Abstract
The aim of this study was to encapsulate hibiscus aqueous extract, which has valuable content in anthocyanins with antioxidant activity. For this purpose, assays were realized using whey protein isolate and polydextrose and a mixture of both as carriers by spray-drying and freeze-drying encapsulation. Statistical analysis indicated that the powder containing only polydextrose by freeze-drying presented the best condition of encapsulation, with retentions of phenolic, anthocyanin and antioxidant activity measured by ABTS, DPPH, and HRSA of 86, 77, 76, 90 and 74%, respectively. Accelerated stability tests (75 and 90% relative humidity, at 40 and 60 °C) performed for 30 days in the powders, showed two periods of losses for polyphenols and antioxidant capacity: a significant decrease (p < 0.05) and its posterior stabilization in all storage conditions. In order to predict the degradation kinetic of anthocyanins encapsulated was used the first-order kinetic model, whose degradation rate constants ranged from 0.0259 to 0.2910 d−1, and increased with the temperature and relative humidity, with the Q10 temperature coefficient values from 1.1 to 2.1. FTIR and TGA assays indicated that the encapsulation occurred by physical incorporation, as well as up to 210 °C, powders presented high thermal stability. The results suggested that the powders may be used as a bioactive ingredient due to its high solubility and thermal stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hibiscus has evoked a great interest in recent times due to its potential health benefits, especially related to bioactive compounds such as anthocyanins and other flavonoids, organic acids and dietary fiber, which may be responsible for its antioxidant, antibacterial, anti-inflammatory, hepatoprotection and anticholesteral activities [1,2,3]. The compounds known for being responsible for these properties as well as for the reddish color of the plant calyces are delphinidin-3-glucoside, cyanidin-3-glucoside, delphinidin-3-sambubioside and cyanidin-3-sambubioside [4]. Thus, the method of extraction and subsequent conservation of the bioactive compounds must be studied and carefully handled, in order to preserve the stability of those molecules when withdraw from the plant matrix.
Up to now, studies have revealed that kinetic parameters such as reaction rate constant, half-life, temperature coefficient, and activation energy are of great importance to evaluate thermal degradation of thermo-sensitive compounds in foods [5]. The two first parameters evaluate the change in concentration of a quality attribute as a function of time at a constant temperature. Besides, the last two parameters describe the constant rate changes of a quality attribute with a temperature [6]. In addition, several authors mention that the stability of anthocyanins obeys to first-order of reaction [5, 7].
Spray‐drying and freeze‐drying are methods commonly used by the food industry for microencapsulation of pigments and anthocyanins [8, 9]. In the first method, bioactive compounds are quickly entangled by carriers. As an advantage, it is considered a low cost method, however, it uses high temperatures for drying [10, 11]. On the other hand, freeze-drying uses low temperatures and is recommended for heat-sensitive components [12], although it is a high cost technology due to the necessity of previously freezing the sample, followed by ice sublimation at low pressures.
In order to define the carrier it is important to consider physicochemical interactions between molecules to be encapsulated as well as the rheological characteristic of the carrier, since it may cause strong effects on the structure, particle size, intermolecular network, and delivery system of the powders [13].
Among the encapsulating agents, whey protein isolate (WPI) may be highlighted as a carrier. It is composed by bioactive peptides located in specific protein regions, with amino acid sequences determining their several biology activities, such as antioxidant, antihypertensive, antithrombotic, antiadipogenic, antimicrobial, anti-inflammatory activities and immunomodulatory effects [14]. This protein is usually recommended as phenolic compounds (PC) carrier [13].
Polydextrose (PD) also presents phenolic compounds carrier characteristics, due to its great solubility, texture, stability, humectation, and PC retention capacity [15]. It is mainly composed by randomly cross-linked glucose units, with a degree of polymerization and a molecular weight average of approximately 12 and 2000 daltons, respectively [16], with low concentrations of sorbitol and bound citric acid [17]. All types of glycosidic linkages with the anomeric carbon of glucose are present (α and β-linked 1–2, 1–3, 1–4, and 1–6), but α-1–6 pyranose glycosidic linkage predominate [18]. In addition, PD is a soluble dietary fiber and has prebiotic potential [10, 12, 16].
Several studies were performed for the encapsulation of hibiscus extract by spray-drying and freeze-drying [8,9,10, 19,20,21]. All studies analyzed the stability of encapsulated anthocyanins and the color of the powder. The extract has been previously obtained by maceration with ethanol or methanol solutions as solvents. However, these researches neither studied the encapsulated powders obtained by aqueous acidulated solution through microwave extraction (MAE) nor the stability of anthocyanins and phenolics encapsulated powder under accelerated stability tests.
The aim of this study was to evaluate the stability of the bioactive compounds of hibiscus calyces aqueous extract obtained by MAE and encapsulated by freeze-drying and spray-drying, using whey protein isolate and polydextrose as carriers. Storage stability of anthocyanins and phenolics as well as the antioxidant capacity of encapsulated powders were also studied through accelerated stability and anthocyanin degradation kinetic tests.
