Abstract
The properties of starch-based edible film prepared in the absence and the presence of citric acid; as cross-linking agent (chemical modification) and/or sunflower oil; as barrier enhancer (physical modifier) were investigated. Films containing citric acid had higher tensile strength (TS) but lower elongation at break (EB), while the films incorporated with sunflower oil showed lower TS but higher EB than the control film (p < 0.05). Moreover, the film in the presence of the combination of sunflower oils and citric acid showed a lower EB, as compared to control. Water vapor permeability was decreased to 2.86–2.93 g mm/m2 day Pa × 10–7 when sunflower oil was added, while control film and the films containing citric acid showed the higher water vapor permeability which are 4.42 and 3.15–3.99 g mm/m2 day Pa × 10–7, respectively. FTIR spectra indicated that films added citric acid induced the film cross-linking, as evidenced by a higher amplitude at the wavenumber of 1746 cm−1. Films added with sunflower oil had opaque, and rough cross-section as compared to other films. Thus, these findings showed that using citric acid or sunflower oil alone to modify the properties of starch-based films had better performance than its combination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accumulation of waste is the major problem of the world. In order to partially replace plastics, edible films are widely investigated. Edible films and coating can be developed using different polymers such as starch, chitosan, and protein [1], which are used to maintain the quality and extend the shelf life of agricultural products. Among polymers, starch is widely used for fruit coating [2], and is produced in plants during photosynthesis [3]. Starch is widely available at low cost and biodegradable in water and soil [4]. However, starch based films are hydrophilic with poor barrier and mechanical properties [5], which limits their application in food coating and as an alternative packaging [5]. In order to overcome these drawbacks, physical and chemical modifications are two main approaches to improve the performance of starch for various applications [6, 7].
Lipids act as a physical modifying agent in increasing the hydrophobicity of the film, which will result in a decrease of their water affinity. Lipids interrupt the polymer matrix which improves the barrier properties of the film [8]. Lipid must be used in an appropriate ratio, as the excessive amount of lipid will migrate to the surface of film and impose undesirable effects on transparency and mechanical properties of the film [2]. Corn oil has been proven to be an effective oil to decrease the water vapor permeability (WVP) of polysaccharide films as compared to other commercial oils [9].
Cross-linking approach improves the performance of starch-based films, wherein the cross-linking agents react with the hydroxyl groups of starch, forming a new chemical bond between starch chains and consequently, forming a different polymer network [10]. Starch reacts with a cross-linking agent can improve the solubility and mechanical properties of the film, while these chemical agents can be toxic, expensive, and even not effective [11]. Citric acid (CA) is used for various coating formulation [12], due to non-toxicity, harmlessness and it is available in citrus fruits. CA can be used as a cross-linking agent [13], the carboxyl groups of CA can form stronger hydrogen bonds with the hydroxyl groups of starch molecules, which prevent re-crystallization and retrogradation of starch [14].
The effects of lipid as physical modifier and CA as a cross-linking agent; chemical modifier have been investigated, while an integrated approach of chemical and physical modifications have not been reported yet. Therefore, this experiment was conducted to characterize and compare the influence of lipid and CA, and their combined effects on the barrier and mechanical properties of starch-based films.
Materials and Methods
Materials
Corn starch, glycerol, CA and Tween 20 (T20) were purchased form Wako FUJIFILM Co, Saitama, Japan. Sunflower oil (SO, 85% oleic acid) was purchased from Tyne Worker. These materials were used for preparing casting solution in this experiment.
Preparation of film forming solution
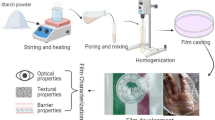
In all film forming solutions, corn starch (S), glycerol (G) and Tween 20 were used in fixed concentrations of 3% (w/v), 40% (w/w of starch) and 25% (w/w of sunflower oil), whereas the concentrations of CA and SO (sunflower oil, 85% oleic acid) varied and symbolized as: Control (S + G), T1 (SO5), T2 (SO10), T3 (CA 0.5), T4 (CA 1), T5 (SO5 + CA 0.5), T6 (SO5 + CA 1), T7 (SO 10 + CA 0.5), and T8 (SO 10 + CA 1), while the numbers beside SO (w/w of starch) and CA (w/v) indicate the percentage of each components.
