Abstract
The present study investigated effects of three different levels of crosslinking using a mixture of sodium trimetaphosphate (STMP) and sodium tripolyphosphate (STPP) on hydroxypropylated barley starches. Hydroxypropylated barley starches crosslinked with 1%, 1.5% and 2% mixture of STMP and STPP were coded as HPCL(1), HPCL(1.5) and HPCL(2.0), respectively. However, the level of hydroxypropylation employed was same for all the modifications i.e. 6%. The results showed that increase in level of crosslinking increased swelling power, solubility and water holding capacity of starches. HPCL(2.0) starch demonstrated noticeably lower percent transmittance, higher percent retrogradation and elevated peak viscosity. Harder gels were produced by dual modified barley starches. A decline was observed in thermal transition temperatures after dual modification. FTIR was unable to detect much difference among different samples. However, peak at 1414 cm−1 associated with hydroxypropylation was detected. Hydroxypropylation followed by crosslinking led to roughness and grooves formation on the surface of barley starch granules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among different cereal crops barley (Hordeum vulgare) has its own unique importance. It is used as a source of food in many parts of the world. Like other grains, barley has majorly 70% carbohydrate. Apart from starch isolation from cereal grains, proper utilization of remnants is equally important too. The leftovers after processing are termed as by-products of food or food wastes which could contain valuable functional components. The remnants of fruits and vegetables yield phenols, carotenoids, dietary fibers etc. while cereal waste generates fibers, Similarly, roots and tuber waste helps in the recovery of organic acids and phenols [1]. Such wastes are treated these days in order to achieve nutraceutical components that act as functional ingredients in food products [2]. The main purpose of development of functional food is prevention from disease and maintaining a healthy body. Exploitation of undervalued components after principle isolation/extraction is governed by “5 Stage Universal Recovery Processing” that explains the whole process form macroscopic pre-treatment to till product formation or encapsulation in five steps namely: (1) macroscopic pre-treatment (2) macro- and micromolecules separation, (3) extraction, (4) isolation-purification (5) product formation [3]. In case of barley, husk that is left after starch extraction is loaded with insoluble fibers that are potentially helpful for intestinal health. Though barley has been known for the production of malt and extraction of betaglucan, but it can also be used for the isolation of a value-added product with diversified uses in food industries i.e. “starch”.
After isolation of starch, there are some constraints in the use of natural/raw (unmodified) barley starch commercially. It is due to native starch’s irresistibility against shear and acidic conditions. To overcome these issues, modification of starch is essentially carried out. Starches can be modified by physical/chemical means or by combination of both. Hydroxypropylation is a chemical modification that involves reaction between the starch and an etherifying agent (propylene oxide) under alkaline conditions. During hydroxypropylation, hydrophilic hydroxypropyl groups are added onto the polymeric starch chain [4]. The hydrophilic nature of hydroxypropyl group weakens internal bond matrix of starch and also traps and binds water in the starch paste therefore, making it freeze thaw stable. Lower temperature of gelatinization, high peak viscosity, and improved paste clarity are some characteristics imparted by hydroxypropylation to starches [5]. During the process of crosslinking ether/ester inter-molecular linkages are formed among hydroxyl groups on two separate starch chains by reacting with multifunctional reagents [6]. Crosslinking is known to reinforce the granular structure of starch [7]. Crosslinked starches exhibit restricted swelling and demonstrate higher tolerance against shear, high temperature and low pH conditions [8].
Hydroxypropyl distarch phosphates are prepared by hydroxypropylation of starches followed by crosslinking to produce dual modified starches which are used in the food industry. Physicochemical properties of dual modified starches demonstrated significant differences when compared with native forms as reported by Hazarika and Sit [9] and Wattanachant et al. [10]. Hydroxypropylation and crosslinking are usually employed together to achieve desirable properties like appropriate gelatinization temperature, viscosity and textural properties. In order to achieve desirable properties of starch the amount of crosslinking reagent to be used depends on factors like type of crosslinking reagent, type of starch, level of hydroxypropylation on starch and efficacy of reaction. Canned, frozen, refrigerated foods, puddings, gravies and salad dressings utilize modified starches (both single and dual).