Materials and methods
Materials
Hibiscus was purchased in the community garden of Lomba do Pinheiro, Porto Alegre, Brazil (GPS location 30°06′49.4"S and 51°06′30"W). The material was selected, sanitized, packed in polyethylene bags and stored at − 18 °C for later use in the extractions. DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS• (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)), Trolox (6-hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid), and gallic acid were acquired from Sigma–Aldrich. Other chemical reagents used were of analytical grade. PD was purchased from MasterSense Ing Alim Ltda. (Brazil) and WPI from Alibra Ingredientes Ltda. (Brazil).
Procedure of extraction
Previously to extraction, calyces were thawed and blanched for 4 min at 100 °C in water vapor and, subsequently, acidified water was added (2% citric acid, w/v, g/mL) in the proportion of 1:5 (calyx:acidified water; w/v, g/mL). Next, the extract underwent microwave-assisted extraction (MAE), using a microwave oven (Electrolux, MEF41), in the frequency of 2450 MHz, and 700 W power during 8 min (parameters of extraction used by Cassol et al. [22], in order to use green recovery technology).
For the extract encapsulation, 225 g of the extract was mixed with 25 g of the coating material (PD or WPI, or a mixture of 12.5 g of PD and 12.5 g of WPI), and homogenized at 6500 rpm in a Ultra-turraz (Ika, T25).
Encapsulation process
Six treatments were evaluated, which resulted in the encapsulated powders by spray-drying and freeze-drying, from dispersions containing 10% of PD (w/w, g/100 g), 10% of WPI (w/w, g/100 g) and a mixture containing 10% of PD and WPI (1:1; w/w, g/100 g).
Spray-drying was performed in a spray dryer (Labmaq, LM MSDI 1.0) using dispersion and drying air volumetric flow rates of 0.6 and 40.5 L h−1, respectively; and inlet and outlet drying air temperatures of 160 and 80 ºC, respectively. For freeze-drying, the samples were previously frozen at − 68 °C in an ultrafreezer (Liotop, URF30), and later dried in a freeze-dryer (Liobras, Liotop L101) at − 57 °C, with vaccum pressure less than 20 µmHg, for 54 h. After drying, the samples were grounded using a mortar and pestle.
The powders obtained were packed in polyethylene bags, covered with aluminum foil for light protection, and stored in a desiccator at 20 ºC until use.
Physicochemical analysis
The moisture of the powders was determined by sample weight difference before and after drying at 105 ºC until reaching constant weight. The water activity was measured by a water activity meter (Aqualab, 3TE-Decagon).
For solubility, 1 g of powder was diluted in 100 mL of distilled water. The dispersion was centrifuged at 3000 × g for 15 min, and 25 mL of the supernatant was dried at 105 °C until constant weight. Solubility was determined by initial and final dry mass weight difference and expressed as g/100 g.
For hygroscopicity, 1 g of the sample was conditioned in a 75% relative humidity environment (NaCl saturated solution) at 25 °C until it reached equilibrium. Hygroscopicity was expressed as weight of adsorbed moisture per 100 g of dry solid (g/100 g) [23].
Spectrophotometric analysis
The structure of the microparticles coating material was previously ruptured in order to release the relevant compounds, following the method of Robert et al. [24]. For the powders containing PD, 10 mL of a metanol:acetic acid:water (50:8:42, v/v/v, mL/mL/mL) solution was added to 1 g of the microparticles. The dispersion was agitated using a vortex (Quimis, Q920A2) for 1 min. Subsequently, the supernatant was centrifuged (Sigma, 4K15) at 3000 × g for 10 min. For the powders containing WPI and the mixture of PD and WPI, 10 mL of acetonitrile and methanol:acetic acid:water (50:8:42, v/v/v, mL/mL/mL) solutions were added in the proportion of 1:1 to 1 g of the sample. Following, the same procedure described for PD was performed.
All the absorbance measures were performed in a spectrophotometer UV–visible (Thermo Scientific, Genesys S10).
Total monomeric anthocyanins (TMA)
The differential pH method was used [25], with absorbances measured at 520 and 700 nm wavelengths, using potassium chloride and sodium acetate buffers of 1.0 and 4.5 pH mixed with the samples, respectively. The anthocyanin content was expressed in mg of delfinidine-3-sambubioside equivalent per g of sample on a dry basis (d.b).
Total phenolic compounds (TPC)
TPC was performed by the Folin-Cicalteu method, with absorbance of 765 nm according to Singleton and Rossi [26], modified by Kuck and Noreña [12]. The results were expressed as mg of gallic acid equivalent (GAE) per g of sample on a dry basis (d.b).
Antioxidant capacity
The antioxidant capacity was measured by ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) according to Re et al. [27], with minor modifications. The absorbance was measured at 734 nm exactly 6 min after the beginning of the reaction. The results were expressed as μmol of Trolox equivalent (TE) per g of sample on a dry basis (d.b).
The measurement of the antioxidant capacity by DPPH (2,2-diphenyl-1-picrylhydrazyl) was performed according the method proposed by Brand-Williams et al. [28]. The results were expressed as μmol of Trolox equivalent per g of sample on a dry basis (d.b).
