Abstract
The main postharvest pathogen of citrus fruit is Penicillium digitatum. The present study looked at the role of Rhodotorula mucilaginosa enhanced with 0.2 mM salicylic acid (SA) on pH, lignin content accumulation and growth dynamics as a resistance mechanism against P. digitatum in orange fruit. Our findings revealed that the increase in lignin content at 20 °C storage temperature resulted in the significant decrease (P ≤ 0.05) in the lesion diameter in fruit treated with R. mucilaginosa enhanced with or without 0.2 mM SA compared to the untreated fruit (control). In addition, the pH values of the fruit treated with R. mucilaginosa enhanced with or without 0.2 mM SA were 4.43 ± 0.07 and 4.15 ± 0.11, respectively, around the infection site compared to the untreated group. Moreover, the in vivo trial showed that the addition of 0.2 mM SA to the antagonist augmented its growth, and subdued substantially the hyphae and spore germination of P. digitatum in vitro. Also, lower levels of malondialdehyde (MDA) and hydrogen peroxide (H2O2) content were observed in fruit treated with the antagonist enhanced with or without 0.2 mM SA compared to the control. The results established that pH, lignin content accumulation and growth of the yeast played a significant role in the control of green mold decay of orange fruit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The application of biological control in the management of postharvest diseases of fruit and vegetables places it above all other methods of disease control [1]. Also, consumers request to reduce chemical residue on fruits and vegetables to tolerable levels makes biological control the ideal method of disease control [2]. Likewise, the human safeness and environmental friendliness of biological control procedures give its credibility over other methods of disease control [3]. For the past year’s advancement in the management of diseases of fruit and vegetables using biological control has been achieved [4, 5]. In realisation to this, diverse biological control agents (BCAs) [6, 7], food additives [8], bioactive compounds [9, 10], as well as techniques involved in biological control have been explored to clarify and develop biocontrol products. Therefore, to improve the capability and intensify the efficacy in disease control it is imperative to understand the mechanisms of action of BCAs [11, 12].
Salicylic acid is a compound found in a wider variety of plants, with a considerable role in growth and development [13, 14]. It exhibits several defense-related genes and have shown some health benefit in the prevention of cardiovascular diseases in human being [15, 16]. Also, studies have shown that SA combines with several microbial antagonist to improve the resistance of fruit to pathogens which includes, Botrytis cinerea in grapes [17], Colletotrichum gloeosporioides in mangoes [18] and Penicillium expansum in sweet cherry [19].
The efficacious ability of the phylloplane yeast R. mucilaginosa has earlier been reported [20]. Previous studies have it that, B. cinerea on geranium seedlings was controlled by the combined application of fungicides with R. mucilaginosa [21]. Also, the use of R. mucilaginosa enhance with phytic acid have been stated to control blue mold decay of apples [22]. Similarly, the management of green mold decay by R. mucilaginosa enhance with SA has also been reported [23]. To further explore the search for alternative approach to control the fungus (P. digitatum), our study chose to administer R. mucilaginosa with SA to further comprehend the mechanism of action that controls green mold decay of oranges.
Lignin accumulation is one of the major properties against fungal infection [24]. Hence, induce lignification has been stated as an active mechanism of plant to biotic and abiotic stress as well as pathogen invasion [24,25,26,27,28].
Oranges form the greater part of citrus fruit grown in the world [29, 30]. As a result, a greater proportion of orange crops grown is used for juice production which is rich in nutrient and biologically active compounds [31]. Nevertheless, orange fruit is vulnerable to many diseases which results in economic losses [32].
Prevailing statistics on the biological control of fruit and vegetables infection suggest that there is scarcity of information relating to the influence of pH, growth dynamics and lignin content accumulation in enhancing disease control. This study, therefore evaluate (1) the effect of enhancing R. mucilaginosa with or without SA on spore germination and hyphae development of P. digitatum in vitro (2) the effect of augmentation of R. mucilaginosa on fruit surface with or without SA (3) the amelioration of lignin content and the effect of pH in orange fruit by the application of R. mucilaginosa with or without SA (4) the effect of the antagonist on MDA and H2O2 activity of orange fruit.
Materials and methods
Fruit
Oranges (Citrus sinensis (L.) Osbeck) cultivar ‘Gongchuan’ were collected from an orchard in Zhenjiang, China. Fruit were of uniform maturity with the absence of injury. The fruit were disinfected with 0.1% sodium hypochlorite for 2 min, rinsed with tap water and dried at room temperature Ahima et al. [23].
Pathogen
The method employed by Li et al. [33] was used in the isolation of P. digitatum. Principally, P. digitatum was maintained on potato dextrose agar (PDA) comprising 200 mL of boiled potatoes extract, 20 g dextrose, 20 g agar and 800 mL of sterile distilled water at 4 °C. Growth was enhanced in PDA prior to use at 25 °C. After 7 days, spores of P. digitatum were scraped from PDA and suspended in distilled water Ahima et al. [23]. The required concentration of 1 × 104 spores/mL was determined using the hemocytometer.