The aim of this investigation was to examine the effects of different levels of crosslinking on morphological, thermal, pasting and functional properties of hydroxypropylated barley starch.
Materials and methods
Materials
Barley grains were procured from local market of Karachi, Pakistan.
Extraction of barley starch
Extraction of barley starch was carried out by following methodology of Mehfooz et al. [11]. Barley kernels (500 g) were slightly ground and were steeped in a mixture of 1000 mL of 0.2% sodium metabisulphite and 5 mL of lactic acid. Kernels were allowed to steep at room temperature, overnight. Steeped barley kernels were then extensively washed with ample amount of water to remove residual chemicals. Kernels were blended with sufficient amount of water and screened through nylon bolting cloth. This step was repeated several times until no more of starch could be released. The starch slurry was then centrifuged at 1200×g for 5 min. The excess water was decanted and brown protein layer on the top was removed with the aid of spatula. The crude starch was suspended in 0.15% (w/v) NaOH solution followed by centrifugation at 1200×g for 5 min. One molar HCl solution was used to neutralize starch solution. After neutralization, starch slurry was again subjected to washing and centrifugation. The obtained starch palate was dried at 40 °C and finally ground to powdered form.
Chemical modifications of barley starch
Preparation of hydroxypropyl starch
Method of Lawal [4] with some modifications was used to prepare hydroxypropylated barley starch. Slurry of 500 g barley starch was prepared by suspending in 1000 mL of distilled water. Afterwards, 100 g of anhydrous sodium sulphate was added to the slurry. The mixture was then stirred for 30 min. Sodium hydroxide (1 M) was used to bring the pH to 10.5–11.0. While continuous stirring, propylene oxide (6% based on starch weight) was added. The mixture was stirred for 24 h at 35 °C in a sealed glass bottle. The slurry was then neutralized with 1 M H2SO4 followed by centrifugation at 2400×g for 5 min. The starch was washed until it gave negative test (absence of precipitates) with 1% BaCl2 solution. The starch was then oven dried at 45 °C.
Crosslinking of hydroxypropylated starch
Hydroxypropylated starch was further modified to form hydroxypropyl crosslinked (HPCL) starch by the procedure of Woo and Seib [12] with some modifications. Sodium sulphate (10 g) was added to 100 g of hydroxypropylated barley starch. The combination of (STMP and STPP) was added in different amounts (1, 1.5 and 2.0 g, on dry basis) in the ratio of 99:1 followed by the addition of 140 mL of distilled water. The pH was adjusted to 11.0 using 1 M NaOH. At 45 °C, the reaction was continued for 3 h. The pH of the suspension was maintained to 6.5 with 1 M HCl and centrifuged at 770×g for 5 min. To remove residual chemicals starch was washed with distilled water. Final drying was done at 45 °C and was then ground to fine powder. The HPCL starches containing (1, 1.5 and 2.0%) of mixture of STMP and STPP were abbreviated as HPCL(1), HPCL(1.5) and HPCL(2.0), respectively.
Morphological properties
The granular surface morphology of native and HPCL modified starches were evaluated using scanning electron microscope (JSM, 6380A, Jeol Japan). The starch samples were mounted on SEM stub and were coated with gold before analysis.
FTIR analysis
Fourier transform infrared spectrometer was used to analyze native and HPCL starches, scanned 64 times over a range of 4000 cm−1 and 400 cm−1 at a resolution of 4 cm−1 using Tensor II Bruker spectrometer.
Determination of percent hydroxypropyl groups and percent phosphorous content
The percent hydroxypropyl groups and percent phosphorous content were determined by using the methods of Lawal [4] and Ashwar et al. [13], respectively.