Analysis of antioxidant capacity by HRSA was performed according to the method described by Meng et al. [29]. One mL of FeSO4 (0.02 M), 45 μL of H2O2 (0.15%) and 1 mL of C7H6O3 (8 mM) were added sequentially. Next, 4 mL of distilled water and 1 mL of the sample were added to the mixture at 37 °C for 30 min. The absorbance was measured at 593 nm and the percentage of free radical scavenging activity was calculated by the Eq. (1):
where: Asample and Acontrol are the absorbances of the sample and the control.
Encapsulation retention
The retention of the encapsulation (R) was calculated according to Eq. (2) [30]:
where: EC and PC represents TMA, TPC or antioxidant capacity (ABTS, DPPH, HRSA) in the extract and in the powders, respectively.
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC)
The thermogravimetric analysis was performed in the TGA (Perkin Elmer, Pyris 1 TGA). Measurements were performed under nitrogen atmosphere of 20 mL min−1, with a constant heat rate of 10 °C min−1, with temperature ranging from 25 to 600 °C. The thermogravimetric curves were later analysed using Pyris Manager Software.
To determine the glass transition temperature (Tg) of the samples, a DSC (Perkin Elmer, 8600) was used. Measurements were carried out at temperatures between − 20 °C and 300 °C, with a heating rate of 40 °C min−1, under nitrogen atmosphere.
Fourier transform infrared spectroscopy (FTIR)
The chemical groups and binding arrangement of the constituents present in the samples were determined in a Fourier Transform Infrared Spectrometer (FTIR) (Shimadzu, 8300). Samples were placed on KBr supports, and analysis was performed ranging from 4000 to 400 cm−1, with resolution adjusted to 4 cm−1 per sample.
Scanning electron microscopy (SEM)
The structural characterization of the particles was determined by a scanning electron microscope (Jeol, JSM 6060). The powders were placed in aluminum samples crucibles, fixed with double-sided tape carbon and metalized with gold under Argon gas. The equipment was operated at 5 kV with 2000 × magnification for spray drying samples and 300 × for freeze drying samples.
Accelerated stability test
The powders were stored in desiccators at 75 and 90% relative humidity and kept in an incubator (Ethik Technology, 411 / FDP) at 40 and 60 °C for 30 days, where weekly determinations of TMA, TPC and antioxidant capacity were performed.
TMA kinetic analysis
The kinetic of TMA degradation was evaluated by the first-order reaction using the following Eq. (3):
where Ct is TMA concentration at different times (t); k is the constant rate and Co is the TMA concentration at time zero.
The half life (t1/2) and the temperature coefficient (Q10) were calculated according to Eqs. (4) and (5) [7]:
where kT2 and kT1 represent the kinetic rate constant at 60 and 40 ºC, respectively.
Statistical analysis
To study the encapsulation process and accelerated stability assays from hibiscus extract powder were used the one-way and two-way completely randomized design, respectively. Data were submitted to analysis of variance (ANOVA) and means comparison by the Tukey test using a significance level of 0.05 and SAS 9.3 statistical software. The experiments were repeated four times.
Results and discussion
Physicochemical analysis
Table 1 shows the values of water activity, moisture, hygroscopicity and solubility of the powders obtained in the different treatments.
The powders containing WPI (T2 and T5) and the mixture of PD and WPI (T3 and T6) presented the lower water activity values (0.11–0.16 aw) and the powders with PD only (T1 and T4) showed the highest values (0.17 and 0.26 aw). The lower values of water activity found in the study are probably due to the fact that WPI is less hygroscopic than polydextrose, contributing to the decrease of the values in the mixture. A study realized in freeze-dried hibiscus calyxes found that at 0.28 aw, hibiscus reaches value minimum integral entropy where there is a high stability of polyphenols [31].
The moisture content of the powders ranged from 3.4 to 6.7/100 g on a dry basis, and the higher moistures corresponded to the samples containing PD (T1, T3, T4 and T6), being significantly higher in the freeze-dried samples (p < 0.05). During spray-drying, due to the exposure to a larger surface area, heat and mass transfer rate increase, leading to lower moisture contents [32]. The higher moisture contents for freeze-dried powders might be due to the fact that, on the fast freezing at low temperatures, the pores formed may act as a barrier to sublimation, preventing a higher mass transfer, with subsequent higher moisture retention [33].
The values of hygroscopicity ranged from 23 to 39 g/100 g, presenting significant differences between all the treatments (p < 0.05). Powders obtained with the use of WPI (T2 and T5) or the mixture (T3 and T6), presented the lower values. On the other hand, since PD is a polymer constituted of glucose molecules (reducing sugar), it has greater capacity of adsorbing moisture of an environment with high relative humidity [23]. Similar values ranging from 17 to 45 g/100 g were obtained for powders using PD at 10% as the carrier [10, 15].
Solubility values were elevated for all powders, higher than 85 g/100 g. The samples containing PD only (T1 and T4) presented the highest values, regardless of the type of drying method used. PD has the characteristic of being a highly water-soluble polysaccharide [12]. Rigon and Noreña [15] and Piovesana and Noreña [10] reported solubility values of 88 and 97 g/100 g for blackberry and hibiscus calyces extract microencapsulated by spray-drying with PD at 10% and air temperatures of 140 and 160 ºC, respectively. To freeze dried hibiscus extract, the solubility was 93 g/100 g [10]. The lower solubility of WPI might be due to its gradual denaturation, leading to a greater tendency of protein aggregation with subsequent decrease of solubility [34].