Starch, glycerol, SO, Tween 20 and CA were mixed at the specified concentrations for 15 min at room temperature, using a stirrer SR-200 (SANSYO, Japan). Then, the suspension was heated to 70–80 °C for 20 min with a constant stirring 100 rpm using a hot plate (RH basic 1, IKA, Germany) to make the starch gelatinized.
Film casting
The solution was cooled down to room temperature in the open air prior to casting the film or measuring pH. Films were casted by placing 10 mL of solution on a silicon plate (5 × 5 cm2) and 9 films were casted for each treatment. The silicon plates were placed at room temperature for 72 h (25 °C) to dry. After drying, the films were removed and conditioned at 25 °C and 53% relative humidity (RH) for at least 72 h in a desiccator containing a saturated magnesium chloride (MgCl2) solution before measurements [15].
pH and film thickness
The pH of film forming solution was measured using a pH meter (LAQUAtwin, HORIBA, Tokyo, Japan) prior to film casting. Film thickness was measured with a micrometer at five points on each film; in the center of the film and in four points along its perimeter, and an average value was used for calculations.
Moisture content and water solubility
Moisture content (MC) was determined according to AOAC Standard Method 19th edition described by Dash et al. [16] with slight modification. Films were cut into (2 × 2 cm2) and the cut samples were weighed and placed in oven set at 105 °C for 24 h for drying. The measurements were done in five replications, and the results were expressed as percentage of MC of samples.
where Mi and Mf are the masses of initial and dried samples, respectively.
Water solubility (WS) was assessed according to Basiak et al. [15] using five samples with an area of (2 × 2 cm2). Samples were dried at 105 °C for 24 h and the weight of each sample was recorded. Each piece of the films was individually placed in 50 mL test tube filled with 20 mL of distilled water. The test tubes were shaken at 60 rpm and 25 °C for 24 h using a shaker (MULTI SHAKER MMS, EYELA, Tokyo, Japan). The remaining pieces were then taken out and dried at 105 °C for 24 h to determine the final weight of dry matter. Loss of total soluble matter was calculated as follow:
Opacity
Opacity was measured using a UV/visible spectrophotometer (Model V-530, JASOCO, Tokyo, Japan). Measurements were done in triplicates and the opacity was calculated based on the absorbance at a wavelength of 600 nm [17].
Scanning electron microscopy
Scanning electron microscopy (SEM) was measured according to Basiak et al. [8] with slight modifications. Briefly, film samples were stored for 14 days in a desiccator containing silica gel (0% RH) to ensure the film is completely dried. The dried samples were coated with osmium (3 nm of thickness) using a plasma coating device (HPC-1SW OSMIUM COATER, VACUUM DEVICE, Ibaraki, Japan) and images were generated using (SEM SO3500, Tokyo, Japan) at accelerating voltage 5 kV and a magnification 3 K for surface and 500 times for cross section.
Mechanical properties
Tensile strength (TS) and elongation at break (EB) were measured by modified ASTM D2370 [18] using a motorized test stand for force gauges (FGS-50E-L Shimpo, Kyoto, Japan) equipped with digital force gauges (FGPX-50, Shimpo, Kyoto, Japan). From each treatment five rectangular strips of (1 × 5 cm2) were mounted in tensile grip. The cross-head speed was set at 25 mm × min−1 and the test was continued until the sample broke.
Water vapor permeability
Water vapor permeability (WVP) of films were measured using a modified ASTME96-80 [19] as described by Basiak et al. [8] with slight modifications. Briefly, 20 mL of water was placed inside WVP cups having a vapor transmission area of 28 cm2, and then films were sealed to the cups. The cups were placed in a 25 °C and 0% RH chamber equipped with a fan having air velocity of 0.8 m × s−1. The cups were weighed until the weight loss reached a steady state for at least 8 h and the calculation was done as:
where W is the weight loss of the cup (g), X is thickness of film (mm), A is exposed area of the films (m2), T is the time of weight gain (sec) and (P2 – P1) is the difference in the vapor pressure across the film (Pa).
Fourier-transform infrared spectroscopy
Fourier transform infrared spectra (FTIR) were measured to confirm the cross-linking of starch film. The spectra were obtained as an average of 32 scans in a range of 550–4000 cm−1 using a FT-IR spectrometer (FT/IR-620, JASCO, Tokyo, Japan). Before performing the test, spectrometer was calibrated with air before measuring and films were washed with water to remove the excess CA [20].