Antagonist
R. mucilaginosa was isolated from peach [22]. Growth was enhanced in nutrient yeast-dextrose agar (NYDA) containing 8 g of nutrient broth, 5 g of yeast extract, 10 g of dextrose, 20 g of agar and 1000 mL of distilled water. After that, nutrient yeast-dextrose broth (NYDB) was prepared (8 g of nutrient broth, 5 g of yeast extract, 10 g of dextrose and 1000 mL of distilled water) with 50 mL each allotted into 250 mL Erlenmeyer flask. Afterward, a bacteriological loop was used to transfer the R. mucilaginosa colonies into each flask and incubated in a rotary shaker at 180 rpm for 20 h at 28 °C. Centrifugation was done at 8000 × g for 10 min, and yeast cells were washed twice with sterile distilled water. Thereafter, re-suspended in sterile distilled water and adjusted to the required concentration with a hemocytometer [23].
Salicylic acid
Salicylic acid was bought from Sangon Co., Shanghai, China.
The influence of SA on R. mucilaginosa on spore germination and mycelial development of P. digitatum in vitro
The method employed by Apaliya et al. [34] with modifications was used to assay the germination of spores and hyphae development of P. digitaum in potato dextrose broth (PDB). Treatments are as follows: To each 250 mL Erlenmeyer flask containing 50 mL PDB was added 1 mL of the following (1) cell suspension of R. mucilaginosa (1 × 108 cells/mL) alone, (2) cell suspension of R. mucilaginosa (1 × 108 cells/mL) + 0.2 mM SA, (3) 0.2 mM SA, and (4) sterile distilled water as a control. At the same time, to each PDB flask, 1 mL of (1 × 104 spores/mL) spore suspension of P. digitatum was added. Incubation was done for 18 h with agitation at 75 rpm at 25 °C. The hyphae length and spore germination were measured using a micrometer and a microscope and approximately 100 spores were observed per treatment. There were three replications and the experiment was conducted three times.
Population growth of R. mucilaginosa enhanced with SA on the surface of oranges stored at 20 °C and 4 °C
This study was assayed using the method employed by Yuan et al. [35]. Briefly, three circles approximately 10 mm in diameter were made at the equator of each fruit with a marker pen. Afterward, 30 μL of R. mucilaginosa suspension (1 × 108 cells/mL) or R. mucilaginosa suspension (1 × 108 cells/mL) + 0.2 mM SA was added to each center and spread evenly with a coated bar. The treated fruit were kept in plastic baskets, wrapped with polythene films and sampled at 0, 1, 2, 3, 4, 5 and 6 days for 20 ℃ and at 0, 3, 6, 9, 12, 15, 18 days for 4 ℃ respectively. Dependent on the different storage time and temperature the growth of R. mucilaginosa on the surface of the fruit were determined as follows.
The surface tissues were detached with a sterilized knife along the marked circles and ground completely in a sterile mortar with 30 mL of sterile 0.85% sodium chloride solution. Serial tenfold dilutions were made and 100 µL of each dilution was spread on nutrient yeast-dextrose agar (NYDA) plate. Counting of colonies were done and expressed as log10 CFU/mL. Each group was composed of 24 oranges with three replications and the experiment was conducted two times.
Assessment of lignin content in the wounded site of orange fruit
The lignin content in the wounded site of orange fruit were determined by the method employed by Mahunu et al. [36]. Three identical and evenly distributed wounds were made and treated as described by the method of Ahima et al. [23]. To each wound 30 µL of (1) R. mucilaginosa (1 × 108 cells/mL) alone, (2) R. mucilaginosa (1 × 108 cells/mL) + 0.2 mM SA, (3) 0.2 mM SA and (4) sterile distilled water as control was inoculated. After 2 h of aeration, 30 µL suspension (1 × 104 spores/mL) of P. digitatum was injected into each wound. Samples were stored by the method employed by Zhang et al. [12]. At different time points of 0, 3, 6, 9, 12, and 15 days the lignin content of orange fruit were determined at storage temperature of 20 °C and a relative humidity of 95%.
For the evaluation of lignin content, the method of Nafussi et al. [37], was employed. Briefly, excises orange disks freeze-dried for 4 days were grounded into a fine powder. Through Whatman 1 filter paper each sample in an orderly manner was washed with the following: water, ethanol, acetone, and diethyl ether until the washed tissue became colorless. Subsequently, a solution of 25% (w/w) acetyl bromide in acetic acid (2.5 mL) and HClO4 (70%, 0.12 mL) was then used to digest 20 mg of each sample after drying the final powder for 1 h at 70 °C and with shaking heated in water bath for 30 min at 70 °C. To the reaction tube, 12 mL of acetic acid was then added to 10 mL of 2 M NaOH after cooling with ice. Afterward, a clear solution was achieved by centrifuging 1.5 mL of the resultant solution at 15, 000 × g for 30 min at 4 °C. For each solution, five serial fold dilution was made and absorbance recorded at 280 nm. The experiment was conducted twice with three replications.
Assessment of pH in the wounded site of orange fruit
The evaluation of pH of individual fruit mesocarp was assayed by the method of Neri et al. [38] with modifications. The pH values were pooled from three wounds along the equatorial section of the fruit. Treatments are as follows: to each wound, 30 μL of the following was inoculated (1) cell suspension of R. mucilaginosa (1 × 108 cells/mL) alone, (2) cell suspension of R. mucilaginosa (1 × 108 cells/mL) + 0.2 mM SA, (3) 0.2 mM SA, and (4) sterile distilled water as a control. Subsequent to 2 h air drying, fruit were inoculated with 30 µL spore suspension (1 × 104 spores/mL) of P. digitatum, before storage at 20 °C and relative humidity (RH) of 95%. The pH of fruit were measured by placing the electrode InLab 427 Mettler Toledo at a depth of 15 mm through the wounds. There were 24 fruit per treatment and the experiment was conducted twice with three replications.
