Abstract
An electrochemical aptamer-based sensor was designed to detect tetracycline (Tet) in chicken ham. First, polyacrylonitrile (PAN) polymer was used to prepare electrospun PAN nanofibers. PAN nanofibers were heat treated to form electrospun carbon nanofiber (CNF) mat. The electrospun CNF mat electrode was modified with Au and the aptamer of Tet was placed on the mat electrode. Raman spectroscopy and scanning electron microscopy equipped with energy-dispersive spectroscopy were used to identify the structure and morphology of electrospun CNFs, respectively. The behavior of CNF mat electrode was evaluated via cyclic voltammetry. The designed aptamer biosensor had a good repeatability, reproducibility, stability, and specific function for the detection of Tet in chicken ham. This biosensor was able to detect the Tet in the linear range of 1 × 10–9 M to 1 × 10–4 M with the detection limit of 1.2 × 10–10 M and preserve about 95% of its primary response after 20 days. In addition, the recoveries of Tet were achieved ranging from 96.92 to 104.31%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tetracyclines (Tet) are important types of antibiotics that are efficient against an extensive range of diseases resulting from Gram-negative and Gram-positive microorganisms. Tet is widely applied as a veterinary medicine for treatment and prevention purposes due to its cheap price, broad-spectrum activity against microorganisms, low toxicity, and good oral absorption [1,2,3]. The wide utilize of Tet seems to lead to Tet buildups in the foods of animal origin, which creates potential risks to public health, such as the liver and dental damage, allergic reactions, and increased antimicrobial resistance [4,5,6,7]. Therefore, the detection of Tet in food has attracted much attention in recent years. Tet residues can be identified in different food products by techniques such as high-performance liquid chromatography coupled to mass spectrometry (HPLC–MS) [8], liquid chromatography-mass spectroscopy (LC–MS) [9], capillary electrophoresis coupled with electrochemiluminescence [10]. These techniques are time-consuming and most of them are carried out in a laboratory environment by qualified personnel. Therefore, the development of an easy, fast, low-cost method with high sensitivity for the detection of Tet is of great importance.
Electrochemical biosensors have attracted much attention not only in the research but also in the commercial sectors [11]. An electrochemical biosensor is composed of an electrode with a biologically active substance to produce a measurable signal for the identification of a special analyte in respects of fast response low cost, organic and easy miniaturization [12,13,14,15]. These electrodes can be easily integrated in electronic accessories for the detection [11]. In recent years, using biosensors for food analysis has attracted considerable attention due to their special properties, including portability, straightforward sample preparation [16, 17]. Despite the different forms of nanomaterials in electrochemical sensing methods in market, reliability of commercial products is yet insufficient to achieve the practical aim [18]. According to previous studies [19,20,21], carbon nanofibers (CNFs) have been proposed for electrochemical biosensors due to advantages such a high ratio of surface-to-volume due to small diameter in mat and high conductivity owing to the binder free process. In addition, these electrodes have a good performance as a transducer in electrochemical biosensors to detect the various targets [22,23,24,25,26,27,28].
The electrospinning method is a simple technique to produce micro or nanofiber [16, 29]. A power supply with high voltage, a collector and a nozzle are used to perform the electrospinning process. The difference of potential between the collector and the nozzle results in the stretch of the polymer solution toward the collector, and the solvent evaporates and polymeric nanofibers form on the collector. Nanofibers produced by the electrospinning process have advantages such as uniformity, a high ratio of surface-to-volume and mechanical strength [23, 30,31,32,33].
AuNPs can be used to immobilize stable and highly dense single-stranded DNA (ssDNA) monolayers on the electrode surface for the preparation of biosensors. Furthermore, AuNPs increase ratio of surface-to-volume and facilitate electron transfer [34].
Aptasensors have been widely used to detect antibiotics due to their good sensitivity and selectivity. Aptamers are single-stranded oligonucleotides (ssDNA or RNA) that have a high affinity and selectivity for binding to different target molecules including nucleic acids, proteins, antibiotics [35,36,37]. Based on previous studies the electrochemical biosensor for the identification of Penicillin in milk was developed by Ebrahimi Vafaye et al., which exhibited high sensitivity [37]. In another research, Wochner et al. chose specific ssDNA aptamers for the detection of doxorubicin and daunomycin [38].
