Abstract
In this research, a novel electrochemical aptasensor for the detection of Penicillin in a milk sample was designed using a Penicillin aptamer as the specific recognition element and an electrospun carbon nanofiber (ECNF) mat electrodeposited gold nanoparticles (AuNPs) as the platform. Firstly, the ECNF mat electrode was fabricated by means of electrospinning and heat treatment method. Secondly, the prepared ECNF mat electrode was modified with electrodeposition of AuNPs to improve the rate of electronic transmission. Finally, a Penicillin aptamer was assembled on the modified electrode. The morphology of AuNPs/ECNF mat electrode was examined using scanning electron microscopy (SEM) equipped with energy dispersive spectroscopy (EDS). The SEM results demonstrated that the electrodeposition of AuNPs on ECNF mat electrode was successfully performed. Cyclic voltammetry (CV) was utilized to indicate the electrochemical performance of the prepared aptasensor. The CV results illustrated a high selectivity, good stability, excellent reproducibility and repeatability as well as wide linear range (1–400 ng/mL) with a low detection limit (0.6 ng/mL). Moreover, the recoveries obtained in this research were in a good agreement with those achieved using the HPLC method. Therefore, a proposed aptasensor with a good electrochemical performance and simple preparation procedure can be proposed for the detection of Penicillin antibiotic in milk samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the most important β-lactam antibiotics is Penicillin extensively prescribed for the prevention and treatment of different bacterial infections such as mastitis in dairy cow [1]. Although Penicillin has beneficial effects, overuse of this antibiotic results in the presence of residues in raw milk and threats human health. Hence, the development of efficient and sensitive method for the detection of antibiotics residues in milk and other dairy products is necessary [2, 3].
To date, several accurate and reliable methods for the antibiotic detection have been used including liquid chromatography [4], high performance liquid chromatography (HPLC) [5], mass spectrometry (MS) [6], liquid chromatograph–mass spectrometry (LC–MS) [7], gas chromatography (GC) [8] and fluorescence [9]. However, the above mentioned approaches are difficult, tedious, and time-consuming process and also require expensive instruments, complex sample preparation, well-trained and experienced operator. Recently, an electrochemical aptasensor due to its easy operation, portable, low cost, fast response and high sensitive has received great attention for antibiotic detection [10,11,12,13].
In general, aptasensor is a specific class of biosensor that utilizes an aptamer as a biological recognition element. Aptamers are short single-stranded oligonucleotides which possess high affinity and selectivity towards various targets such as antibiotics. Due to such benefits, aptamers are promising candidate for most electrochemical aptasensor applications. Aptamers can be identified through an in vitro process called systematic evolution of ligands by exponential enrichment (SELEX) [14].
The detection limit of electrochemical aptasensors depends on the amount of aptamers immobilized on electrode surface. Hence, the modification of electrode surface can results in increment of immobilized aptamers and therefore amplification of signal intensity. In recent years, the gold electrode has been extensively used for the immobilization of aptamer [15]. However, the low specific surface area of these electrodes decreases a sensitivity of aptasensor. Therefore, it is necessary to develop a facile method to modify the electrode for the improvement of aptasensors performance. Up to now, efforts have been made to modify the electrode surface [16,17,18]. For example, Zhao et al. developed an electrochemical aptasensor for the detection of antibiotic penicillin using a composite film consisting of a magnetic graphene nanocomposite and a poly (3,4 ethylenedioxythiophene)—gold nanoparticle composite as a modified electrode for the immobilization of the penicillin aptamer [19]. In another research, an electrochemical aptasensor based on the utilization of a graphite electrode modified with reduced graphene oxide and gold nanoparticles for the detection of penicillin was fabricated by Ghasemi-Varnamkhasti et al. [20].
On the other hand, carbon nanofiber mat as an electrode are promising in the field of aptasensing because of their easy surface modification [21]. Among different methods used for the fabrication of carbon nanofiber mat, electrospinning technique due to its simplicity and cost effective has been the subject of considerable interest [22, 23].
The combination of gold nanoparticles (AuNPs) as surface modifier and electrospun carbon nanofiber (ECNF) mat as an electrode can provide more sites for the immobilization of aptamer, high electron transfer capability and therefore better electrochemical performance. Nevertheless, to the best of the authors’ knowledge, the assembly of Penicillin aptamer on ECNF mat electrode electrodeposited with AuNPs has not been evaluated for the detection of Penicillin antibiotic in raw milk. Therefore, a facile process to prepare the Penicillin aptamer/AuNPs/ECNF mat electrode is attractive for the development of high performance electrochemical aptasensor.