Assessment of the extent of the lesion in the wounded site of orange fruit
The lesion diameter on the equatorial sections of each fruit wound were measured by the method employed by Morales et al. [39] with modifications. Treatments are as follows: to each wound 30 μL of the following was inoculated (1) cell suspension of R. mucilaginosa (1 × 108 cells/mL) alone, (2) cell suspension of R. mucilaginosa (1 × 108 cells/mL) + 0.2 mM SA, (3) 0.2 mM SA, and (4) sterile distilled water as a control. Subsequent to 2 h air drying of inoculated wounds 30 μL (1 × 104 spores/mL) spore suspension of P. digitatum was injected before storage at 20 °C and relative humidity (RH) of 95%. Three perpendicular diameters from individual lesions were measured from each fruit using a pair of calipers. Each group was composed of 24 fruit with three replications and the experiment was conducted two times.
Effect of R. mucilaginosa and R. mucilaginosa enhanced with SA on MDA and H2O2 activities in oranges
Malondialdehyde (MDA)
Lipid peroxidation was expressed as MDA content and assayed using the method employed by Bradford [40]. Firstly, 1 mL of enzyme extract was mixed with 2 mL of 0.5% thiobarbituric acid (TBA) in 15% trichloroacetic acid. The mixture was then subjected to heat in a water bath at 95 °C for 20 min, followed by cooling in ice for 5 min. Afterward, centrifugation of the mixture was carried out at 10,000 × g for 15 min at 4 °C, and absorbance measured at 532 nm. The specific absorbance was deducted from a non-specific absorbance (600 nm) and expressed as unit per gram fresh weight.
Hydrogen peroxide (H2O2)
The hydrogen peroxide (H2O2) content of orange fruit were measured in accordance with the method described by Patterson et al. [41]. Briefly, 3 g of orange tissues was used to extract H2O2. Five milliliter (5 mL) of 100% cold acetone was used to homogenize the sample and then centrifuged at 12,000 × g for 20 min at 4 °C. Absorbance was recorded at 412 nm and reported as mg/kg fresh weight.
Statistical analysis
All data were subjected to one-way analysis of variance (ANOVA) using Minitab version 17 statistical package (Pennsylvania State University, USA). The mean values of treatments were compared by Tukey test and assessed at P ≤ 0.05 level.
Results and discussion
Effects of R. mucilaginosa enhanced with SA on spore germination and hyphae development of P. digitatum in vitro
Our earlier research using R. mucilaginosa with diverse concentrations of SA exhibited a promising result against P. digitatum with 0.2 mM SA been the best concentration. It was observed that R. mucilaginosa enhanced with 0.2 mM SA was able to reduce natural decay and upheld the organoleptic properties of orange fruit [23]. In addition, the treatment was able to improve the growth of R. mucilaginosa in orange wounds at 20 °C and 4 °C, whiles subduing the growth of P. digatum in vitro. Again, results from the scanned electron micrograph showed that R. mucilaginosa enhanced with 0.2 mM SA hindered the growth of P. digitatum. This is accredited to literature as competition for nutrient and space with the pathogen [42, 43]. Furthermore, our study showed that the combined treatment significantly boosted the defense enzyme activities in orange fruit. We, therefore, decided to investigate further the ability of the combined treatment of R. mucilaginosa and 0.2 mM SA on the influence of spore germination and hyphae development, population growth on the surface of orange fruit, lesion diameter, pH, lignin content accumulation, MDA and H2O2 activity.
The elucidation of mechanisms involved in postharvest control of disease is dependent on the use of biological control agents [4, 11, 44]. As pointed out by Paster et al. [45] complex mechanism of action is attained by the combination of more treatments which interrupts the possible development of pathogen resistance. From our study, it was revealed that R. mucilaginosa enhanced with or without 0.2 mM SA subdue the growth of spores of P. digitatum compared to the untreated group. The results showed that the percentage of P. digitatum spores that was incubated in the PDB containing R. mucilaginosa enhanced with 0.2 mM SA was 15% whiles those spores in PDB containing only R. mucilaginosa, 0.2 mM SA and the control were 21%, 29.5% and 80% respectively (Fig. 1a). Furthermore, as shown in (Fig. 1b) it was noticed that the hyphae length of P. digitatum was markedly controlled by R. mucilaginosa enhanced with 0.2 mM SA compared to R. mucilaginosa, 0.2 mM SA and the control treatments. This indicates that the spore germination and hyphae of P. digitatum were better controlled by R. mucilaginosa enhanced with 0.2 mM SA. A significant difference (P ≤ 0.05) was observed among the treatments (Fig. 1a, b). Droby [46] detailed that the initial upsurge in BCA’s population after inoculation into the host allows for good inhibition of hyphae and spore germination [47]. This finding is in line with our results as the hyphae and spore of P. digitatum were significantly inhibited due to the earlier increase in the population of the antagonist. These results demonstrated that SA may have improved the viability of R. mucilaginosa.