Swelling power (SP) and solubility
To determine swelling power (SP) and solubility of native and dual modified starches methodology of Ali and Hasnain [14] was used. Starch (0.6 g) was weighed in centrifuge tubes. Thirty millilitres of distilled water was added to the tubes. Tubes were allowed to heat at 90 °C for 30 min with occasional stirring. After cooling, tubes to room temperature, starch suspension was centrifuged at 6500×g for 15 min. Swelling power was calculated by the formula:
where W2 is the weight of residue after centrifugation, W1 is the weight of empty dry centrifuge tube and W is the Weight of dried starch before heating.
For determination of solubility 5 mL aliquots of supernatant (obtained after centrifugation) was dried at 110 °C to constant weight. The obtained weight is the amount of solubilised starch at 90 °C and was used to calculate solubility of starch.
Water holding capacity (WHC)
Water holding capacity (WHC) was calculated by following the procedure of Ali and Hasnain [15]. Starch (0.5 g) was measured in centrifuge tubes. Ten millilitres of distilled water was added to the tubes followed by heating at 90 °C for 15 min. Tubes were centrifuged at 2350×g for 10 min after cooling to room temperature. The tubes were weighed with the obtained starch cake. The formula used to calculate WHC was:
where W is the weight of starch + centrifuge tube (dry basis) and W1 is the weight of swollen grains + centrifuge tube.
Percent light transmittance
Modified method of Lawal [16] was used to determine percent light transmittance of native and dual modified barley starches. One percent (w/v) starch slurry was heated in a boiling water bath for 30 min with occasional mixing. The slurry was allowed to cool to room temperature. Light transmittance (%T) of slurry was measured at 650 nm against distilled water as blank using UV–Visible Spectrophotometer (Model V670, JASCO Corporation, Tokyo, Japan). The samples were refrigerated and %T was measured on 1, 3 and 7 days in comparison to %T at 0 day.
Temperature sweep measurements
HR-1 Hybrid Rheometer (TA Instruments, USA) was used to determine temperature sweep measurements following methodology of Li et al. [17]. A cone-plate geometry (40 mm, 2°) was used. The gap, strain and frequency were set at 1000 μm, 1.0% and 10.0 rad/s, respectively. Ten percent starch suspension (w/w) was loaded on the Peltier plate and covered with a thin layer of silicon oil to avoid evaporation losses. The starch sample was subjected to a temperature ramp ranging from 25 to 95 °C at a rate of 5 °C/min. The sample was again cooled from 95 to 25 °C at the rate of 5 °C/min in order to obtain viscosity profile during heating and cooling. The temperature at which the first rise in viscosity was observed is known as pasting temperature (PT) while peak viscosity (PV) was the maximum dynamic viscosity observed during the heating ramp. Time to reach peak viscosity (TPV) was directly recorded from the graph. V95 °C was defined as viscosity at 95 °C during the heating cycle. Whereas, V25 °C was observed at 25 °C (i.e. at the end of cooling cycle). The Trios (V.4.1.031739) software was used to find the above-mentioned parameters.
Textural analysis
Texture of starch gels was analyzed in accordance with the methodology of Mehfooz et al. [11]. Gels were prepared using 10% (w/w) starch solution by heating in a water bath at 90 °C with occasional stirring. Starch gels were poured in plastic cups of 3.5 cm diameter. Two cyclic penetration test was performed. Cylinder probe having a diameter of 1 cm was used with a preload speed of 10 mm/min. Hardness, springiness, cohesiveness, adhesiveness and gumminess were some observed parameters. Test was performed on freshly prepared gels and after 7 days of refrigerated storage of gels.
Thermal properties
Method of Ali and Hasnain [18] was used to study thermal properties of starches using a differential scanning calorimeter (DSC Q10, TA Instruments, USA). The retrogradation studies were conducted after 14 days storage of hermetic pans at 4 °C. Two milligrams of starch was placed in aluminium pans. Six micro litre of distilled water was added to the pan with the aid of microliter syringe and then sealed hermetically using Tzero hermetic lid. The sealed pans were heated from 30 to 110 °C at a heating rate of 10 °C/min. The pans were rescanned after 14 days of refrigerated storage. Onset, peak and conclusion temperature and enthalpy of gelatinization were some observed parameters. Percent retrogradation was calculated using formula:
where ∆Hret is the enthalpy of retrogradation and ∆Hgel is the enthalpy of gelatinization.
