75% US amplitude treatments, respectively, after 12 h storage in the osmotic solution. The considerable TSP loss that was noticed during the OD period may be ascribed to mass transfer and physical migration of phenolic compounds into the sucrose solution. In addition, higher TSP loss for sonicated samples and also, for higher US amplitude may be ascribed to cavitation phenomenon and more cell structure rupture which along with concentration gradient in osmotic process causing more leakage of solutes from the Saturn peach tissues and losses of the phenolics compounds during the OD [34, 35]. These outcomes were in agreement with the previous findings of Osae et al. [11].
Changes in total phenols (mg/100 g) of Saturn peach samples. Error bars indicate the standard deviation of total phenols in sonicated (at 50 and 75% amplitude) and unsonicated (control) Saturn peach samples during 12 h of osmotic dehydration. The same lowercase letters are not significantly different between various treatments within the same study hour at P > 0.05
The antioxidant capacity of Saturn peach samples undergoing UAOD was determined by the model of scavenging the stable DPPH radical (Fig. 4). A similar trend was observed for antioxidant capacity, as noticed for TSP. This means that US pretreatment of samples did not markedly (P > 0.05) affect the antioxidant capacity, while OD significantly decreased (P < 0.05) the antioxidant capacity of samples. Moreover, sonicated samples showed less antioxidant capacity than the control sample during the OD, and this decline enhanced with increasing the US amplitude. Wong et al. [6] reported that ultrasonic pretreatment has no significant impact on the antioxidant activity of treated blackberry juice. The decrease in TSP during OD somehow revealed that antioxidant capacity and soluble phenolics are related to each other. Similarly, Rahaman et al. [27] stated that the loss of antioxidant capacity of plum fruit may be due to cell destruction and higher diffusion of compounds during OD which exhibit antioxidant activity. Also, Hamedi et al. [36] mentioned that higher water loss and higher leakage of compounds with antioxidant properties happened as a result of high power US during the UAOD of food model enriched with phenolics compounds of pomegranate peel. So it can be concluded that the amount of phenolics and antioxidant capacity of osmo-treated samples depends on OD period and power of US. Besides, studies exhibited other factors like concentration of osmotic solution, type of osmotic solute and temperature of treatment [36,37,38].
Changes in DPPH radical-scavenging effects (mg/g) of Saturn peach samples. Error bars indicate the standard deviation of DPPH in sonicated (at 50 and 75% amplitude) and unsonicated (control) Saturn peach samples during 12 h of osmotic dehydration. The same lowercase letters are not significantly different between various treatments within the same study hour at P > 0.05
Conclusion
Based on the findings, the application of US pretreatment alone can effectively alleviate the studied pathogens. Although higher US amplitude resulted in more bacterial death, it had no significant effect on moisture content, TSP and antioxidant capacity of sonicated samples prior to OD. Moreover, when samples underwent OD treatment, remarkable decrease was observed in the number of pathogens, amount of MC, TSP and DPPH radical scavenging activity compare to samples subjected to US pretreatment alone. Also, further reduction was noticed in these parameters in sonicated samples as compared to the unsonicated ones due to the microscopic channels formation and higher water/solute transition. It was also found that higher US power caused more reduction in the mentioned parameters. Hence, the obtained data confirmed that UAOD method could be used as a non-thermal technology to decrease the pathogenic bacteria in Saturn peach, in which higher US amplitude at longer OD periods was more effective. However, further research efforts are necessary to evaluate the impacts of UAOD method on other microorganisms and on the quality parameters of the treated products.
Data availability
Research data are not shared.