In this research, a novel aptasensor based on immobilization of the Penicillin aptamer on AuNPs/ECNF mat electrode for the detection of Penicillin antibiotic was developed. For the fabrication of this aptasensor, ECNF mat electrode was firstly prepared using the electrospinning and heat treating method. After that, the electrodeposition of AuNPs was carried out on ECNF mat electrode. The Penicillin aptamer was finally immoblized onto AuNPs/ECNF mat electrode.
The resulting electrode was characterized using SEM, EDS and Raman spectroscopy. The electrochemical behavior of the aptasensor was evaluated using CV technique and the results were compared with HPLC.
Experimental
Reagents and materials
Polyacrylonitrile (PAN) with a molecular weight of 150,000 g/mol was obtained from Polyacryl company (Iran). Dimethylformamide (DMF) used as solvent for PAN was received from Merck.
Sulfuric acid (H2SO4), sodium phosphate dibasic (Na2HPO4), hydrogen tetracholoroaurate (HAuCl4), potassium phosphate monobasic (KH2PO4), potassium chloride (KCl), sodium chloride (NaCl), potassium ferrocyanide (K4[Fe(CN)6]), potassium ferricyanide (K3[Fe(CN)6]) and bovine serum albumin (BSA) were bought from Sigma-Aldrich. Penicillin aptamer sequence with the following sequence was purchased from Faza Biotech Co. (Tehran, Iran): (5′-thiol-(CH2)6-CTG AAT TGG ATC TCT CTT CTT GAG CGA TCT CCA CA-3′). All solutions were prepared with ultra pure water.
Fabrication of AuNPs/ECNF mat electrode
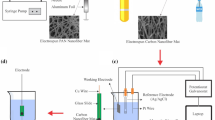
The ECNF was prepared according to the procedure as described in previous reports [24, 25]. Briefly, PAN solution (12 wt%) was achieved through dissolution of PAN polymer in DMF under continuous magnetic stirring at 40 °C for 12 h. The prepared solution was then placed in a 10 mL plastic syringe fitted with an 18-gauge needle as nozzle and electrospun utilizing Electroris (Fanavaran Nano Meghyas Ltd., Co., Tehran, Iran) with high-voltage power of 20 KV, flow rate of 1 mL/h and distance between tip to collector (covered with an aluminum foil) of 10 cm. Electrospun PAN nanofiber mat were put into a tube furnace and stabilized at 290 °C for 4 h under constant heating rate of 1.5 °C min−1 in an air atmosphere and consequently carbonized at 1000 °C for 1 h under constant heating rate of 4 °C min−1 in an nitrogen atmosphere. The obtained ECNF mat was punched to get a circle with diameter of 5 mm and then connected with a copper wire and straightly utilized as a platform. The ECNF mat platform was dipped into an electrolyte solution containing 3 mM HAuCl4 and 0.1 M H2SO4, and electrodeposition was conducted potentiostatically at − 0.4 V versus Ag/AgCl for 90 seconds.
Immobilization of Penicillin aptamer onto AuNPs/ECNF mat electrode
The immobilization process was carried out by adding 4 µL of 8 µM Penicillin aptamer on the surface of AuNPs/ECNF mat electrode. The unbounded aptamer on the surface of modified electrode was removed by washing with ultra pure water. After that, 5 µL of phosphate buffered saline (PBS) solution containing 10% BSA was dropped onto the electrode surface and kept for 2 h at room temperature to block remaining binding sites. Finally, the electrode was rinsed with ultra pure water and dried for next use.
Characterization of nanofibers
Scanning electron microscopy (SEM)
The morphology of the eletrospun nanofibers and AuNPs electrodeposited onto ECNF mat was characterized using a Vega Tescan scanning electron microscope (SEM) equipped with energy dispersive X-ray spectrometer (EDS).
Raman spectroscopy
Raman spectroscopy of the carbon nanofibers was performed using a Senterra (Bruker, Germany) in the spectral range of 0–4000 cm−1 at a room temperature. The laser excitation wavelength, spectral resolution and optical power were 758 nm, 3 cm−1 and 25 mW, respectively. The measurement was carried out at three different areas on the sample to ensure the uniformity of the CNFs.