Statistical analysis
The reported data was the mean of triplicate measurements. The SPSS software (Version 17.0. Inc., Chicago, USA) was used for statistical treatments. Analysis of variance was performed at significance level of 0.05. Duncan’s multiple range test was used to compare results at 95% confidence intervals.
Results and discussion
Morphological properties
Morphological properties were studied to understand the surface structure of starch granules. Oval shaped starch granules were observed for native and HPCL starches. The scanning electron micrographs are given as Figs. 1 and 2. The granules of native and HPCL starches ranged between 10.6 to 26.0 µm. NB starches were found to have smoother surface as compared to the HPCL starches. However, at higher magnification some grooves were observed in the NB granule. In case of dual modified HPCL starches, more grooves and surface erosion/roughness was observed at higher magnification as compared to NB starches due to reaction with harsh chemicals under alkaline conditions.
FTIR analysis
Figure 3 shows the FTIR spectra of native and hydroxypropylated crosslinked barley starches. Much similarity in spectrum was observed for native and modified starches. Characteristic peaks of starch include absorption bands at 3200–3900 cm−1, 1641 cm−1 and 900–1250 cm−1 corresponding to O–H stretching, O–H bending of the absorbed water and C–H stretching, respectively. A new peak at 1414 cm−1 was detected i.e. exclusive to HPCL modified starches due to C–H bending [19]. However, the spectra (native and modified starches) observed between the wavelength of 400 cm−1 and 4000 cm−1 did not show much diversity. The possible reason for this could be the introduction of only –C–O–C– ether linkages to the starch chain after hydroxypropylation. The anhydroglucose unit present in native starch is linked by the glycosidic linkages which also contains hemiacetal group [20]. Due to lower level of crosslinking in dual modified starches, its characteristic peak was not detected. The absence of P–O and P–O–C inherent peaks was also reported by [21,22,23].
Percent hydroxypropyl content and percent phosphorous groups
Percent hydroxypropyl content of HPCL starches were found to be 0.577% as the level of hydroxypropylation employed was the same for all three batches. However, significant rise in percent phosphorous groups were observed with the increase in use of mixture of STMP and STPP. The % phosphorus groups were found to be 0.0045, 0.0061 and 0.0155% for HPCL(1), HPCL(1.5) and HPCL(2.0), respectively (See Table 1). Both hydroxypropyl content and phosphorous groups were found to be in accordance with the limits set by JECFA i.e. less than 7% and 0.4% for hydroxypropyl groups and phosphorus content, respectively.
Swelling power (SP) and solubility
Swelling power (SP) and solubility of native and dual modified starches are presented in Table 1. It was observed that SP and solubility declined with the increase in level of crosslinking. Native barley starch (NB) showed lower values for SP and solubility in contrast to their modified forms. However, NB and HPCL(2.0) starches differed insignificantly. The increase in SP and solubility in comparison to NB is due to the introduction of hydroxypropyl group that is capable of disrupting inter and intra molecular hydrogen bonds in the starch chains. This results in the weakening of starch’s granular structure by easing water percolation [9]. Crosslinking strengthens bonds between starch chains, thus restricting water uptake by granules and restricts swelling [24]. It could be observed from Table 1 that increase in level of modifications significantly decreased SP and solubility among HPCL starches. Since, level of hydroxypropylation was same for all HPCL modified starches, it could be assumed that reduction of SP and solubility was mainly governed by the phenomenon of crosslinking. It is a known fact that hydroxypropylated starches are hydrophilic in nature and tends to absorb more water. However, the cross-linking between two different hydroxyl groups causes the granules to become compact and absorb less water than that of native starch. For HPCL(1) starch both SP and solubility increased as compared to NB. However, with the increase in level of crosslinking (HPCL(2.0)) SP and solubility showed a decline. Another possible reason for this could be due to the weakening of starch’s internal structure by hydroxypropylation that allows more penetration of crosslinking reagent. Similar trend was also observed by Hazarika and Sit [9].