Statistical analysis
The data were compared using analysis of variance (ANOVA) through R 3.6.2 statistical software. The means were measured using Duncan’s test at P < 0.05 level.
Results and discussions
Fourier-transform infrared spectroscopy
Fourier-transform infrared spectroscopy is used to show the functional groups in a molecule by producing infrared absorption spectrum. FTIR spectra of the nine composite films are shown in Fig. 1. All the spectra showed similar peaks, except a peak at 1746 cm−1; the peak at 3300 cm−1 indicates the stretching of OH group, which was present in starch, glycerol and water [20]. The peak at 2920 cm−1 represents CH stretching present in either molecules [21]. The peak at 2360 cm−1 indicates CO2 present in the air.
Films containing CA have an additional peak at 1746 cm−1 which is an indication of carbonyl group (C=O) stretching bond. Since the un-reacted citric acid were removed by water, the presence of (C=O) peak in the spectra can be ascribed to the ester bond formed between starch, CA and glycerol, and confirming the cross-linking reaction in the film [11, 20]. The height of this peak can be an indication of the esterification degree. These results are in agreement with Reddy and Yang [11] who used citric acid to cross-link starch and improve the mechanical properties of film and Ghanbarzadeh et al. [28] who investigated the reaction between starch, CA and glycerol during the gelatinization stage. Besides, T2 also showed a small peak at 1746 cm−1, which could be due to the presence of C=O group in the structural formula of oleic acid. Similar results have been reported by Chen et al. [22]. These results confirmed the cross-linking reaction in the films containing CA.
pH
The pH of film forming solutions were recorded for the control = 4.88, T1 = 4.77, T2 = 4.76, T3 = 3.73, T4 = 3.55, T5 = 3.66, T6 = 3.57, T7 = 3.65 and T8 = 3.52. Clearly, T8 had the lowest pH of 3.52, while control had the highest pH of 4.88 among the treatments. Since CA is an acid, as expected, the pH of the solution decreased with the increasing level of CA. On the other hand, the pH of T1 and T2 also decreased to 4.77 and 4.65, respectively, which can be attributed to the acidic nature of oleic acid. These findings are supported by Yang Qin et al. [23] which used CA as a cross-linking agent in hydroxypropyl high amylose starch and Kowalczyk et al. [21] indicated that the increasing level of ascorbic acid resulted in acidification of potato starch film forming solution and film. These findings indicate that film containing SO will not affect the sensory properties of the product when applied in practice.
Moisture content and water solubility
MC is an important parameter to determine the amount of moisture in the film. As it can be seen in Fig. 2, the MC of films decreased with addition of CA and SO, which is due to the hydrophobicity of SO, as it can affect the ability of film to retain water. Oleic acid in the SO reacts with functional group of starch that restricts the water-polysaccharide interaction, thus causing a decrease in film MC. These results were in agreement with Ghasemlou et al. [24] and Mohammad Amini et al. [25]. Meanwhile, CA decreases the intra and intermolecular interaction between starch–starch chains and strengthens the hydrogen bonding interaction between the hydroxyl groups of starch-CA, leading to a reduction in available OH, thus preventing the absorption of water molecules and resulting a reduction in MC [14, 26]. The little increase caused by higher concentration of CA, may be due to the excess amount of CA, which might have reacted with water molecules instead of starch. In combined treatments, the effect of SO was sensible at higher concentration, where T7 and T8 had the lowest MC among all the treatments. This indicated that lipid supported the cross-linked film to reduce the MC of the film significantly. These results indicate the combined and single treatment of SO and CA were effective to reduce MC as compared to control films, and their effect intensity can be ranked as: combined treatments and single treatment of SO and CA, respectively.