Electrochemical measurements
All electrochemical experiments were conducted in a µStat 400 potentiostat/galvanostat (DropSens, Spain) with a conventional three-electrode system composed Ag/AgCl as reference electrode, a platinum wire as auxiliary electrode and a modified electrode as the working electrode. The electrochemical measurements were carried out in PBS solution (pH 7) containing ferri/ferrocyanide ([Fe(CN)6] −3 /−4) at 1 mM.
Milk sample preparation procedure for electrochemical analysis
Since milk fat can have negative effect on the electrochemical measurements, the milk purchased from a local market was centrifuged at 5000 rpm for 15 min and the fatty layer in the top of the centrifuge tube was carefully removed. The milk separated from the fat was then diluted with 20% PBS and spiked with 10 µL drop of Penicillin antibiotic at different concentrations. After that, the aptasenor was put into the prepared solution for binding of Penicillin antibiotic to the modified electrode. Next, the aptasensor was removed from the solution and rinsed with ultra pure water to eliminate the unfixed Penicillin antibiotic molecules and other unbounded materials. Finally, the aptasensor was dipped into PBS solution containing [Fe(CN)6]−3/−4 and the electrochemical tests were carried out according the previous section.
Results and discussion
Characterization of AuNPs/ECNF mat electrode
Figure 1a shows the SEM micrographs of electrospun PAN nanofibers and Fig. 1b presents the same nanofibers after carbonization. As seen in Fig. 1a, a porous structure has been composed by randomly oriented nanofibers. The heat treatment, stabilization and carbonization, had no significant effect on the morphology of mat (Fig. 1b). Stabilization step is the most important process during conversion of PAN nanofibers to carbon nanofibers. During this process, the reaction of macromolecules of nanofibers with oxygen of air leads to formation of ladder structure that can tolerate the high temperature without melting and preserve the morphology of nanofibers in the next heat treatment [26]. During carbonization process, the non-carbon elements as volatile gases such as N2, HCN and H2O are removed. Consequently, the process results in increment of carbon content and reduction of nanofiber diameter [27]. This observation is in good agreement with previous report presented by Zhou et al. [28].
Raman characterization is useful as a non destructive measurement apparatus that can be used to indentify carbon nanostructures. Raman spectra, in the range of Raman shift from 0 to 4000 cm−1, of carbon nanofibers is shown in Fig. 2. Two sharp peaks were illustrated at 1350 cm−1 (D band) and 1580 cm−1 (G band). The D band is corresponding to structural imperfection of graphite, whereas the G band is attributed to ordered graphitic structures [29].
Figure 3 shows the SEM image of the AuNPs/ECNF mat electrode. As seen in Fig. 3a, b, the electrodeposited AuNPs are uniform and well-dispersed on the entire surface of ECNF mat electrode. Moreover, the chemical composition of electrodeposited AuNPs obtained by EDS (3c) confirms that modified electrode is composed of carbon and metallic gold.
Electrochemical measurements of pDNA/AuNPs/ECNFs aptasensor
To evaluate the electrochemical behaviour of the proposed aptasensor, cyclic voltammetric results of the ECNF mat electrode, AuNPs/ECNF mat electrode and pDNA/AuNPs/ECNF mat electrode, and Penicilline/pDNA/AuNPs/ECNF mat electrode are presented in Fig. 4. As seen in this figure, the reduction/oxidation process in range of – 0.3 to 0.7 V related to reduction/oxidation of the pair Fe[(CN)6] −3/−4 occurred for all electrodes. Furthermore, the change in peak current of cyclic voltammograms is also observed that can be attributed to the electron transfer resistance. For instance, the electrodeposition of AuNPs on ECNF mat electrode surface led to increase in the voltammetric response of [Fe(CN)6]−3/−4 redox. Generally, there are several reports regarding the enhancement of peak current of CV in presence of AuNPs [20, 30]. The reason is that the AuNPs improve electron transfer efficiency. After immobilizing pDNA onto AuNPs/ECNF mat electrode, voltammetric peak current of [Fe(CN)6]−3/−4 redox decreased because pDNA act as a barrier for the charge transfer between [Fe(CN)6]−3/−4 and the modified electrode. A further reduction in peak current was observed for Penicilline/pDNA/AuNPs/ECNF mat electrode compared to pDNA/AuNPs/ECNF electrode, indicating that the rate of charge transfer between [Fe(CN)6]−3/−4 and the electrode surface was decreased. These observations are in good agreement with results reported by Zhao et al. [19].