Water holding capacity (WHC)
The water holding capacity (WHC) is known as the ability to absorb water and to hold it even after treatment with external forces. Table 1 represents WHC of native and modified starches. Native barley starch was observed to have the least WHC as compared to its modified forms. Modified starches showed improved WHC with respect to NB due to the incorporation of hydrophilic hydroxypropyl groups. Increment in WHC after hydroxypropylation is also reported by Waliszewski et al. [22]. However, WHC declined significantly with the increase in level of crosslinking due to the addition of intra and intermolecular bonds at various positions in starch granules.
Percent light transmittance
Table 2 demonstrates clarity of native and hydroxypropylated crosslinked starches. Dual modification led to substantial decrease in percent transmittance from 52.10 to 18.52%. More pronounced reduction was observed with the increase in the level of crosslinking. Similar results were also observed by Hazarika and Sit [9], Wattanachant et al. [25] and Van Hung and Morita [26]. This might be due to the formation of diphosphate crosslinks. It could also be suggested that structure of modified starches seemed to be almost intact, resulting in higher turbidity. The higher turbidity facilitates the phenomenon of reflection rather than transmittance resulting in lower clarity [27].
Temperature sweep measurements
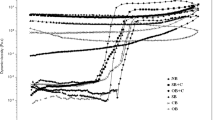
Table 3 represents pasting profile of native and HPCL starches. The pasting profile of starches evaluates the suitability of starch for commercialization. Pasting temperature (PT i.e. temperature that marks first rise in viscosity) of HPCL modified starches was lesser than that of NB. However, the decline was insignificant among the modified forms. Similar trend was also reported by Chuenkamol et al. [28], Lawal et al. [29] and Hazarika and Sit [9]. Increment in peak viscosity (PV) was observed for HPCL(1.5) and HPCL(2) starches. Higher PV can be ascribed to highly integrated granules of starch. Increased level of crosslinking provides more resistance towards disintegration due to formation of intra and inter-bonding among the granules corresponding to high viscosity [12]. Native barley starch took longer time (TPV) to reach peak viscosity. Modifications significantly reduced TPV owing to the ease in gelatinization manifested by decrease in organizational structure of starch due to insertion of functional groups. Viscosities at 95 °C (V 95 °C) and 25 °C (V 25 °C) i.e. hot and cold viscosities were insignificantly different for native and different modified forms of barley starches.
Thermal properties
The DSC parameters are presented in Table 4. Transition temperatures (To, Tp and Tc) declined significantly with modifications. Similar results were also obtained by Thirathumthavorn and Trisuth [30]. Decrease in thermal transition temperatures was attributed to the addition of hydroxypropyl groups that disrupts the internal structure, resulting in decreased crystallinity and ease in gelatinization. The values for To, Tp and Tc were found to be higher with the increasing crosslinking levels. Jyothi et al. [31] also observed the same for crosslinked cassava starch. Gelatinization enthalpy (ΔHgel) presents the measure of overall crystallinity that indicates disruption in molecular order within the granule [30]. Theoretically, ΔHgel of modified starches should have decreased compared to native starch. However, in this study, the enthalpy of HPCL(1.5) and HPCL(2.0) was not significantly different compared to native barley starch as expected decline in enthalpy due to hydroxypropylation was counterbalanced by crosslinking which usually increases the enthalpy due to formation of diphosphate links in starch. Only HPCL(1.0) demonstrated decline in enthalpy compared to NB which could be speculated as HPCL(1.0) had comparatively lower level of crosslinking compared to HPCL(1.5) and HPCL(2.0) (See Table 1). Similar results were also reported by Morikawa and Nishinari [32]. Starch molecules recrystallize when the gel is stored. A new endothermic transition was achieved when these aged gels were exposed to heat. This new transition is caused by melting of crystallized amylopectin. The retrogradation enthalpy corresponds to the order–disorder transition of crystallites in the areas with low crystallinity [33]. Percent retrogradation (%R) of HPCL(1) and HPCL(1.5) starches were insignificantly different from NB. However, HPCL(2.0) showed higher %R as compared to other samples. The results obtained for HPCL(2.0) negated the fact that hydroxypropylation and crosslinking has synergistic effects on reducing retrogradation of gelatinized starch as reported by Kaur et al. [34] and Yook et al. [35]. However, the higher value for % R could be due higher level of crosslinking. According to Jyothi et al. [31], crosslinking results in well-organized structure in starch which consequently increases the degree of retrogradation.