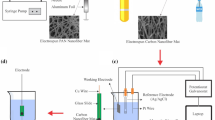
Based on our knowledge, an electrospun CNF mat electrode which is directly used as a transducer for the detection of Tet in chicken ham has not been used. In this study, CNF mat were fabricated by electrospinning method and heat-treatment process. These CNF mats were directly applied as both an electrode and matrix for the immobilization of bioreceptors. Afterwards, AuNPs were electrodeposited on the CNF mat electrode and aptamers were then self-assembled on the AuNPs modified CNF mat electrode. Eventually, the biosensor of electrochemical was employed to detect Tet in chicken ham by CV as shown in Fig. 1.
2 Materials and methods
2.1 Chemicals
In this study, polyacrylonitrile (PAN) was obtained from Polyacryl Company (Iran). Dimethylformamide (DMF), potassium ferrocyanide (K4[Fe(CN)6]), potassium ferricyanide (K3[Fe(CN)6]), and hydrogen tetracholoroaurate (HAuCl4) were bought from Merck Chemical company (Germany). Tetracycline (98%), bovine serum albumin (BSA), potassium phosphate monobasic (KH2PO4), sodium phosphate dibasic (Na2HPO4), sodium chloride (NaCl) were obtained from Sigma-Aldrich company. KH2PO4 (0.1 M) and Na2HPO4 (0.1 M) were employed to prepared of phosphate buffer solutions (PBS). Ultra-pure water was used to prepare all solutions. The aptamer of Tet was obtained from Faza Biotech Company (Iran) and its sequence was the following: 5′-thiol-(CH2)6-CGTACGGAATTCGCTAGCCCCCCGGCAGGCCACGGC TTGGGTTGGTCCCACTGCGCGTGGATCCGAGCTCCACGTG-3′.
2.2 Preparation of the modified electrode
The CNF was fabricated base on the previous works [37, 39]. In brief, 1.1 g of PAN polymer was dissolved in 8.9 mL of DMF. The solution was stirred for 9 h at 45 °C by magnetic stirring at 1000 rpm. The electrospinning process of solution of PAN was carried out with an Electroris device (Fanavaran Nano Meghyas Ltd, Co., Iran) at room temperature. The PAN solution was inserted into a syringe equipped with a 16-gauge needle, power supply with high voltage was applied to eject the solution of PAN from the spinneret to a collector. A high-voltage power supply (19 kV) was employed to create the potential difference between the solution of PAN and the collector, and as a result, the solution of PAN was ejected from the needle to the collector. In this study, the distance between the rotating collector and the nozzle was 10 cm and the rotation speed of the collector was set to 100 rpm. Then, to convert the produced nanofibers into carbon nanofibers, two stages of stabilization and carbonization were performed. For the stabilization step, the PAN nanofibers was placed in a tube furnace in an atmosphere of air for 4 h at 290 °C; and then the carbonization of stabilized nanofibers was then performed in an inert atmosphere for 1 h at 1000 °C. The heating rate for stabilization and carbonation steps were 1.5 °C min−1 and 4 °C min−1, respectively. The CNFs electrodes were spherically perforated (5 mm) and attached to copper wire for electrical contact. Finally, the electrode of CNFs was immersed in a 0.1 M gold solution and a voltage of -0.4 kV was applied for 45 s.
In the next step, the aptamer of Tet is self-assembled on the modified electrode by adding 5 μL Tet aptamer (10 μM) on the surface of Au/CNF and dried. Unbound Tet aptamer was removed from the Au/CNF electrode surface by rinsing with ultrapure water. Then, to block the remaining binding sites, PBS (5 μL) containing 10% BSA was poured on the electrode surface and kept at room temperature for 2 h. Eventually, for later use, the electrode was washed with ultrapure water and dried.
2.3 Chicken ham sample preparation
Chicken ham samples were prepared according to Prestes et al. [40]. The raw material was comminuted in a commercial blender. The base formulation consisted of 70% skinless thighs and drumsticks, about 2.1% sodium chloride, 0.5% sodium polyphosphate, 0.5% semi-refined kappa-carrageenan, 0.5% glucose, 0.1% monosodium glutamate, 0.015% sodium nitrite, 0.1% sodium erythorbate and 25% water. The suspension of chicken ham was diluted with PBS (20%) and spiked with different concentrations of Tet antibiotics (10 μL).