Encapsulation Retention (R)
The contents of TMA, TPC and antioxidant capacity by ABTS, DPPH and HRSA of the hibiscus calyxes’ aqueous extract obtained by MAE were 1.24 mg delphinidin-3-sambubioside/g, 23.62 mg GAE/g, 93.21 µmol TE/g, 99.04 µmol TE/g and 19.25%, respectively.
For anthocyanins contents in the powders (Table 2) after drying, the highest retention was found in treatment T4 (77%) and treatment T3 had the lowest retention rate (38%), corresponding to 0.95 and 0.40 mg GAE/g of sample on a dry basis, respectively.
Our results suggest that retentions found in the freeze-drying were higher than in the spray-drying method. However, when WPI was only used, there were no significant differences between the drying methods (p > 0.05). This fact might be a consequence of lower temperatures applied during the freeze-drying process. In addition, spray-drying used higher temperatures (160 ºC) as well as exposition to oxygen, which may have caused anthocyanins degradation by thermal and oxidation reactions.
Tsai and Huang [35] reported a possible formation of polymeric anthocyanins during drying. The protocol of analysis used, it is not able to quantify polymeric anthocyanins, since it may only quantify monomeric anthocyanins.
The phenolic contents were significantly higher (p < 0.05) in the hibiscus extract when compared to the microparticles produced with different wall materials (Table 2). TPC decreased to values ranging from 21.06 to 10.02 mg GAE/g in the powders, indicating retentions from 89 to 42%, respectively. The higher phenolic concentrations were obtained in treatments T6 and T4 (21.06 and 20.36 mg GAE/g, respectively).
The phenolic compounds losses in the spray-drying process might be due to the high temperatures used, which may have caused the degradation and polymerization of these compounds [36]. Tonon et al. [23] mention that shorter periods of time and lower temperature exposures are important factors to reduce the loss of these compounds.
When it comes to the powder obtained with the mixture of PD with WPI, Thongkaew et al. [37], mention that polysaccharides have the ability to link to phenolic compounds in order to prevent its interaction with proteins, leading to a phenolic compounds binding competition between them [38].
The dispersions containing only WPI (T2 and T5) had lower retentions (51.6 and 42%). Even so, they are widely used as emulsifiers due to their amphipathic nature, forming a strong and cohesive protective film that helps to prevent droplet aggregation [39], they may lose encapsulation efficiency due to protein aggregation or precipitation [40]. Jia et al. [41] also mentioned that the protein binding capacity depends on the chemical structure of both molecules, since not all phenolic compounds are able to bind to a protein matrix. Several researches have shown that the interaction between proteins and phenolic compounds are affected by mixing ratio, chemical structure and concentration of phenolic compounds, pH and temperature [37]. Le Bourvellec and Renard [38] mention that the main phenolics-protein interactions are of non-covalent type, such as hydrogen bonds and hydrophobic interactions. However, due to their unfolded structure, they may produce instability [38].
Piovesana and Noreña [10] pointed out that low encapsulation efficiency results in lower stability of phenolics since those compounds might be on the surface of the powder without encapsulation.
When evaluating the three antioxidant capacity methods together, it was possible to verify that treatments T4 and T6 presented higher significant activities (p < 0.05). For T4, the retention values were 76, 90 and 74% for ABTS, DPPH and HRSA, respectively.
From all the assessments performed, it may be verified that the powders obtained by freeze-drying, containing only PD or the mixture with WPI, showed the greatest retention values. However, freeze-dried powder using PD (T4), presented the best behavior for the retentions of TMA, TPC and antioxidant capacity by ABTS, DPPH and HRSA.
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC)
The thermogravimetric analysis reports the mass change of a sample in accordance with temperature or time. Figure 1 shows the thermal behavior, which produces weight loss or the powder produced by spray-drying and freeze-drying when exposed to heat up to 600 °C.
Thermogravimetric analysis curves (TGA) of the hibiscus calyces aqueous extract microencapsulated with PD and WPI by spray-drying and freeze-drying. (T1: spray-drying, 10% (g/100 g) PD; T2: spray-drying, 10% (g/100 g) WPI; T3: spray-drying, 5% (g/100 g) PD and 5% (g/100 g) WPI; T4: freeze-drying, 10% (g/100 g) PD; T5: freeze-drying, 10% (g/100 g) WPI; T6: freeze-drying, 5% (g/100 g) PD and 5% (g/100 g) WPI)
The thermogram analysis shows that in the first stage of mass loss, from 25 °C, all the microencapsulated samples had a similar behavior. Around 100 °C, there was a mass loss, which might be due to bound water and the volatile compounds vaporization [32, 42].
In the second stage, from 214.7 to 229.4 °C, the powders suffered a higher mass loss, corresponding to around 10% of the total weight, which may be a consequence of phenolic compounds degradation and protein denaturation [43].
In the third stage, in the temperature range from 287.7 to 306.1 °C, all samples suffered mass losses around 25% possibly due to the depolymerization and decomposition of the polysaccharides [44].