Textural analysis
Starch plays a pivotal role in developing texture of products. Texture of freshly prepared and stored gels is presented in Table 5. Hardness is the force necessary to bite a sample. Modified starches showed significant increment in hardness. Similar trend was also observed for stored gels. This increment is governed by strengthening of bonds due to crosslinking [36]. Springiness also termed as ‘elasticity’ is the rate at which a deformed sample regains its original size and shape. Springiness did not show significant differences between native and modified forms for both fresh and stored starches.
Cohesiveness represents strength of internal bonds in the sample. Fresh NB starch formed the most cohesive gel however, cohesiveness of NB gel reduced significantly on cold storage. On the other hand modified starches demonstrated significantly lower cohesiveness as compared to NB starch. Modified starch gels, upon storage, retained their integrity represented by higher values of cohesiveness as compared to NB starch gels which could be due to formation of diphosphate crosslinks. The starches with lower values of cohesiveness are suitable for use in canned products and soups as it reduced the aggregation of starch chains for a longer period of time. Adhesiveness is a surface property that determines sticking ability of starch gels to other materials [37]. Freshly prepared HPCL(1) and HPCL(1.5) starch gels were found to be more adhesive than HPCL(2.0) starch gel, which might be due to the stronger internal matrix that curtails stickiness towards probe [38]. Starch with lower adhesiveness favours the development of imitation cheese which sticks less to the packaging material [39]. Gumminess is the amount of energy needed to disintegrate the gel. The least gummy gels were formed by NB.
Conclusion
Dual modification of barley starch through hydroxypropylation and crosslinking led to significant changes in the chemical and physical properties of starch granules. The outcome of higher substitution level of crosslinking was also prominently observed as the level of hydroxypropylation was same for all the three dual modifications. Crosslinking of hydroxypropylated starches improved functional properties making starch suitable for a wide range of potential applications. Hydroxypropylated distarch phosphates can be utilized as thickening agents in toppings, fillings, fruit preserves and soups. HPCL starches are resistant towards high temperature and low pH which make it capable for use in retort products. Modification also alters solubility, water holding capacity, swelling power, clarity, pasting temperature, peak viscosity etc. However, the extent of crosslinking should be carefully selected in order to achieve modified starch for specialty products.
References
C.M. Galanakis, Trends Food Sci. Technol. 26, 68–87 (2012)
C.M. Galankis, Food Bioprod. Process. 91, 575–579 (2013)
C.M. Galankis, Trends Food Sci. Technol. 42, 44–63 (2015)
O.S. Lawal, LWT-Food Sci. Technol. 44(3), 771–778 (2011)