Water solubility is an important property of film; coating material may require water insolubility to enhance product integrity and water resistance. Both SO and CA diminished the WS of the film with increasing concentration of SO showed a reduction, whereas increasing concentration of CA showed an increment trend (Fig. 2). This reduction in WS caused by CA could be attributed to the reaction of carboxyl group of CA with OH groups of starch that creating a strong hydrogen bond, and gave the film a compact structure that resulting in decreased film solidity [27]. However, WS increased from 23.2 to 28.6% as the amount of CA increased to 1%. Some CA have remained un-reacted in the film, which was dissolved by water, thereby causing higher WS of the film. These results are in alignment with Wilpiszewska et al. [20], which investigated the hydrophilicity of carboxymethyl cellulose film using CA. SO had a stronger effect on WS compared to CA, whereas T2 film showed the lowest (16.08%) among all treatments. It is due to the hydrophilic groups of oleic acid that react with starch, which may limit reacting sites of water, thus providing a more hydrophobic film [25]. In the combined treatments, it seems that SO had no significant effect on the WS. A possible reason for this could be the strong network/structure created by cross-linking reaction has limited the interaction of SO with the film matrix, and thus limiting the effect of SO. Therefore, we concluded that SO alone has a stronger effect in reducing WS of starch-based films.
Opacity
Film transparency (opacity) has a great impact on acceptability of the product, and low opacity value indicates more transparency. In this experiment, control treatment film has the lowest opacity of 0.1%, whereas the opacity of the film significantly increased with the addition of SO and CA (Fig. 3). The increment of opacity was in parallel with SO concentration, but an increase in the concentration of CA produced a less opaque film. SO opacity is due to the dispersed droplets of SO in film-forming solution, which deflects the transmitted light in a different direction, thus inducing opacity to films [17, 28]. On the other hand, it has been found that the strong network created by CA due to the cross-linkages, increases the absorbance rate compared with the control film, which promotes the opacity. The increasing concentration of CA caused a decrease in opacity and is proportional to moisture content of the film. Water diluted the opaque color which decreased the absorption bands in visible spectra and caused the transparency in the film. In combined treatment, the effect of oil droplets is more pronounced to induce opacity. As SO interferes the strong network of the cross-linked films, it produces cracks in the structure and resulting in a less opacity of combined treatment as compared to single treatment of SO films. Furthermore, water content of the film also has it proportional effect to reduce opacity in combined treatments. These results indicate that opacity of film is strongly related to the lipid particles present in the structure of the film, which can restrict the light transmission rate at a higher level as compared to control and cross-linked films.
Scanning electron microscopy
Scanning electron microscopy is used to evaluate film homogeneity, surface smoothness, cracks and gives a better understanding of WVP and mechanical properties of films [24]. Figure 4 illustrates the surface and cross-sectional images of composite film. Control film (a) has the smoothest surface among all the treatment, while cross-linked and lipid-incorporated film promoted roughness on the film surface. This roughness was more pronounced in combined treatment, and cross-linked films, respectively. In combined treatments splotches of different size can be visualized which may be due to the diffusion of oil droplets to the surface of the film [22]. The roughness caused by cross-lining reaction was observed by Sharma et al. [26] who developed edible film by cross-linking sesame protein. The roughness promoted by SO is probably due to the coalescence of oil droplets during drying [28, 29]. By using any concentration in single treatment, films had no cracks, whereas in the combined treatment cracks can be seen. Moreover, the combined treatments exhibited protuberances, which indicated a phase separation. Especially in films with low concentration of CA and high concentration of SO (T7). The phase separation can weaken the adhesion between matrix and lipid particles, consequently reducing the tensile strength of the film [30]. These results are in agreement with Taqi et al. [17] who evaluated composite film of oleic acid and Laurus nobilis L. oil incorporated into apple pectin/cassava starch, and Sharma et al. [26] which used CA, malic acid and succinic acid to cross-link sesame protein film.
When visualizing the cross section, it seems that SO films have a more compact structure; a lower concentration of oil (T1) was more compact as compared to T2, which could be due to the homogenous suspension of the SO in the starch matrix. The chemically modified films seemed to have a tighter structure as compared with control and combined treatments which indicates a structural integrity of film that promotes good mechanical properties [31]. In the combined treatment, fractures were seen in the cross section which indicated that tight structure of the film promoted by cross-linking was destroyed by the oil droplets. The images clearly indicate, with the addition of CA and SO, and their combination induces roughness to the surface of the films, while single treatment of CA and SO increases the structural integrity of films. The combined treatments showed cracks and fractures in their structure.