References
J.F. Romeu, M.C. Sánchez, J. GarcíaBrunton, Potential productivity evolution of flat peach cultivars (Prunus persica var. platycarpa) grown in different climatic conditions of southeast of Spain. Sci. Hortic. 197, 687–696 (2015)
S.M.B. Hashemi, D. Jafarpour, The efficacy of edible film from Konjac glucomannan and saffron petal extract to improve shelf life of fresh-cut cucumber. Food Sci. Nutr. 8(7), 3128–3137 (2020)
CDC, Centers for Disease Control and Prevention (CDC)—surveillance summaries. Morb. Mortal. Wkly. Rep. 49, 1–51 (2000)
B.S. Yadav, R.B. Yadav, M. Jatain, Optimization of osmotic dehydration conditions of peach slices in sucrose solution using response surface methodology. J. Food Sci. Technol. 49(5), 547–555 (2012)
Y. Mille, L. Beney, P. Gervais, Compared tolerance to osmotic stress in various microorganisms: towards a survival prediction test. Biotechnol. Bioeng. 92(4), 479–484 (2005)
E. Wong, F. Vaillant, A. Pérez, Osmosonication of blackberry juice: impact on selected pathogens, spoilage microorganisms, and main quality parameters. J. Food Sci. 75, M468–M474 (2010)
S.M.B. Hashemi, A.M. Khaneghah, M. Koubaa, F.J. Barba, E. Abedi, M. Niakousari, J. Tavakoli, Extraction of essential oil from Aloysia citriodora Palau leaves using continuous and pulsed ultrasound: kinetics, antioxidant activity and antimicrobial properties. Process Biochem. 65, 197–204 (2018)
J. Tavakoli, M.J. Rashidi, S.M.B. Hashemi, Evaluating antioxidative activity of the peel of Cucurbita pepo cultivated in two areas of Mazandaran. Iran. Curr Nutr Food Sci. 13(4), 319–322 (2017)
United States Food and Drug Administration (U.S. FDA), Kinetics of microbial inactivation for alternative food processing technologies (2000). www.fda.gov/downloads/food/foodborneillnesscontaminants/ucm545175.pdf.
I. Filipović, S. Markov, V. Filipović, J. Filipović, V. Vujačić, L. Pezo, The effects of the osmotic dehydration parameters on reduction of selected microorganisms on chicken meat. J. Food Process. Preserv. 43(10), e14144 (2019)
R. Osae, C. Zhou, W. Tchabo, B. Xu, E. Bonah, E.A. Alenyorege, H. Ma, Optimization of osmosonication pretreatment of ginger (Zingiber officinale Roscoe) using response surface methodology: effect on antioxidant activity, enzyme inactivation, phenolic com-pounds, and physical properties. J. Food Process. Eng. 42(10), 1–17 (2019)
AOAC, Official Methods of Analysis, 15th edn. (Association of Official Analytical Chemists, Washington, DC, 1999).
T. Swain, W.E. Hillis, The phenolic constituents of Prunus domestica. J. Sci. Food Agric. 10, 63–68 (1959)
S.M.B. Hashemi, A.M. Khaneghah, M.G. Ghahfarrokhi, I. Eş, Basil-seed gum containing Origanum vulgare subsp. viride essential oil as edible coating for fresh cut apricots. Postharvest Biol. Technol. 125, 26–34 (2017)
W. Brand-Williams, M.E. Cuvelier, C. Berset, Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28, 25–28 (1995)
M. Kordowska-Wiater, D.M. Stasiak, Effect of ultrasound on survival of gram negative bacteria on chicken skin surface. Bull. Vet. Inst. Pulawy. 55, 207–210 (2011)
M.H. Dehghani, Effectiveness of ultrasound on the destruction of E. coli. Am. J. Environ. Sci. 1, 187–189 (2005)
H. Sagong, S. Lee, P. Chang, S. Heu, S. Ryu, Y. Choi, D. Kang, Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. Int. J. Food Microbiol. 145, 287–292 (2011)
A. Patist, D. Bates, Ultrasonic innovations in the food industry: from the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 9, 147–154 (2008)
M.I. Piñon, A.D. Alarcon-Rojo, A.L. Renteria, L.M. Carrillo-Lopez, Microbiological properties of poultry breast meat treated with high-intensity ultrasound. Ultrasonics 102, 105680 (2020)
G.J. Tortora, B.R. Funke, C.L. Case, Microbiology: An Introduction, 11th edn. (Pearson Education Inc, Glenview, 2013).