2.4 Characterization of nanofibers
2.4.1 SEM analysis
SEM (Philips XL-30) was used to evaluate the morphology of CNFs with an accelerating voltage of 40 kV. The presence of gold particles was also examined using an SEM equipped with EDS.
2.4.2 Raman spectroscopic analysis
Electrospun CNFs were analyzed using a Raman spectrophotometer (Senterra, Bruker, Germany) at room temperature under the following conditions: 25 mW of laser power, 758 nm of laser wavenumber and 3.0 cm−1 of resolution.
2.4.3 Electrochemical behavior
A μStat 400 potentiostat/galvanostat (DropSens, Spain) was used for all electrochemical experiments. In these experiments, platinum wire was applied as an auxiliary electrode. Besides, Ag/AgCl electrode was used as reference electrode.
3 Results and discussion
3.1 Morphology of CNFs and Raman spectroscopy
The morphology of electrospun CNFs is shown in Fig. 2a which has a porous structure with randomly oriented nanofibers. Raman spectroscopy is an efficient tool to identify carbon nanostructures. The Raman spectrum of CNFs was recorded and showed two main peak at around 1572 cm−1 (G band) and 1388 cm−1 (D band) (Fig. 2b). The characteristic peak at a wavenumber of 1388 cm−1 was attributed to disorder features of carbon structures, whereas the graphitic structures were represented by a peak at wavenumber 1572 cm−1 [22].
Figure 3a exhibits the SEM image of the AuNPs/ECNF electrode and indicates to somehow uniform patterns of dispersion of AuNPs on the ECNF mat electrode. Figure 3b shows the composition of chemical of electrode which was taken using EDS and indicated to the presence of carbon and Au.
3.2 Electrochemical behavior
3.2.1 CV characterization
CV is a useful technique to evaluate the behaviour of the electrode. In this study, the cyclic voltammograms behaviour of CNF electrode, Au modified CNF (Au/CNF) electrode, aptamer modified Au/CNF (ssDNA/Au/CNF) electrode, and Tet modified ssDNA/Au/CNF (Tet/ssDNA/Au/CNF) electrode were investigated. As presented in Fig. 4, the reduction/oxidation of [Fe(CN)6]−3/−4 pair led to a reduction/oxidation process in the range of -0.3 to 0.7 V for all electrodes. As shown in Fig. 4, after modification of the surface of the electrode with Au, the cyclic voltammetric response of [Fe(CN)6]−3/−4redox increased which was in agreement with the results of previous work [37]. The ssDNA/Au/CNF electrode had a lower peak current compared to the Au/CNF because the presence of ssDNA play a barrier role for the charge transfer among[Fe(CN)6]−3/−4and the modified electrode surface. The Tet/ssDNA/Au/CNF electrode also showed a lower cyclic voltammetric response compared ssDNA/Au/CNF, which is attributed to the reduction in charge transfer between [Fe(CN)6]−3/−4and the modified electrode surface.
3.2.2 Selectivity, stability, repeatability, reproducibility and detection limit of the Au/CNF electrode
The selectivity of the biosensor was investigated in presence of other antibiotics including acetaminophen, diclofenac, and ciprofloxacin. As presented in Fig. 5a, the current of ssDNA/Au/CNF electrode did not show a significant change in the presence of acetaminophen, diclofenac, and ciprofloxacin, indicating that no significant adsorption happen between the ssDNA and these antibiotics. However, the amount of current in the presence of Tet considerably decreased, indicating Tet attached to ssDNA/Au/CNF electrode. The change of current in the presence of Tet confirmed that the electrode had a selective function for this substance.
The linear range was evaluated and shown in Fig. 5b for the detection of Tet. According to this curve (Fig. 5b), the biosensor was able to detect the Tet in the range of 1 × 10–4 to 1 × 10–9 M with the detection limit of 1.2 × 10–10 M.
To evaluate the repeatability and reproducibility of the aptasensor, four independent electrodes were fabricated and the activity of each one was examined three times. As shown in Fig. 5c, the reproducibility (A) and repeatability (B) of the aptasensor was investigated by measuring CV current peaks and no remarkable difference was recognized in the current of these electrodes. The relative standard deviation (RSD) of the biosensors for reproducibility and repeatability was approximately 2.13% and 2.92%, which confirm the good preparation of these electrodes.