Effect of R. mucilaginosa enhanced with SA on a spore germination and b hyphae length of P. digitatum. Treatments are as follows: CK = sterile distilled water, SA = 0.2 mM SA, Y = R. mucilaginosa (1 × 108 cells/mL) alone, Y + SA = R. mucilaginosa (1 × 108 cells/mL) + 0.2 mM SA. Bars represent standard error of means from three independent experiments. Data in columns with different letters are significantly different (P ≤ 0.05) according to the Tukey test
The influence of SA on the growth of R. mucilaginosa on the surface of orange fruit stored at 20 °C and 4 °C
The high growth displayed by R. mucilaginosa enhanced with 0.2 mM SA demonstrated its ability to flourish, survive and colonize fruit surface [35]. The growth of R. mucilaginosa augmented with or without 0.2 mM SA on fruit surface stored at 20 °C increased from 0 to 3 days. Thereafter, the growth of the antagonist enhanced with or without 0.2 mM SA decreased from the 3 to 6 days, but remained higher than the initial value of (log10 6.05 CFU/mL) (Fig. 2a). A significant difference (P ≤ 0.05) was observed among the treatments from 3 to 6 days. A similar trend was observed at the incubation temperature of 4 °C with the growth of the antagonist enhanced with or without 0.2 mM SA showing no significant effect (P ≥ 0.05) from 0 to 3 days. Nonetheless, a significant effect (P ≤ 0.05) was observed between the treatments from 6 to 18 days. The growth of both treatments declined from the 6 to 18 days, with the population of R. mucilaginosa as a standalone treatment been lower than the initial value of (log10 5.99 CFU/mL), whiles R. mucilaginosa enhanced with 0.2 mM SA still remaining higher (Fig. 2b). The speedy increase in the growth of R. mucilaginosa enhanced with 0.2 mM SA compared to R. mucilaginosa as a standalone treatment is an advantage in biocontrol activity against pathogenic infection [4]. This may explain the reason why there was a significant difference in the biological control between the two treatments. The growth dynamics of the enhanced yeast support other reports such as Sharma et al. [9], Romanazzi [43], and Droby et al. [48] that defined the phenomenon of initial colonization as an advantage to biological control agents in the competition for space and nutrients.
Population growth of R.mucilaginosa inoculated on the fruit surface, and incubated at a 20 °C for 7 days b 4 °C for 18 days. Treatments are as follows: Y + SA = R. mucilaginosa (1 × 108 cells/mL) + 0.2 mM SA and Y = R. mucilaginosa (1 × 108 cells/mL) alone. Bars represent standard error of means expressed in (log10 CFU/mL) from two independent experiments. The different letters indicate significant differences (P ≤ 0.05) according to Tukey test
At 4 °C the population achieved by R. mucilaginosa enhanced with 0.2 mM SA is indicative of the fact that SA may have impacted positively on the growth. It was also observed that the colonies formed were larger in size compared to the standalone treatment. This finding is evocative of the fact that at storage temperature as low as 4 °C R. mucilaginosa enhanced with 0.2 mM SA had the ability to endure and adapt [49]. We, therefore, submit that the increase in the growth of R. mucilaginosa enhanced with 0.2 mM SA on the fruit surface stored at 4 °C could be attributed to the nourishment of the yeast cells by SA.
Extension of lesion of the wounded site of treated fruit
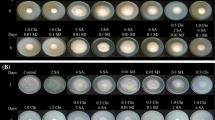
The result on lesion diameter as shown in (Fig. 3) revealed that R. mucilaginosa enhanced with 0.2 mM SA exhibited the highest inhibition in controlling green mold decay in orange fruit. There was a significant difference (P ≤ 0.05) between treated samples. R. mucilaginosa enhanced with 0.2 mM SA displayed the least lesion diameter of (13.20 mm), followed by R. mucilaginosa alone (16.69 mm), SA (23.51 mm) and the controlled treated fruit (63.42 mm) at a storage temperature of 20 °C. This result is revealing that under the existing storage conditions the growth of P. digitatum could be hindered by the treatment applied, which may be attributed to the ability of the yeast cells stimulated to augment the fruit resistance against the stress imposed by P. digitatum. This is in agreement with the report of Qin et al. [17] who found that SA improved the biocontrol efficacy of H. uvarum against B. cinerea. Similar report by Qin et al. [19] showed that the bio-efficacy of R. glutinis and C. laurentii were heightened due to the amelioration with SA. Furthermore, Linlin and Yu [50] mentioned that the combined treatment of SA and Ca2+ improved disease resistance to gray mold decay caused by B. cinerea by increasing the expression levels of the pathogenesis-related proteins of tomato. Likewise, Yu and Zheng [51] stated that the enhancement of C. laurentti with SA improved the viability of the antagonist to hinder blue mold decay of apple fruit. Therefore, the improvement achieved in the lesion diameter by the antagonist enhanced with 0.2 mM SA may be attributed to the ability to outcompete P. digitatum in the host apart from the elevated heights in lignin content.