In a fourth stage, when temperatures ranged from 352.1 to 379.7 °C, there were losses of around 50%, probably due to the partial decomposition of the carbon skeleton [45]. At temperatures higher than 400 °C, the residual mass found may be due to the inorganic fraction of the compounds present in the sample and part of the organic compounds not yet degraded [42].
The glass transition temperature (Tg) in the spray-dried sample from the mixture of PD and WPI (T3) showed a Tg of 3.23 °C, while the freeze-dried sample with the mixture of PD and WPI (T6) presented a Tg of 15.59 °C. The values of Tg may relate to the stability during the storage, increasing the values of Tg with the addition of the wall material [10]. In addition, the Tg is affected by several factors, such as molar mass, chemical structure and moisture content.
Fourier transform infrared spectroscopy (FTIR)
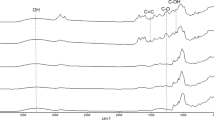
FTIR is a tool that allows the analysis of possible interactions between the mixture of polymers and functional ingredients. The infrared spectra of the hibiscus extract, PD, WPI and their spray-dried and freeze-dried dispersions are shown in Fig. 2. The spectrum of the hibiscus extract exhibited a peak between 1317 cm−1 and 1462 cm−1 which might be assigned to the hydroxycinnamic acids present in the extract [46]. The peak at 1644 cm−1 represents C = C stretching bands typical of aromatic compounds. The peak at 1212 cm−1 may be related to the presence of proanthocyanidins.
FTIR spectra of the hibiscus extract, PD, WPI, and spray-drying and freeze-drying powders. (T1: spray-drying, 10% (g/100 g) PD; T2: spray-drying, 10% (g/100 g) WPI; T3: spray-drying, 5% (g/100 g) PD and 5% (g/100 g) WPI; T4: freeze-drying, 10% (g/100 g) PD; T5: freeze-drying, 10% (g/100 g) WPI; T6: freeze-drying, 5 (g/100 g) % PD and 5% (g/100 g) WPI)
In the WPI infrared spectrum it was possible to identify the peaks of amide I (1639 cm−1) and II (1568 cm−1). Those amide I and II bands are the two most prominent vibrational bands of the proteins. The PD spectrum presented a typical band of carbohydrate in the region of 1200 and 870 cm−1, reported as absorption bands of C–O, C–C and C-O–H bonds. It was also observed a broader absorption band between 3700 cm−1 and 3000 cm−1 which represents O–H and hydroxyl (OH) stretching, while the band at 2922 cm−1 is assigned to the C-H stretching.
The spray-dried powders presented similar spectra that freeze-dried samples. However, in powders containing only WPI (T2 and T5) it can be observed the presence of strong bands of amide I and II, besides of stretching bands of 2930 cm−1 and 3400 cm−1 which may be attributed to the interactions of hydrogen bondings with active groups of WPI. Additionally, it is possible to observe the strong absorption band at 1028 cm−1 in T2 and T5, that is assigned to C-H stretching of the aromatic ring, suggesting the presence of aromatic compounds from phenolics in the extract. The same band is present, although with lower intensity, in formulations containing PD (T1, T3, T4 and T6), which may indicate a higher presence of these compounds in the formulations with PD, resulting in a greater retention of the encapsulation, as shown in Table 2. The peaks of 1639 cm−1 and 1550 cm−1 from WPI were also present in the formulations containing WPI (T2, T3, T5 and T6).
The FTIR results showed the effect of DP and WPI as carriers in powders. In general, it is possible to observe that the encapsulation of the hibiscus extract with these wall materials did not create considerable changes in the obtained frequency of the peaks and intensity of transmission. This behavior indicates that the chemical bonds characteristic of the composition of the extract and the carriers have been preserved, and the encapsulation was induced by physical interactions, since the formation of new chemical bonds in the powders spectra was not observed. Therefore, spray drying and freeze-drying did not change the structure of the polymer matrix formed [42], since no significant changes were observed in the obtained spectra.
Scanning electron microscopy (SEM)
Figure 3 shows the structure of each one of powders. The PD obtained by spray-drying (Fig. 3, T1), showed particles in the form of a spheroid, a wall without cracks and few concavities and roughness, which suggests that particles are less permeable and susceptible to degradation reactions [12].
Micrographs of the hibiscus calyces aqueous extract microencapsulated with PD and WPI by spray-drying and freeze-drying. (T1) spray-drying, 10% (g/100 g) PD; (T2) spray-drying, 10% (g/100 g) WPI; (T3) spray-drying, 5% (g/100 g) PD and 5% (g/100 g) WPI; (T4) freeze-drying, 10% (g/100 g) PD; (T5) freeze-drying, 10% (g/100 g) WPI; (T6) freeze-drying, 5% (g/100 g) PD and 5% (g/100 g) WPI)
The spray-dried powders containing WPI and the mixture with PD (Fig. 3, T2 and T3) presented irregular forms, with roughened surfaces, free of cracks and visible pores. Jouenne and Crouzet [47] mentioned that this characteristic is typical from WPI since β-lactoglobulin presents emulsifying properties. The roughness form of the spray-dried powders may be due to the quick remotion of the water during drying and subsequent cooling [48]. Ramírez et al. [49] also mentioned that during the spray-drying process, the fast evaporation of water might result in cracks or concavities on the surface of the particles, which was observed mostly in the spray-dried powders containing WPI (T2 and T3).