A suitable electrode should preserve its efficiency over time. For this purpose, the stability of the fabricated electrode was examined for a period of 20 days. As shown in Fig. 5d, no significant difference in the amount of current was observed during the storage, and this electrode preserved about 95% of its primary current after 20 days, exhibiting satisfactory stability.
3.2.3 Determination of Tet in chicken ham samples
For the determination of aptasensor efficiency for the Tet detection in chicken ham sample, four different Tet concentrations including 1 × 10–4, 1 × 10–5, 1 × 10–6 and 1 × 10–7 M were dropped in the diluted chicken ham samples and the investigations were performed via the proposed aptasenor. According to Table 1, recovery variations ranging from 96.92 to 104.31% exhibited that the reliability of the electrochemical biosensor was acceptable. Besides, the detection performance of the prepared aptasensor was compared with several reported biosensors and the results are presented in Table 2. The maximum residue limit of Tet in meat and eggs has set 220 and 440 nM, respectively, in European Union to preserve consumer health [41] and this aptasensor can be proposed to detect Tet for the detection in meat and eggs. In addition, this aptasensor have good repeatability and reproducibility compared to other sensors in Table 2. In this aptasensor, AuNPs modified CNF mat electrode was used to increase sensitivity of electrode due to electrical conductivity and high electrochemical activity compared to conventional bulk metal electrodes such as gold and platinum. Because, oxide layers may form on these conventional bulk metal electrodes and limit their applications in electroanalytical methods. On the other hand, using carbon paste electrodes decrease their conductivities owing to oil [27]. Therefore, several problems such as poor sensitivity and low ratio of surface-to-volume in these electrodes in electrochemical sensing are defects to be commercialized such sensing techniques. To solve these issues, several efforts have been performed to use nanomaterials for the construction of a transduction platform in electrochemical sensors. Among these nanomaterials, CNF mat in electrochemical biosensors can be directly used as an electrode to have high conductivity owing to the binder free process and a high ratio of surface-to-volume due to small diameter in mat.
4 Conclusion
To the best of the author’s knowledge, this is the first research to fabricate the electrospun CNF mat electrode for the detection of Tet with the linear range of 1 × 10–9 M to 1 × 10–4 M and the detection limit of 1.2 × 10–10 M. The present study indicated that aptasensor based on Au/CNF was a good choice for Tet detection. In this study, the designed biosensor had good reproducibility and repeatability and also exhibited good stability over time and had a good selectivity for the detection of Tet compared to other antibiotics. Therefore, the electrospun CNF mat can be used directly as an electrode which has high potential for the immobilization of bioreceptors. Despite the high potential of Au/CNF mat electrodes in electrochemical biosensor, reliability of commercial product is yet insufficient to be used in food industries.
Data availability
The data for this study are available from the corresponding author upon reasonable request.
References
N.E.A. El Hassani, A. Baraket, S. Boudjaoui, E.T.T. Neto, J. Bausells, N. El Bari, B. Bouchikhi, A. Elaissari, A. Errachid, N. Zine, Development and application of a novel electrochemical immunosensor for tetracycline screening in honey using a fully integrated electrochemical Bio-MEMS. Biosens. Bioelectron. 130, 330–337 (2019)
M. Pérez-Rodríguez, R.G. Pellerano, L. Pezza, H.R. Pezza, An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 182, 1–21 (2018)