Assessment of lesion diameter in the wounded site of orange fruit. To each wound (5 mm diameter and 3 mm deep) the following was inoculated 30 µL of (1) cell suspension of R. mucilaginosa alone (1 × 108 cells/mL), (2) cell suspension of R. mucilaginosa (1 × 108 cells/mL) + 0.2 mM SA, (3) 0.2 mM SA and (4) Control (sterile distilled water). Subsequent to aeration for 2 h fruit were inoculated with 30 µL (1 × 104 spores/mL) spore suspension of P. digitatum. Storage was done for 15 days at 20 °C and relative humidity (RH) of 95%. Bars represent the standard error of the mean. Each value is the mean of two independent experiments. Data in columns with different letters are significantly different (P ≤ 0.05) according to the Tukey test
Effect of pH of the wounded site of treated fruit
pH is one of the major environmental parameters that regulate the interaction between the pathogen and the host cells [52]. Therefore, the change in lesion diameter extension in fruit and vegetables could be changed by various factors including pH [53]. Data from this study showed that at storage temperature of 20 °C, there was a decline in pH in all tested samples. There was no significant difference (P ≥ 0.05) among samples treated with R. mucilaginosa enhanced with or without 0.2 mM SA on 3 days. However, a significant difference (P ≤ 0.05) was observed among all treated samples throughout the trial period (Fig. 4). This noticed in pH was much evident on the 15 days with samples inoculated with R. mucilaginosa enhanced with 0.2 mM SA showing the highest value of (pH 4.43 ± 0.07), followed by R. mucilaginosa alone (pH 4.15 ± 0.11), SA (pH 3.51 ± 0.04) and the control with (pH 2.98 ± 0.13).
Assessment of pH in the wounded site of orange fruit. To each wound (5 mm diameter and 3 mm deep) the following was inoculated 30 µL of (1) cell suspension of R. mucilaginosa alone (1 × 108 cells/mL), (2) cell suspension of R. mucilaginosa (1 × 108 cells/mL) + 0.2 mM SA, (3) 0.2 mM SA and (4) Control (sterile distilled water). Subsequent to 2 h drying fruit were inoculated with 30 µL (1 × 104 spores/mL) spore suspension of P. digitatum. pH of fruit were recorded at time points of 0, 3, 6, 9, 12, and 15 days after storage at 20 °C (RH 95%). Bars represent the standard error of the mean according Tukey test at (P ≤ 0.05). Each value is the mean of two independent experiments. The different letters indicate significant differences (P ≤ 0.05) according to Tukey test
The pH exhibited by most fruit is normally between 3.32 and 4.39 Manteau et al. [54], and during fungal invasion, most of the identified proteins are proteolysis [55]. As reported by Peñalva et al. [56] pathogens have the ability to change and adapt to acidic or alkaline pH environment by degrading the structural plant cell or antifungal proteins secreted by the plant host to attain full control [52, 57]. This is in concord with Tardi-Ovadia et al. [58] who reported that the native pH observed at the infected site of potato tubers increased from 6.0 to 7.4 to 8.0 when inoculated with H. solani and C. coccodes. Similarly, Smilanick et al. [59], demonstrated that in an in vitro trail the spores of P. digitatum germinated without any inhibition at a pH between 4.0 and 7.0 but were inhibited at a higher pH. The result of our study exhibited a similar trend where orange fruit inoculated with R. mucilaginosa enhanced with 0.2 mM SA gave the highest pH value compared to the untreated group. Even though all the pH values of treated samples exhibited a decline, that of the antagonist enhanced with 0.2 mM SA kept a higher curve with pH value greater than 4.0 at the end of the trail which is in agreement with the findings of Moss [60]; Mahunu et al. [36]. Similarly, a pH value between 4.0 and 6.0 in a non-decayed tissue of apples and a lower pH of 3.6–4.1 in decayed tissues after injection with P. expansium between 4 and 6 days was reported [61]. Furthermore, a study conducted by Neri et al. [38] detected a pH value lesser than 4.0 at the infected site of kiwifruit and pear tissues. Penicillium spp. is reported to be assisted by a decline in pH which aids fungi invasion where changes in pH can modulate the pathogenicity around the infection site [62]. It is, therefore, suggestive that perhaps the amelioration of the antagonist with SA help restricted the rapid growth of the pathogen resulting in the drift of pH observed. Certainly, as mentioned by Neri et al. [38], that the less belligerent growth of a pathogen will limit the dropping of pH in a substrate, whiles the more belligerent growth will significantly decrease the pH.
Effect of treatment on lignin content of the wounded site of treated fruit
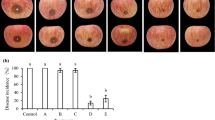
The results on lignin content as shown in (Table 1) revealed that fruit treated with R. mucilaginosa enhanced with 0.2 mM SA gave the highest lignin content compared to R. mucilaginosa alone and the untreated group. This significant increase in lignin content was much evident on 15 days with wound treated with R. mucilaginosa enhanced with 0.2 mM SA with value of (0.3207 ± 0.02) followed by R. mucilaginosa alone (0.2858 ± 0.01), SA (0.2592 ± 0.07) and control treated sample exhibiting the least value (0.1923 ± 0.03).