Tensile strength and elongation at break
Tensile strength is the maximum stress endured by a film before it breaks. This parameter indicates the strength of a film in resisting the mechanical damage. In the experiment, SO and CA had an inverse effect on TS and EB; SO reduced the TS and increased EB, whereas CA enhanced the TS and diminished the EB of film, but higher amount of the CA reduced the TS (Fig. 5). These finding were supported by Taqi et al. [17]. Addition of oleic acid induced discontinuity in the polymer matrix, which resulted in a less cohesive matrix, thus reduced TS and increased EB. Lipid droplet size and their distribution can also affect the TS and EB of film [22, 32]. On the other hand, CA through cross-linking can provide a better intermolecular interaction, which improves the TS [11]. CA can act as a plasticizer in the starch film [13], therefore as the concentration of CA increased to 1%, the un-reacted CA acted as the plasticizer [27] and reduced the interaction among macromolecules, thus TS decreased and EB increased. Similar results have been reported by Shi et al. [33], which stated that a concentration higher than 30% (w/w of starch) caused a significant decrease in the TS and increase in EB. In combined treatment, the effect of both (SO and CA) were apparent, but because the SO had disrupted the structure of film, the effect of SO was more sensible. These findings showed that CA is effective in improving the TS of film and reducing the EB. On the contrary, SO improved the EB and diminished the TS, while the combined treatments did not improve any of these mechanical properties.
Water vapor permeability
Water vapor permeability of film is an important characteristic, wherein films with a lower WVP are preferable in order to avoid moisture transfer between product and surrounding atmosphere. Figure 6 shows the WVP of composite films, which indicates that SO at both concentrations decreased the WVP, but higher concentration of SO did not have a significant effect. On the other hand, T3 showed a significant decrease in WVP, while the increasing CA concentration increased the WVP. In combined treatments the values were significantly smaller than control but higher as compared to single treatment values. In combined treatments, as the concentration of SO increased, the WVP showed a small decrement. When starch films were cross-linked with CA, a tight and strong structure was formed, which interrupted water passage and reduced the WVP. However, when CA increased from 0.5% to 1%, the WVP also increased from 3.16 to 3.99 g mm/m2 day Pa × 10–7. This could be due to the effect of excessive CA, which acts as a plasticizer, as it has been discussed previously. These results are supported by Ghanbarzadeh et al. [27] and Reddy and Yang [11]. The decline caused by SO is explained by Taqi et al. [17] which states the decline may be due to the formation of an interconnection lipid network within the film matrix, and these hydrophobic particles of lipid make a difficult path way for water, thus decreased the WVP. Increasing SO concentration seem to have no effect on WVP, similar results have been reported by Guacamole et al. [24] who used different concentration of oleic acid in kefiran-based films. The increase in the combined treatments may be due to the interference of oil droplets in the strong network of cross-linked films as stated in SEM section. Among the treatments, SO had the lowest WVP followed by CA and combined treatments, but in general all the treatments showed lower WVP as compared to control.
Conclusion
Chemical, physical, and their integrated approach for modification of starch-based films were evaluated by means of chemical, mechanical and barrier properties. According to these results, the presence of lipid did not affect the cross-linking reaction caused by CA but it can be observed that the integrated modification approach adversely affected the barrier and mechanical properties of composite films. Among all treatments of physical modification, minimum concentration of SO used (T1) showed the most superior characteristics as compared to other treatment. Although this approach induced opacity to the film, it significantly reduced WVP and enhanced mechanical properties of the film. Thus, T1 could be recommended to apply in the real system. Further research should be conducted to evaluate the effectiveness of this composition on food system in practice.