J. Hu, X. Yang, Q. Peng, F. Wang, Y. Zhu, X. Hu, B. Zheng, J. Du, D. Xiao, A highly sensitive visual sensor for tetracycline in food samples by a double-signal response fluorescent nanohybrid. Food Control 108, 106832 (2020)
A. Önal, Overview on liquid chromatographic analysis of tetracycline residues in food matrices. Food Chem. 127, 197–203 (2011)
F. Muriuki, W. Ogara, F. Njeruh, E. Mitema, Tetracycline residue levels in cattle meat from Nairobi slaughter house in Kenya. J. Vet. Sci. 2, 97–102 (2001)
K. Bahmani, Y. Shahbazi, Z. Nikousefat, Monitoring and risk assessment of tetracycline residues in foods of animal origin. Food Sci. Biotechnol. 29, 441–448 (2020)
Z. Rouhbakhsh, A. Verdian, G. Rajabzadeh, Design of a liquid crystal-based aptasensing platform for ultrasensitive detection of tetracycline. Talanta 206, 120246 (2020)
E.V. Pokrant, A.E. Maddaleno, C.E. Araya, B.V.S. Martín, J. Cornejo, In-house validation of HPLC-MS/MS methods for detection and quantification of tetracyclines in edible tissues and feathers of broiler chickens. J. Braz. Chem. Soc. 29, 659–668 (2018)
A. Desmarchelier, S. Anizan, M. Minh Tien, M.-C. Savoy, C. Bion, Determination of five tetracyclines and their epimers by LC-MS/MS based on a liquid-liquid extraction with low temperature partitioning. Food Addit. Contam. Part A 35, 687–695 (2018)
B. Deng, Q. Xu, H. Lu, L. Ye, Y. Wang, Pharmacokinetics and residues of tetracycline in crucian carp muscle using capillary electrophoresis on-line coupled with electrochemiluminescence detection. Food Chem. 134, 2350–2354 (2012)
K. Mahato, P.K. Maurya, P. Chandra, Fundamentals and commercial aspects of nanobiosensors in point-of-care clinical diagnostics. 3 Biotech 8, 1–14 (2018)
G.S. Wilson, R. Gifford, Biosensors for real-time in vivo measurements. Biosens. Bioelectron. 20, 2388–2403 (2005)
D. Grieshaber, R. MacKenzie, J. Vörös, E. Reimhult, Electrochemical biosensors-sensor principles and architectures. Sensors 8, 1400–1458 (2008)
Y. Zhang, X. Jiang, J. Zhang, H. Zhang, Y. Li, Simultaneous voltammetric determination of acetaminophen and isoniazid using MXene modified screen-printed electrode. Biosens. Bioelectron. 130, 315–321 (2019)
L. Zhang, K. Khan, J. Zou, H. Zhang, Y. Li, Recent advances in emerging 2D material-based gas sensors: potential in disease diagnosis. Adv. Mater. Interface 6, 1901329 (2019)
L.A. Mercante, V.P. Scagion, F.L. Migliorini, L.H. Mattoso, D.S. Correa, Electrospinning-based (bio) sensors for food and agricultural applications: A review. TrAC Trends Anal. Chem. 91, 91–103 (2017)
R. Rapini, G. Marrazza, Biosensor Potential in Pesticide Monitoring, Comprehensive Analytical Chemistry (Elsevier, Amsterdam, 2016), pp.3–31
R. Gupta, N. Raza, S.K. Bhardwaj, K. Vikrant, K.-H. Kim, N. Bhardwaj, Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 401, 123379 (2021)
K. Cui, Y. Song, Q. Guo, F. Xu, Y. Zhang, Y. Shi, L. Wang, H. Hou, Z. Li, Architecture of electrospun carbon nanofibers–hydroxyapatite composite and its application act as a platform in biosensing. Sens. Actuators B Chem. 160, 435–440 (2011)
V. Vamvakaki, K. Tsagaraki, N. Chaniotakis, Carbon nanofiber-based glucose biosensor. Anal. chem. 78, 5538–5542 (2006)
L. Wu, J. Lei, X. Zhang, H. Ju, Biofunctional nanocomposite of carbon nanofiber with water-soluble porphyrin for highly sensitive ethanol biosensing. Biosens. Bioelectron. 24, 644–649 (2008)
A. Niri, R. Faridi-Majidi, R. Saber, M. Khosravani, M. Adabi, Electrospun carbon nanofiber-based electrochemical biosensor for the detection of hepatitis B virus. Biointerface Res. Appl. Chem. 9, 4022–4026 (2019)
M. Adabi, M. Adabi, Electrodeposition of nickel on electrospun carbon nanofiber mat electrode for electrochemical sensing of glucose. J. Dispers. Sci. Technol. 42, 262–269 (2021)
Y. Liu, J. Huang, H. Hou, T. You, Simultaneous determination of dopamine, ascorbic acid and uric acid with electrospun carbon nanofibers modified electrode. Electrochem. Commun. 10, 1431–1434 (2008)