This indication with our findings is in accordance with Mahunu et al. [36] who pointed out that an increase in lignin levels could possibly hinder infiltration of the pathogen and increased disease resistance. Comparable report by Njoroge et al. [63], support the findings that an upsurge in lignin content was associated with the resistance of Verticillium dahliae infection of broccoli and cauliflower. Likewise, Vilanova et al. [64] demonstrated that in the evaluation of wound response in orange fruit P. digitatum showed a lower decay incidence and disease severity owing to an increase in lignin content. Additionally, Barros et al. [24] reported that resistance to green mold decay during de-greening increased as a result of an upsurge in lignin content. They further stated that the synthesis of lignin resulted in the formation of phenolics which possess fungitoxic properties and played a substantial role in the prevention of infiltration. Higher lignin and phenolic content in fruit tissue is reported to be connected with higher induction in disease resistance [28]. Results from our previous research indicated an increase in the phenolic compounds in fruit wound treated with R. mucilaginosa enhanced with 0.2 mM SA [23]. Therefore, the presence of both phenolic and lignin content in the wound played a pivotal role in the mechanism of defense against P. digitatum. Mahunu et al. [36] pointed out that hydroxylated and methoxylated cinnamoyl alcohols played a significant role in disease resistance expression as a result of stimulation of lignin of plant cells by polymerization of phenolic phenylpropanoid precursors. Walter et al. [65] stated that lignification in many plants is observed as a reaction to pathogenic infection as it augments the mechanical strength of cell walls and discourages pathogen attacks. Mechanically lignification is reported to add a substantial reinforcement to any cell wall by providing additional tensile strength of 25–75 MPa and a Young’s modulus of 2.5–3.7 GPa [66]. Also, it has been reported that the adaptation of plant to the environment for proper functioning is essential with regards to the timing and localization of lignin deposition [24]. Valentines et al. [28] established that through the deed of POX enzymes lignification can be associated with H2O2 which is a general procedure implicated in the plant protection. Moreover, in previous reports, it is stated that the interrelation of locally accumulated lignin and phenolics regulates the cell wall thickening around wounds Spotts et al. [67] and it relies on the wound healing response through the creation of wound periderm [64]. Consequence to these reports it is acknowledged that lignification or lignin deposition is unique to the mechanisms of disease resistance in fruit, which seems to impact on the curative and preventive action on green mold decay in citrus fruit [28, 37].
The influence of SA on R. mucilaginosa on MDA and H2O2 activities in orange fruits
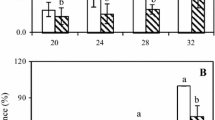
MDA activity in fruit treated with R. mucilaginosa enhanced with or without 0.2 mM SA was low throughout the trail (Fig. 5a). It was observed that there was no significant effect (P ≥ 0.05) among the treatments from 0 to 3 days, yet significantly different from the 4 to 6 days. Nevertheless, MDA activity in the controlled and SA treated samples increased throughout the test period with the controlled samples exhibiting the highest level of MDA. All the treatments were significantly different (P ≤ 0.05) in comparison with the control.
Time course change of a malondialdehyde (MDA), and b Hydrogen peroxide (H2O2) in orange fruit incubated at 20 °C. Treatments are as follows CK = sterile distilled water; SA = 0.2 mM SA; Y = R. mucilaginosa (1 × 108 cells/mL) alone; Y + SA = R. mucilaginosa (1 × 108 cells/mL) + 0.2 mM SA. Bars represent the standard error of the mean and each value is the mean of two independent experiments. The different letters indicate significant differences (P ≤ 0.05) according to Tukey test
As shown in (Fig. 5b), H2O2 content in fruit treated with R. mucilaginosa enhanced with or without 0.2 mM SA was found generally low with a maximum value below 1.5 mg/kg FW. There was no significant difference (P ≥ 0.05) among the samples on the 3 days. However, H2O2 content in both the controlled and SA treated samples increased sharply from 0 to 3 days and became steady throughout the test period. There was no significant difference (P ≥ 0.05) in H2O2 content detected among the fruit treated with control and SA. However, there was a significant effect (P ≤ 0.05) among all tested samples throughout the trail. Oxidative damage of fruit is reported to depend on the amount of MDA which is the final product of lipid peroxidation [68]. The MDA content in fruit treated with R. mucilaginosa enhanced with or without 0.2 mM SA was low and did not vary in MDA content indicating that R. mucilaginosa has no effect on MDA activity in orange fruit [10]. This is in accordance with Sharma et al. [69] who pointed out that a sharp increase in POD activity led to the decline of ROS in plant. Similarly, the comparatively low level of H2O2 in treated fruit which comes as a result of the increase in both SOD and POD activity.
Conclusion
In conclusion, it has been established that even though R. mucilaginosa as a stand-alone treatment inhibited the growth of P. digitatum in the in vivo and in vitro test, however, its combination with 0.2 mM SA showed an improved effect. Thus, under the existing storage condition, the increase in lignin content and the reduction in lesion extension of orange fruit by the antagonist enhanced with exogenous SA demonstrated here is suggestive of the fact that the use of R. mucilaginosa enhanced with SA is a possible agent for the management of postharvest disease of orange fruit.