The effect of Penicillin concentration on the peak current of [Fe(CN)6]−3/−4 redox was investigated by means of cyclic voltammetric technique (Fig. 5). There is a good linear relationship between Penicillin concentration and peak current in the range of 1–400 ng/mL with a regression equation of I (mA) = − 0.301c + 156.8 (ng/mL) (R2 = 0.99). The detection limit for aptasensor on the basis of signal to noise ratio (S/N = 3) was 0.6 ng/mL. In addition, the detection performance of the prepared aptasensor was compared to some of the reported aptasensor and the results were presented in Table 1. According to Table 1, it was found that the detection limit of the developed aptasensor was better than other sensors reported in previous studies.
Selectivity, reproducibility, repeatability and stability of the pDNA/AuNPs/ECNFs aptasensor
One of the most important factors to evaluate the performance of aptamer is selectivity. Hence, the selectivity of the proposed aptasensor was investigated in presence of three other antibiotics as probable interferences including tetracycline, amphotericin B and kanamycine. As shown in Fig. 6, penicillin showed a strong current decrease whereas all interferences had a negligible effect on the response signal, demonstrating that the aptamer can successfully distinguish between Penicillin and other interferences.
Another key issue to investigate an aptasensor is reproducibility. For this purpose, the current response of the four electrodes prepared at the same conditions was measured (Fig. 7a). The results displayed that the relative standard deviation (RSD) of electrochemical response for four electrodes was about 2.81%, indicating that the aptasensor had a good reproducibility. Meanwhile, the RSD obtained from six successive measurements for each electrode was approximately 2.78%, suggesting acceptable repeatability. To study the stability of the modified electrode, the peak current of pDNA/AuNPs/ECNFs aptasensor was measured for 21 days. As shown in Fig. 7b, it was found that almost 95% of the initial value was remained after the storage for 21 days, indicating a satisfactory stability.
Determination of Penicillin in milk samples
The determination of apatsensor efficiency for the Penicillin detection in milk sample has a great important. Thus, four different Penicillin concentrations (5, 10, 15 and 20 ng/mL) were firstly dropped in the diluted milk samples and the analyses were then carried out using the proposed aptasenor. As can be seen in Table 2, recovery variations ranging from 97.93 to 102.5% demonstrated that the reliability of the aptasensor was acceptable. The results validation was performed by means of HPLC method to ensure overall correctness. As shown in Table 2, there is a good correlation between the aptasensor and HPLC. Hence, the aptasensor shows great potential for the practical use in the Penicillin residues detection in milk samples.
Conclusion
In this work, a new strategy was used to develop an electrochemical aptasensor for the detection of Penicillin antibiotic via a pDNA aptamer as a particular recognition element on an AuNPs/ECNF mat electrode. The cyclic voltammetry technique was utilized to evaluate the fabrication stages and performance of aptasensor. The electrochemical results of pDNA/AuNPs/ECNF mat electrode represent remarkable analytical properties like excellent reproducibility, great repeatability, a high selectivity, long term stability wide linear range and a low detection limit toward Penicillin antibiotic. Furthermore, recovery results achieved using the prepared aptasensor were in good agreement with HPLC method. Therefore, the proposed aptasensor can have great potential for monitoring Penicillin antibiotic in milk samples.