H.R. Rahmani, M. Adabi, K. Pooshang Bagheri, G. Karim, Effect of processing variables on the performance of electrochemical aptasensor for determination of aflatoxin M1. Nanomed. Res. J. 5, 378–382 (2020)
S. Ebrahimi Vafaye, A. Rahman, S. Safaeian, M. Adabi, Investigation of effective parameters on electrochemical aptasensor for detection of Penicillin antibiotic. Nanomed. Res. J. 6, 73–78 (2021)
M. Adabi, S.S. Esnaashari, M. Adabi, An electrochemical immunosensor based on electrospun carbon nanofiber mat decorated with gold nanoparticles and carbon nanotubes for the detection of breast cancer. J. Porous Mater. 28, 415–421 (2021)
M. Adabi, Detection of lead ions using an electrochemical aptasensor. Nanomed. Res. J. 4, 247–252 (2019)
R. Nayak, R. Padhye, Nano fibres by electro spinning, properties and applications. J. Text. Eng. Fash. Technol. 2, 486–497 (2017)
S.-H. Tan, R. Inai, M. Kotaki, S. Ramakrishna, Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer 46, 6128–6134 (2005)
S.S. Esnaashari, S. Rezaei, E. Mirzaei, H. Afshari, S.M. Rezayat, R. Faridi-Majidi, Preparation and characterization of kefiran electrospun nanofibers. Int. J. Biol. Macromol. 70, 50–56 (2014)
N. Bhardwaj, S.C. Kundu, Electrospinning: a fascinating fiber fabrication technique. Biotechnol. Adv. 28, 325–347 (2010)
S. Torres-Giner, E. Gimenez, J.M. Lagaron, Characterization of the morphology and thermal properties of zein prolamine nanostructures obtained by electrospinning. Food Hydrocoll. 22, 601–614 (2008)
J.M. Pingarrón, P. Yañez-Sedeño, A. González-Cortés, Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 53, 5848–5866 (2008)
R. Stoltenburg, C. Reinemann, B. Strehlitz, SELEX—a (r) evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 24, 381–403 (2007)
A.D. Ellington, J.W. Szostak, In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 (1990)
S.E. Vafaye, A. Rahman, S. Safaeian, M. Adabi, An electrochemical aptasensor based on electrospun carbon nanofiber mat and gold nanoparticles for the sensitive detection of Penicillin in milk. J. Food Meas. Charact. 15, 876–882 (2021)
A. Wochner, M. Menger, D. Orgel, B. Cech, M. Rimmele, V.A. Erdmann, J. Glökler, A DNA aptamer with high affinity and specificity for therapeutic anthracyclines. Anal. Biochem. 373, 34–42 (2008)
H.R. Rahmani, M. Adabi, K.P. Bagheri, G. Karim, Development of electrochemical aptasensor based on gold nanoparticles and electrospun carbon nanofibers for the detection of aflatoxin M1 in milk. J. Food Meas. Charact. 15, 1826–1833 (2021)
R.C. Prestes, A. Graboski, S.S. Roman, A.P. Kempka, G. Toniazzo, I.M. Demiate, M. Di Luccio, Effects of the addition of collagen and degree of comminution in the quality of chicken ham. J. Appl. Poult. Res. 22, 885–903 (2013)
A. Mohammad-Razdari, M. Ghasemi-Varnamkhasti, S. Rostami, Z. Izadi, A.A. Ensafi, M. Siadat, Development of an electrochemical biosensor for impedimetric detection of tetracycline in milk. J. Food Sci. Technol. 57, 4697–4706 (2020)
A.O. Rad, A. Azadbakht, An aptamer embedded in a molecularly imprinted polymer for impedimetric determination of tetracycline. Microchim. Acta 186, 1–10 (2019)
D. Chen, D. Yao, C. Xie, D. Liu, Development of an aptasensor for electrochemical detection of tetracycline. Food Control 42, 109–115 (2014)
M. Bougrini, A. Florea, C. Cristea, R. Sandulescu, F. Vocanson, A. Errachid, B. Bouchikhi, N. El Bari, N. Jaffrezic-Renault, Development of a novel sensitive molecularly imprinted polymer sensor based on electropolymerization of a microporous-metal-organic framework for tetracycline detection in honey. Food Control 59, 424–429 (2016)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammadi, S., Pooshang Bagheri, K., Mousavi Nadushan, R. et al. Nanoarchitectonics of electrochemical aptasensor based on electrospun carbon nanofibers and gold nanoparticles for tetracycline detection in chicken ham. Appl. Phys. A 129, 176 (2023). https://doi.org/10.1007/s00339-023-06416-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06416-4