References
C. Mellinas, A. Valdés, M. Ramos, N. Burgos, M. Del Carmen Garrigós, A. Jiménez, J. Appl. Polym. Sci. (2016). https://doi.org/10.1002/app.42631
M. Sapper, A. Chiralt, Coatings 8, 152 (2018)
T. Niranjana Prabhu, K. Prashantha, Polym. Compos. 39, 2499 (2018)
M. Carissimi, S.H. Flôres, R. Rech, Algal Res. 32, 201 (2018)
P. Cazón, G. Velazquez, J.A. Ramírez, M. Vázquez, Food Hydrocoll. 68, 136 (2017)
T. Mehfooz, T.M. Ali, A. Hasnain, J. Food Meas. Charact. 13, 1058 (2019)
M. Kaushik, B.S. Yadav, R.B. Yadav, N. Dangi, J. Food Meas. Charact. 14, 1623 (2020)
E. Basiak, F. Debeaufort, A. Lenart, Food Chem. 195, 56 (2016)
S. Sahraee, J.M. Milani, B. Ghanbarzadeh, H. Hamishehkar, LWT - Food Sci. Technol. 76, 33 (2017)
L.C. Núñez-Bretón, L.C. Cruz-Rodríguez, M.L. Tzompole-Colohua, J. Jiménez-Guzmán, M.J. de Perea-Flores, W. Rosas-Flores, F.E. González-Jiménez, J. Food Meas. Charact. 13, 2862 (2019)
N. Reddy, Y. Yang, Food Chem. 118, 702 (2010)
M. Zalewska, M. Marcinkowska-Lesiak, A. Onopiuk, J. Food Process. Preserv. 42, 1 (2018)
M. Yıldırım-Yalçın, M. Şeker, H. Sadıkoğlu, Food Chem. 292, 6 (2019)
P.G. Seligra, C. Medina Jaramillo, L. Famá, S. Goyanes, Carbohydr. Polym. 138, 66 (2016)
E. Basiak, A. Lenart, F. Debeaufort, Polymers (Basel) 10, 412 (2018)
K.K. Dash, N.A. Ali, D. Das, D. Mohanta, Int. J. Biol. Macromol. 139, 449 (2019)
A. Taqi, L. Mutihac, I. Stamatin, J. Food Process. Preserv. 38, 1982 (2014)
ASTM D2370, 06, 8 (2002)
ASTM E 96, i, 1 (1995)
K. Wilpiszewska, A.K. Antosik, M. Zdanowicz, J. Polym. Environ. 27, 1379 (2019)
D. Kowalczyk, W. Kazimierczak, E. Zięba, M. Mężyńska, M. Basiura-Cembala, S. Lisiecki, M. Karaś, B. Baraniak, Carbohydr. Polym. 181, 317 (2018)
G. Chen, B. Zhang, J. Zhao, Materials (Basel) 8, 2346 (2015)
Y. Qin, W. Wang, H. Zhang, Y. Dai, H. Hou, H. Dong, Materials (Basel) 12, 1705 (2019)
M. Ghasemlou, F. Khodaiyan, A. Oromiehie, M.S. Yarmand, Int. J. Biol. Macromol. 49, 378 (2011)
A. Mohammad Amini, S.M.A. Razavi, Y. Zahedi, Int. J. Food Sci. Technol. 50, 1137 (2015)
L. Sharma, H.K. Sharma, C.S. Saini, J. Food Sci. Technol. 55, 532 (2018)
B. Ghanbarzadeh, H. Almasi, A.A. Entezami, Ind. Crops Prod. 33, 229 (2011)
S. Acosta, A. Jiménez, M. Cháfer, C. González-Martínez, A. Chiralt, Food Hydrocoll. 49, 135 (2015)
S. Shojaee-Aliabadi, H. Hosseini, M.A. Mohammadifar, A. Mohammadi, M. Ghasemlou, S.M. Ojagh, S.M. Hosseini, R. Khaksar, Int. J. Biol. Macromol. 52, 116 (2013)
Q. Sun, T. Xi, Y. Li, L. Xiong, PLoS ONE 9, 1 (2014)
M.A. García, A. Pinotti, M.N. Martino, N.E. Zaritzky, Characterization of Starch and Composite Edible Films and Coatings (Springer, New York, 2009).
A. Jiménez, M.J. Fabra, P. Talens, A. Chiralt, Food Hydrocoll. 26, 302 (2012)
R. Shi, J. Bi, Z. Zhang, A. Zhu, D. Chen, X. Zhou, L. Zhang, W. Tian, Carbohydr. Polym. 74, 763 (2008)
Acknowledgements
We thank Japan International Cooperation Agency (JICA) for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wardak, M.H., Kingwascharapong, P., Aryan, S. et al. Preparation and characterization of corn starch-based film: effect of citric acid or sunflower oil and its combination. Food Measure 15, 1907–1915 (2021). https://doi.org/10.1007/s11694-020-00786-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00786-6