E. Wong, A.M. Perez, F. Vaillant, Combined effect of osmotic pressure and sonication on the reduction of Salmonella spp. in concentrated orange juice. J. Food Saf. 28, 499–513 (2008)
E. Wong, F. Vaillant, E. Chaves-Olarte, Synergistic effect of sonication and high osmotic pressure enhances membrane damage and viability loss of Salmonella in orange juice. Food Res. Int. 45, 1072–1079 (2012)
C.L. Luchese, P.D. Gurak, L.D. Ferreira Marczak, Short communication: osmotic dehydration of physalis—influence of ultrasound pretreatment. Food Eng. Rev. 7, 193–197 (2015)
M. Nowacka, U. Tylewicz, S. Romani, M. Dalla Rosa, D. Witrowa-Rajchert, Influence of ultrasound-assisted osmotic dehydration on the main quality parameters of kiwifruit. Innov. Food Sci. Emerg. Technol. 41, 71–78 (2017)
S. Shamaei, Z. Emam-djomeh, S. Moini, Ultrasound-assisted osmotic dehydration of cranberries: effect of finish drying methods and ultrasonic frequency on textural properties. J. Texture Stud. 43(2), 133–141 (2012)
A. Rahaman, X.A. Zeng, A. Kumari, M. Rafiq, A. Siddeeg, M.F. Manzoor, Z. Bloch, Z. Ahmad, Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason. Sonochem. 58, 104643 (2019)
J. Lee, L. Lim, Osmo-dehydration pretreatment for drying of pumpkin slice. Int. Food Res. 18(4), 1223 (2011)
J. Garcia-Noguera, F.I.P. Oliveira, M.I. Gallão, C.L. Weller, S. Rodrigues, F.A.N. Fernandes, Ultrasound-assisted osmotic dehydration of strawberries: effect of pretreatment time and ultrasonic frequency. Drying Technol. 28, 294–303 (2010)
D. Çağlayan, I. Barutçu Mazı, Effects of ultrasound -assisted osmotic dehydration as a pretreatment and finish drying methods on the quality of pumpkin slices. J. Food Process. Preserv. 42, e13679 (2018)
D. Bermúdez Aguirre, T. Mobbs, G.V. Barbosa-Cánovas, Ultrasound applications in food processing, in Ultrasound Technologies for Food and Bioprocessing. ed. by H. Feng, G.V. Barbosa-Cánovas, J. Weiss (Springer, New York, 2011), pp. 65–105
H.R. Alighourchi, M. Barzegar, M.A. Sahari, S. Abbasi, Effect of sonication on anthocyanins, total phenolic content, and antioxidant capacity of pomegranate juices. Int. Food Res. J. 20(4), 1703–1709 (2013)
R. Bhat, N.S.B.C. Kamaruddin, L. Min-Tze, A.A. Karim, Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem. 18(6), 1295–1300 (2011)
Z. Karami, Gh. Yousefi, Z. Emam-Djomeh, Modeling and optimization of ultrasound-assisted osmotic dehydration with finished freeze drying on black cherries—the effect on antioxidant activities. J. Food Biosci. Technol. 3, 11–22 (2013)
S.M.B. Hashemi, D. Jafarpour, Ultrasound and malic acid treatment of sweet lemon juice: microbial inactivation and quality changes. J. Food Process. Preserv. 44(11), e14866 (2020)
F. Hamedi, M. Mohebbi, F. Shahidi, E. Azarpazhooh, Ultrasound-assisted osmotic treatment of model food impregnated with pomegranate peel phenolic compounds: mass transfer, texture, and phenolic evaluations. Food Bioprocess Technol. 11(5), 1060–1074 (2018)
S.M.B. Hashemi, D. Jafarpour, Fermentation of bergamot juice with Lactobacillus plantarum strains in pure and mixed fermentations: chemical composition, antioxidant activity and sensorial properties. LWT Food Sci. Technol. 131, 109803 (2020)
J. Tavakoli, K.H. Soq, A. Yousefi, P. Estakhr, M. Dalvi, A.M. Khaneghah, Antioxidant activity of Pistacia atlantica var mutica kernel oil and it’s unsaponifiable matters. J. Food Sci. Technol. 56(12), 5336–5345 (2019)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Ethical approval
Ethics approval was not required for this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hashemi, S.M.B., Jafarpour, D. Antimicrobial and antioxidant properties of Saturn peach subjected to ultrasound-assisted osmotic dehydration. Food Measure 15, 2516–2523 (2021). https://doi.org/10.1007/s11694-021-00842-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00842-9