References
S.M. Sanzani, M. Reverberi, R. Geisen, Postharvest Biol. Technol. 122, 95–105 (2016)
R. Castoria, L. Mannina, R. Durán-Patrón, J. Agric. Food Chem. 59(21), 11571–11578 (2011)
S.M. Yu, Y.H. Lee, J. Basic. Microbiol. 55(7), 898–906 (2015)
S. Droby, M. Wisniewski, N. Teixidó, D. Spadaro, Postharvest Biol. Technol. 122, 22–29 (2016)
H. Zhang, L. Wang, Y. Dong, S. Jiang, H. Zhang, X. Zheng, Int. J. Food. Microbiol. 126(1–2), 167–171 (2008)
H. Zhang, L. Wang, L. Ma, Y. Dong, S. Jiang, B. Xu, X. Zheng, Biol. Control. 48(1), 79–83 (2009)
J. Cao, H. Zhang, Q. Yang, R. Ren, Int. J. Food Microbiol. 162(2), 167–173 (2013)
T. Lai, X. Bai, Y. Wang, J. Zhou, N. Shi, T. Zhou, Sci. Hortic. 187, 108–114 (2015)
R.R. Sharma, D. Singh, R. Singh, Biol. Control. 50(3), 205–221 (2009)
D. Zhang, H. Wang, Y. Hu, Y. Liu, J. Agric. Food Chem. 63(33), 7399–7404 (2015)
A. Di Francesco, C. Martini, M. Mari, Eur. J. Plant Pathol. 145, 1–7 (2016)
Q. Zhang, L. Zhao, Z. Li, C. Li, B. Li, X. Gu, X. Zhang, H.Y. Zhang, Biol. Control. 133, 26–33 (2019)
B. Meena, T. Marimuthu, R.J. Velazhahan, J. Mycol Plant Pathol. 31, 139–145 (2001)
L. Wang, S. Chen, W. Kong, L. Shaohua, D. Douglas, Postharvest Biol Technol. 41, 244–251 (2006)
Y. Mo, D. Gong, G. Liang, R. Han, J. Xie, W. Li, J. Sci. Food Agric. 88, 2693–2699 (2008)
W.G. Deng, K.H. Ruan, M. Du, M.A. Saunders, K.K. Wu, FASEB J 15(13), 2463–2470 (2001)
X. Qin, H. Xiao, C. Xue, Z. Yu, R. Yang, Z. Cai, L. Si, Postharvest Biol. Technol. 100, 160–167 (2015)
Z.D.C. Joyce, H. Wearing, L. Coates, L. Terry, Aust. J. Exp. Agr. 41(6), 805–813 (2001)
G.Z. Qin, S.P. Tian, Y. Xu, Y.K. Wan, Physiol Mol. Plant. 62, 147–154 (2003)
R.P. Li, H.Y. Zhang, W.M. Liu, X.D. Zhang, Int J Food Microbiol. 146, 151–156 (2011)
J.W. Buck, Phytopathology 94, 196–202 (2004)
Q. Yang, H.Y. Zhang, X. Zhang, X. Zheng, J. Qin, Front. Microbiol. 6, 1296 (2015)
J. Ahima, X. Zhang, Q. Yang, L. Zhao, A.M. Tibiru, H.Y. Zhang, Biol. Control. 135, 23–32 (2019)
J. Barros, H. Serk, I. Granlund, E. Pesquet, Ann Bot. 115(7), 1053–1074 (2015)
M. Aoun, Int. J. Bot. 13(2), 82–102 (2017)
Z. Ma, L. Yang, H. Yan, J.F. Kennedy, X. Meng, Carbohdr. Polym. 94, 272–277 (2013)
C. Zhang, J. Wang, J. Zhang, C. Hou, G. Wang, Postharvest Biol. Technol. 61, 145–151 (2011)
M.C. Valentines, R. Vilaplana, R. Torres, J. Usall, C. Larrigaudiere, Postharvest Biol. Technol. 36, 227–234 (2005)
Y.Q. Liu, E. Heying, S.A. Tanumihardjo, Compr. Rev. Food. Sci. Food Safety. 11, 530–545 (2012)
V.K. Joshi, D.K. Sandhu, N.S. Thakur, in Food Fermentation, vol. 2, ed. by V.K. Joshi, A. Pandey (International Biotech, Abohar, 2000), pp. 647–732
S. Rafiq, R. Kaul, S.A. Sofi, N. Bashir, F. Nazir, G.A. Nayik, J. Saudi, Soc. Agric. Sci. 17(4), 351–358 (2018)
F. Fallanaj, A. Ippolito, A. Ligorio, F. Garganese, C. Zavanella, S.M. Sanzani, Postharvest Biol. Technol. 115, 18–29 (2016)
W. Li, H.Y. Zhang, P. Li, M.T. Apaliya, Q. Yang, Y. Peng, X. Zhang, Biol. Control. 103, 30–38 (2016)
A.M. Tibiru, H.Y. Zhang, X. Zheng, Q. Yang, G.K. Mahunu, E. Kwaw, J. Sci. Food. Agric. 98(12), 4665–4672 (2018)
Y. Yuan, X. Zhang, X. Zheng, A.M. Tibiru, Q. Yang, L. Zhao, X. Gu, H.Y. Zhang, Postharvest Biol. Technol 136, 124–131 (2018)
G.K. Mahunu, H.Y. Zhang, A.M. Tibiru, Q. Yang, X. Zhang, L. Zhao, Postharvest Biol. Technol. 141, 1–7 (2018)
B. Nafussi, S. Ben-Yehoshua, V. Rodov, J. Peretz, B.K. Ozer, G. D’hallewin, J. Agric. Food Chem. 49(1), 107–113 (2001)