References
C. Macarov, L. Tong, M. Martínez-Huélamo, M. Hermo, E. Chirila, Y. Wang, D. Barrón, J. Barbosa, Food Chem. 135, 2612 (2012)
T.M. do Prado, M.V. Foguel, L.M. Gonçalves, T.S. Maria del Pilar. Sens. Actuators B 210, 254 (2015)
G. Hui, Y. Ying, Trans. ASABE 60, 1439 (2017)
M. Bailón-Pérez, A. García-Campaña, M. del Olmo-Iruela, L. Gámiz-Gracia, C. Cruces-Blanco, J. Chromatogr. A 1216, 8355 (2009)
R.B.D. Brito, R.G. Junqueira, Braz. Arch. Biol. Technol. 49, 41 (2006)
Z. Xu, H.-Y. Wang, S.-X. Huang, Y.-L. Wei, S.-J. Yao, Y.-L. Guo, Anal. Chem. 82, 2113 (2010)
A. Junza, R. Amatya, D. Barrón, J. Barbosa, J. Chromatogr. B 879, 2601 (2011)
E.N. Evaggelopoulou, V.F. Samanidou, Food Chem. 136, 1322 (2013)
Q. Yang, L. Zhou, Y.-X. Wu, K. Zhang, Y. Cao, Y. Zhou, D. Wu, F. Hu, N. Gan, Anal. Chim. Acta 1020, 1 (2018)
Y. Zhou, C. Mahapatra, H. Chen, X. Peng, S. Ramakrishna, H.S. Nanda, Curr. Opin. Biomed. Eng. 13, 16–24 (2019)
T. Wang, H. Yin, Y. Zhang, L. Wang, Y. Du, Y. Zhuge, S. Ai, Talanta 197, 42 (2019)
S.M. Taghdisi, N.M. Danesh, M.A. Nameghi, M. Ramezani, M. Alibolandi, K. Abnous, Biosens. Bioelectron. 133, 230 (2019)
Z. Xiaohong, Z. Zhidong, L. Xiongwei, L. Jian, H. Guohua, J. Food Meas. Charact. 11, 548 (2017)
A. Mehlhorn, P. Rahimi, Y. Joseph, Biosensors 8, 54 (2018)
J.M. Pingarrón, P. Yañez-Sedeño, A. González-Cortés, Electrochim. Acta 53, 5848 (2008)
Y. Li, Y.-Y. Song, C. Yang, X.-H. Xia, Electrochem. Commun. 9, 981 (2007)
L. Luo, L. Zhu, Z. Wang, Bioelectrochemistry 88, 156 (2012)
X. Niu, Y. Li, J. Tang, Y. Hu, H. Zhao, M. Lan, Biosens. Bioelectron. 51, 22 (2014)
J. Zhao, W. Guo, M. Pei, F. Ding, Anal. Methods 8, 4391 (2016)
A. Mohammad-Razdari, M. Ghasemi-Varnamkhasti, Z. Izadi, A.A. Ensafi, S. Rostami, M. Siadat, Microchim. Acta 186, 372 (2019)
S.G. Kim, J.S. Lee, J. Jun, D.H. Shin, J. Jang, ACS Appl. Mater. Interfaces 8, 6602 (2016)
Z. Dong, S.J. Kennedy, Y. Wu, J. Power Sources 196, 4886 (2011)
M. Adabi, Nanomed. Res. J. 4, 247 (2019)
M. Adabi, M. Adabi, J. Dispers. Sci. Technol. (2019). https://doi.org/10.1080/01932691.2019.1678483
A. Niri, R. Faridi-Majidi, R. Saber, M. Khosravani, M. Adabi, Biointerface Res. Appl. Chem. 9, 4022 (2019)
S.R. Dhakate, A. Gupta, A. Chaudhari, J. Tawale, R.B. Mathur, Synth. Met. 161, 411 (2011)
N. Yusof, A. Ismail, J. Anal. Appl. Pyrol. 93, 1 (2012)
Z. Zhou, C. Lai, L. Zhang, Y. Qian, H. Hou, D.H. Reneker, H. Fong, Polymer 50, 2999 (2009)
C. Lai, Z. Zhou, L. Zhang, X. Wang, Q. Zhou, Y. Zhao, Y. Wang, X.-F. Wu, Z. Zhu, H. Fong, J. Power Sources 247, 134 (2014)
J.I.A. Rashid, N.A. Yusof, Sens. Biosens. Res. 16, 19 (2017)
Z. Luo, Y. Wang, X. Lu, J. Chen, F. Wei, Z. Huang, C. Zhou, Y. Duan, Anal. Chim. Acta 984, 177 (2017)
G. Rosati, M. Ravarotto, M. Scaramuzza, A. De Toni, A. Paccagnella, Sens. Actuators B 280, 280 (2019)
N. Karaseva, T. Ermolaeva, Talanta 120, 312 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ebrahimi Vafaye, S., Rahman, A., Safaeian, S. et al. An electrochemical aptasensor based on electrospun carbon nanofiber mat and gold nanoparticles for the sensitive detection of Penicillin in milk. Food Measure 15, 876–882 (2021). https://doi.org/10.1007/s11694-020-00684-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00684-x