The freeze-dried powders (Fig. 3, T4, T5, and T6) showed irregular forms, such as broken glass or splinter, probably as a consequence of the milling performed after drying [50]. The pores represent mainly the spaces that were occupied by ice, which has been sublimed during the freeze-drying [51]. The formation of these pores produced during sublimation and the milling after drying might lead to the degradation of phenolic compounds in the powders due to the occurrence of oxidation reactions [12].
Accelerated stability testing
For accelerated storage condition, Fig. 4a and c show that at 60 °C and 90% of relative humidity, the anthocyanins were completely degraded in the second week when WPI was used (T2 and T5), suggesting that it is not recommended to use only WPI as encapsulating agent. For the other treatments, two periods were observed. In the first two weeks, significant decreases in the concentration have occurred (p < 0.05), however, after that period, no significant changes were seen (p > 0.05). The losses in the first period may be due to the non-encapsulated phenolic compounds which were retained on the surface of the carrier.
Effect of temperature and relative humidity on total monomeric anthocyanins and total phenolic compounds of the hibiscus calyces aqueous extract microencapsulated with PD and WPI by spray-drying (a, b) and freeze-drying (c, d). (●) PD: 75%, (■) WPI: 75%, (▼) PD/WPI: 75%, (◆) PD- 90%, ( ) WPI: 90%, (▲) PD/WPI: 90%, (○) PD: 75%, (□) WPI: 75%, (▽) PD/WPI: 75%, (◇) PD: 90%, (
) WPI: 90%, (▲) PD/WPI: 90%, (○) PD: 75%, (□) WPI: 75%, (▽) PD/WPI: 75%, (◇) PD: 90%, ( ) WPI: 90%, (△) PD/WPI: 90% (closed symbol: 40 ºC, open symbol: 60 ºC)
) WPI: 90%, (△) PD/WPI: 90% (closed symbol: 40 ºC, open symbol: 60 ºC)
It was also observed a significant effect of the interaction between the increase of the temperature and the relative humidity on the decrease of the concentration of anthocyanins (p < 0.05). When only the less drastic storage condition was analyzed (40 ºC; 75%), the sample with greater stability found was the powder obtained by freeze-drying using only PD as encapsulating (T4), showing retentions at the end of the storage period of up to 53% (0.51 mg delphinidin-3-sambubioside/·g of sample on a dry basis), which represents the percentage obtained in relation to the concentration in the begining of the storage stability test.
Moura et al. [46] mention that the increase of the storage temperature may decrease the stability of anthocyanins during storage, leading to losses due to degradation, such as observed in this paper. Robert et al. [24] observed that anthocyanins from pomegranate encapsulated by spray-drying, when stored at 60 °C, showed degradation rates lower when maltodextrin was used as a carrier instead of isolated soy protein.
For phenolic compounds (Figs. 4b and d), it was also observed a significant effect of the interaction of temperature and relative humidity on the decrease of the contents in spray-dried and freeze-dried powders (p < 0.05), as well as the two periods of losses which were described previously: a significant decrease on the phenolic contents in the first week (p < 0.05), and its posterior stabilization in all storage conditions. In the final period of storage at 40 ºC and 75%, the stability was higher in powders containing PD (T1 and T4), showing retentions of 80.9 and 74.9%, when encapsulated by spray-drying and freeze-drying, respectively.
For antioxidant capacity losses (Fig. 5a–f), it was also observed two stages. The first stage was significant until it reached the second week (p < 0.05), associated with the decrease of phenolic compounds and monomeric anthocyanins [7]. After that period, the antioxidant capacity did not change significantly (p > 0.05). In the end of the storage period (40 ºC, 75%), measurements of antioxidant capacity were performed by DPPH and HRSA, and powders with PD presented the best behavior, with retentions of 86% for freeze-drying (T4) and 82% for spray-drying (T1) by DPPH, and 27 and 26% for HRSA, respectively. However, when the activity was measured by ABTS, the mixture of PD and WPI by freeze-drying (T6) showed the best retention value (64.7%), which may be due to peptides, such as α-lactoalbumin and β-lactoglobulin present in WPI, that also present antioxidant capacity [52].
Effect of temperature and relative humidity on the antioxidant activity by ABTS, DPPH e HRSA of the hibiscus calyces aqueous extract microencapsulated with PD and WPI by spray-drying (a–c) and freeze-drying (d–f). (●) PD: 75%, (■) WPI: 75%, (▼) PD/WPI: 75%, (◆) PD- 90%, ( ) WPI: 90%, (▲) PD/WPI: 90%, (○) PD: 75%, (□) WPI: 75%, (▽) PD/WPI: 75%, (◇) PD: 90%, (
) WPI: 90%, (▲) PD/WPI: 90%, (○) PD: 75%, (□) WPI: 75%, (▽) PD/WPI: 75%, (◇) PD: 90%, ( ) WPI: 90%, (△) PD/WPI: 90% (closed symbol: 40 ºC, open symbol: 60 ºC)
) WPI: 90%, (△) PD/WPI: 90% (closed symbol: 40 ºC, open symbol: 60 ºC)
During extended heating or storage, the occurance of phenolic degradation, including monomeric anthocyanins, may form new compounds through hydrolysis or polymerization which may totally or partially compensate the loss of antioxidant capacity [7]. Sadilova et al. [53] also mention that due to the degradation of the products during heating, the antioxidant capacity may increase.