S. Senanayake, A. Gunaratne, K.K. Ranaweera, A. Bamunuarachchi, Int. J. Food Sci. 2014, 148982 (2014). https://doi.org/10.1155/2014/148982
R. Wongsagonsup, T. Pujchakarn, S. Jitrakbumrung, W. Chaiwat, A. Fuongfuchat, S. Varavinit, S. Dangtip, M. Suphantharika, Carbohydr. Polym. 101, 656–665 (2014)
V. Acquarone, M. Rao, Carbohydr. Polym. 51(4), 451–458 (2003)
H.-J. Chung, K.-S. Woo, S.-T. Lim, Carbohydr. Polym. 55(1), 9–15 (2004)
B.J. Hazarika, N. Sit, Carbohydr. Polym. 140, 269–278 (2016)
S. Wattanachant, S.K.S. Muhammad, D.M. Hashim, R.A. Rahman, Songklanakarin J. Sci. Technol 24(3), 439–450 (2002)
T. Mehfooz, T.M. Ali, A. Hasnain, J. Food Meas. Charact. 13, 1058–1069 (2019)
K. Woo, P. Seib, Cereal Chem. 79(6), 819 (2002)
B.A. Ashwar, A. Gani, A. Shah, F.A. Masoodi, Int. J. Biol. Macromol. 105, 471–477 (2017)
T.M. Ali, A. Hasnain, Int. J. Polym. Anal. Charact. 16(3), 187–198 (2011)
T.M. Ali, A. Hasnain, Int. J. Food Prop. 17(3), 523–535 (2014)
O.S. Lawal, Carbohydr. Res. 339(16), 2673–2682 (2004)
Y. Li, C.F. Shoemaker, J. Ma, X. Shen, F. Zhong, Food Chem. 109(3), 616–623 (2008)
T.M. Ali, A. Hasnain, Int. J. Polym. Anal. Charact. 17(3), 227–234 (2012)
T. Woggum, P. Sirivongpaisal, T. Wittaya, Int. J. Biol. Macromol. 67, 490–502 (2014)
D.-H. Kim, S.-K. Na, J.-S. Park, J. Appl. Polym. Sci. 88(8), 2100–2107 (2003)
L. Yang, Y. Zhou, Y. Wu, X. Meng, Y. Jiang, H. Zhang, H. Wang, Carbohydr. Polym. 137, 305–313 (2016)
B.Z. Li, L.J. Wang, D. Li, Y.L. Chiu, Z.J. Zhang, J. Shi, X.D. Chen, Z.H. Mao, J. Food Eng. 92(3), 255–260 (2009)
D.M. Suflet, G.C. Chitanu, V.I. Popa, React. Funct. Polym. 66(11), 1240–1249 (2006)
S.H. Koo, K.Y. Lee, H.G. Lee, Food Hydrocoll. 24(6–7), 619–625 (2010)
S. Wattanachant, K.M.A.T. Muhammad, D.M. Hashim, R.A. Rahman, Food Chem. 80(4), 463–471 (2003)
P. Van Hung, N. Morita, Carbohydr. Polym. 59(2), 239–246 (2005)
K.N. Waliszewski, M.A. Aparicio, L.A. Bello, J.A. Monroy, Carbohydr. Polym. 52(3), 237–242 (2003)
B. Chuenkamol, C. Puttanlek, V. Rungsardthong, D. Uttapap, Food Hydrocoll. 21(7), 1123–1132 (2007)
O.S. Lawal, O.O. Ogundiran, E.K. Adesogan, B.M. Ogunsanwo, O.A. Sosanwo, Starch Stärke 60(7), 340–348 (2008)
D. Thirathumthavorn, T. Trisuth, Int. J. Food Prop. 11(4), 858–864 (2008)
A.N. Jyothi, S.N. Moorthy, K.N. Rajasekharan, Starch Stärke 58(6), 292–299 (2006)
K. Morikawa, K. Nishinari, Carbohydr. Polym. 43(3), 241–247 (2000)
C. Perera, R. Hoover, Food Chem. 64(3), 361–375 (1999)
L. Kaur, N. Singh, J. Singh, Carbohydr. Polym. 55(2), 211–223 (2004)
C. Yook, U.-H. Pek, K.-H. Park, J. Food Sci. 58(2), 405–407 (1993)
H. Liu, L. Ramsden, H. Corke, Starch Stärke 51(7), 249–252 (1999)
M. Pons, S.M. Fiszman, J. Texture Stud. 27(6), 597–624 (1996)
G. Bultosa, J.R.N. Taylor, Starch Stärke 56(1), 20–28 (2004)
N.A. Butt, T.M. Ali, S. Arif, A. Hasnain, J. Food Process Eng 42, e13297 (2019)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehfooz, T., Ali, T.M., Ahsan, M. et al. Morphological, functional and thermal characteristics of hydroxypropylated-crosslinked barley starches. Food Measure 15, 237–246 (2021). https://doi.org/10.1007/s11694-020-00624-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00624-9