F. Neri, I. Donati, F. Veronesi, D. Mazzoni, M. Mari, Int. J. Food Microbiol. 143, 109–117 (2010)
H. Morales, S. Marín, A. Rovira, V. Sanchis, Lett. Appl. Microbiol. 44, 30–35 (2006)
M.M. Bradford, Anal. Biochem 72(1–2), 248–254 (1976)
B.D. Patterson, E.A. MacRae, I.B. Ferguson, Anal. Biochem. 139(2), 487–492 (1984)
R. Trias, L. Bañeras, E. Montesinos, E. Badosa, Int. Microbiol. 11(4), 231–236 (2008)
G. Romanazzi, New Trends. Postharvest Man. Fresh Produce, vol. 4 (1) (Global Science Books Ltd, London, 2010), pp. 111–115
V. Hershkovitz, N. Sela, L. Taha-Salaime, J. Liu, G. Rafael, C. Kessler, R. Aly, M. Levy, M. Wisniewski, S. Droby, BMC Genom. 14(1), 168 (2013)
N. Paster, R. Barkai-Golan, World Mycotoxin J. 1(4), 385–396 (2008)
S. Droby, Int. Symp. Nat. Preserv. Food Syst. 709, 45–52 (2005)
B.Q. Li, Z.W. Zhou, S.P. Tian, Biol. Control. 46(2), 187–193 (2008)
S. Droby, M. Wisniewski, D. Macarisin, Postharvest Biol. Technol 52, 137–145 (2009)
Q. Yang, H. Wang, H. Zhang, X. Zhang, A.M. Tibiru, Postharvest Biol. Technol. 126, 15–22 (2017)
L. Linlin, Z. Yu, Emir. J. Food. Agric. 29(1), 78–82 (2017)
T. Yu, X.D. Zheng, J. Plant. Growth Regul. 25(2), 166–174 (2006)
H. Li, Y. Chen, Z. Zhang, B. Li, G. Qin, S. Tian, Food Qual. Safety. 2(3), 111–119 (2018)
S. Drusch, S. Kopka, J. Kaeding, Food Chem. 100, 192–197 (2007)
S. Manteau, S. Abouna, B. Lambert, L. Legendre, FEMS Microbiol. Ecol. 43(3), 359–366 (2003)
B.Q. Li, W.H. Wang, Y.Y. Zong, G.Z. Qin, S.P. Tian, J. Proteome Res. 11, 4249–4260 (2012)
M.A. Peñalva, J. Tilburn, E. Bignell, H.N. Arst Jr., Trends Microbiol. 16(6), 291–300 (2008)
C.P. Kubicek, T.L. Starr, N.L. Glass, Annu. Rev. Phytopathol. 52, 427–451 (2014)
R. Tardi-Ovadia, R. Linker, L. Tsror Lahkim, Phytopathology. 107(1), 132–137 (2017)
J.L. Smilanick, M.F. Mansour, D.A. Margosan, F. Mlikota Gabler, W.R. Goodwine, Plant Dis. 89, 640–648 (2005)
M.O. Moss, in Mycotoxins and Animal Foods, ed. by J.E. Smith, R.S. Henderson (CRC Press, Boca Raton, 1991), pp. 37–56
D. Prusky, J.L. McEvoy, R. Saftner, W.S. Conway, R. Jones, Phytopathology 94(1), 44–51 (2004)
D. Prusky, S. Barad, N. Luria, D. Ment, Postharvest Pathology (Springer, Cham, 2014), pp. 11–25
S.M.C. Njoroge, G.E. Vallad, S.Y. Park, S. Kang, S.T. Koike, Phytopathology. 101(5), 523–534 (2011)
L. Vilanova, N. Teixidó, R. Torres, J. Usall, I. Viñas, Int. J. Food Microbiol. 157, 360–367 (2012)
S. Walter, P. Nicholson, F.M. Doohan, New Phytol. 185(1), 54–66 (2010)
L.J. Gibson, J.R. Soc, Interface. 9(76), 2749–2766 (2012)
R.A. Spotts, P.G. Sanderson, C.L. Lennox, D. Sugar, L.A. Cervantes, Postharvest Biol. Technol. 13, 27–36 (1998)
Q. Zhou, C. Ma, S.C. Cheng, B. Wei, X. Liu, S. Ji, Postharvest Biol. Technol. 88, 88–95 (2014)
P. Sharma, A.B. Jha, R.S. Dubey, M. Pessarakli, J. Bot. (2012). https://doi.org/10.1155/2012/217037
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31571899; 31772369), the National key research project (sub project) of China (Grant No. 2016YFD0400902-04), and the Agricultural Independent Innovation Fund in Jiangsu Province [Grant No. CX (18) 2018]
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahima, J., Zhang, H., Apaliya, M.T. et al. The mechanism involved in enhancing the biological control efficacy of Rhodotorula mucilaginosa with salicylic acid to postharvest green mold decay of oranges. Food Measure 14, 3146–3155 (2020). https://doi.org/10.1007/s11694-020-00559-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00559-1