Except for ABTS, all analysis performed in the powders, suggested that the one containing PD were more stable to the different accelerated storage conditions studied. Specifically, in the condition of 40 ºC and 75% relative humidity, powders containing PD demonstraded the best behavior during storage.
TMA kinetic of degradation
The model adequately adjusted the degradation data with coefficients of determination (R2) higher than 0.93 (Table 3). Several authors have reported that the thermal degradation of anthocyanin followed the first-order model [7]. This model was also successfully used to evaluate assays of degradation of anthocyanins from hibiscus calyx in beverages using accelerated thermal conditions [54]. Table 3 also shows that the rate constant values ranged from 0.0259 to 0.2910 d−1, and increased with the temperature and relative humidity. In addition, for each condition of temperature and relative humidity the k values were lower when PD was used as a carrier. The half-lives of anthocyanins ranged between 2.4 to 26.7 d. The t1/2 is known as the time period which causes 50% of degradation of TMA. The Q10 values ranged between 1.1 and 1.9. The Q10 value is usually close to 2 for the degradation of natural pigments [7].
Conclusion
Our study showed that it was possible to use PD and WPI as carrier in order to obtain microencapsulated powders from hibiscus calyx’s aqueous extract obtained by microwave extraction. The encapsulation resulted from physical interactions between bioactive compounds and carriers. The chemical analysis results, structural assays and accelerated storage tests suggested that the powder obtained by freeze-drying using PD as a carrier was the best treatment, showing characteristic of high solubility and thermal resistance. Anthocyanin degradation kinetics showed to be of first order and, for the powder before mentioned, the rate constants were the smallest found in this study.
Microencapsulated powders are a source of functional compounds and due to its high solubility and thermal stability may be interesting alternative ingredients in order to develop new healthier foods, such as beverages, soup, sauces, and creams.
References
I. Da-Costa-Rocha, B. Bonnlaender, H. Sievers, I. Pischel, M. Heinrich, Food Chem. 165, 424–443 (2014)
A. Formagio, D. Ramos, M. Vieira, S.R. Ramalho, M.M. Silva, N.A.H. Zárate, J.E. Carvalho, Braz. J. Biol. 75, 69–75 (2015)
D.M. Amaya-Cruz, I.F. Perez-Ramirez, D. Ortega-Diaz, M.E. Rodriguez-Garcia, R. Reynoso-Camacho, J. Food Meas. Charact. 12, 135–144 (2018)
I. Borrás-Linares, S. Fernández-Arroyo, D. Arráez-Roman, P.A. Palmeros-Suárez, R. Del Val-Díaz, I. Andrade-Gonzáles, A. Segura-Carretero, Ind. Crops Prod. 69, 385–394 (2015)
J. Sang, Q. Ma, M. Ren, S. He, D. Feng, X. Yan, C. Li, J. Food Meas. Charact. 12, 937–948 (2018)
B. Ling, J. Tang, F. Kong, E.J. Mitcham, S. Wang, Food Bioprocess Technol. 8, 343–358 (2015)
L.S. Kuck, J.L. Wesolowski, C.P.Z. Noreña, Food Chem. 230, 257–264 (2017)
S. Gonzalez-Palomares, M. Estarrón-Espinosa, J.F. Gómez-Leyva, I. Andrade-González, Plant Food Hum. Nutr. 64, 62–67 (2009)
Z. Idham, I.I. Muhamad, M.R. Sarmidi, J. Food Process Eng. 35, 522–542 (2012)
A. Piovesana, C.P.Z. Noreña, Int. J. Food Eng. 4, 1–9 (2018)
S. Darniadi, I. Ifie, P. Ho, B.S. Murray, J. Food Meas. Charact. 13, 1599–1606 (2019)
L.S. Kuck, C.P.Z. Noreña, Food Chem. 194, 569–576 (2016)
D.A. Popović, D.D. Milinčić, M.B. Pešić, A.M. Kalušević, Ž.L. Tešić, V.A. Nedović, Elsevier (2019)
C.B. Ahn, Y.S. Cho, J.Y. Je, Food Chem. 168, 151–156 (2015)
R.T. Rigon, C.P.Z. Noreña, J. Food Sci. Technol. 53, 1515–1524 (2016)
M.H. Auerbach, S.A.S. Craig, J.F. Howlett, K.C. Hayes, Nutr. Rev. 65, 544–549 (2007)
S. Hull, R. Re, K. Tiihonen, L. Viscione, M. Wickham, Appetite. 59, 706–712 (2012)
S.J. Lahtinen, K. Knoblock, A. Drakoularakou, M. Jacob, J. Stowell, G.R. Gibson, A.C. Ouwehand, Biosci. Biotechnol. Biochem. 74, 2016–2021 (2010)
H.A. Al-Kahtani, B.H. Hassan, J. Food Sci. 55, 1073–1076 (1990)
K. Duangmal, B. Saicheua, S. Sueeprasan, LWT-Food. Sci. Technol. 41, 1437–1445 (2008)
G. Gradinaru, C.G. Biliaderis, S. Kallithraka, P. Kefalas, C. Garcia-Viguera, Food Chem. 83, 423–436 (2003)
L. Cassol, E. Rodrigues, C.P.Z. Noreña, Ind. Crops Prod. 133, 168–177 (2019)
R.V. Tonon, C. Brabet, M.D. Hubinger, Food Res. Int. 43, 907–914 (2008)
P. Robert, T. Gorena, N. Romero, E. Sepulveda, J. Chavez, C. Saen, Int. J. Food Sci. Technol. 45, 1386–1394 (2010)
D.H. Lee, F.J. Francis, HortScience. Stanford. 7, 83–84 (1972)
V.L. Singleton, J.A. Rossi, Am. J. Enol. Viticult. 16, 144–158 (1965)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Free Radic. Biol. Med. 26, 1231–1237 (1999)
W. Brand-Williams, M.E. Cuvelier, C. Berset, LWT - Food Sci. Technol. 28, 25–30 (1995)
J.F. Meng, Y.L. Fang, M.Y. Qin, X.F. Zhuang, Z.W. Zhang, Food Chem. 134, 2049–2056 (2012)
C.C. Ferrari, S.P.M. Germer, J.M. Aguirre, Dry. Technol. 30, 154–163 (2012)
Y.I. Maldonado-Astudillo, J. Jiménez-Hernández, G. Arámbula-Villa, V. Flores-Casamayor, P. Álvarez-Fitz, M. Ramírez-Ruano, R. Salazar, J. Food Meas. Charact. 13, 687–696 (2019)
S.Y. Hundre, P. Karthik, C. Anandharamakrishnan, Food Chem. 174, 16–24 (2015)
P.N. Ezhilarasi, D. Indrani, B.S. Jena, C. Anandharamakrishnan, J. Food Eng. 117, 513–520 (2013)
C.E. Lupano, Food Res. Int. 33, 691–696 (2000)
P. Tsai, H. Huang, Food Res. Int. 37, 313–318 (2004)
Q. Chang, Z. Zuo, M.S.S. Chow, W.K.K. Ho, Food Chem. 98, 426–430 (2006)
C. Thongkaew, M. Gibis, J. Hinrichs, J. Weiss, Food Hydrocolloid. 41, 103–112 (2014)
C. Le Bourvellec, C.M.G.C. Renard, Crit. Rev. Food Sci. Nutr. 52, 213–248 (2012)
B. Shah, S. Ikeda, P.M. Davidson, Q. Zhong, J. Food Eng. 113, 79–86 (2012)
K.S. Sonu, M. Bimlesh, S. Rajan, K. Rajesh, Res. Rev. J. Food Dairy Technol. 5, 7–16 (2017)
Z. Jia, M. Dumont, V. Orsat, Food Biosci. 15, 87–104 (2016)
L.F. Ballesteros, M.J. Ramirez, C.E. Orrego, J.A. Teixeira, S.I. Mussatto, Food Chem. 237, 623–631 (2017)
Y. Jafari, H. Sabahi, M. Rahaie, Food Chem. 211, 700–706 (2016)
L.F. Ballesteros, M.A. Cerqueira, J.A. Teixeira, S.I. Mussatto, Carbohydr. Polym. 127, 347–354 (2015)
A. Gundogdu, C. Duran, H.B. Senturk, M. Soylak, M. Imamoglu, Y. Onal, J. Anal. Appl. Pyrolysis. 104, 249–259 (2013)
S.C.S.R. Moura, C.L. Berling, S.P.M. Germer, I.D. Alvim, M.D. Hubinger, Food Chem. 241, 317–327 (2018)
E. Jouenne, J. Crouzet, J. Agric. Food Chem. 48, 5396–5400 (2000)
W. Wang, Y. Jiang, W. Zhou, J. Food Eng. 119, 724–730 (2013)
M.J. Ramírez, G.I. Giraldo, C.E. Orrego, Powder Technol. 277, 89–96 (2015)
K.M. Khazaei, S.M. Jafari, M. Ghorbani, A.H. Kakhki, Carbohydr. Polym. 105, 57–62 (2014)
C. Anandharamakrishnan, C.D. Rielly, A.G.F. Stapley, Dairy. Sci Technol. 90, 321–334 (2010)
H.C. Liu, W.L. Chen, S.J.T. Mao, J. Dairy Sci. 90, 547–555 (2007)
E. Sadilova, R. Carle, F.C. Stintzing, Mol. Nutr. Food Res. 51, 1461–1471 (2007)
Y. Zhang, J. Sang, F. Chen, J. Sang, C. Li, J. Food Meas. Charact. 12, 2475–2483 (2018)
Acknowledgment
The authors thank FAPERGS, CAPES, and CNPq for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cassol, L., Noreña, C.P.Z. Microencapsulation and accelerated stability testing of bioactive compounds of Hibiscus sabdariffa. Food Measure 15, 1599–1610 (2021). https://doi.org/10.1007/s11694-020-00757-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00757-x