Abstract
Soursop (Annona muricata) is a tropical fruit that can be infected by Colletotrichum gloeosporioides and Rhizopus stolonifer. Traditional methods used for postharvest disease control include the application of fungicides, however due to their excessive use, as well as their persistence in the environment, the development of new strategies that control pathogens are required. The application of chitosan (Chi), salicylic acid (SA) and methyl jasmonate (MJ) is an environmentally-friendly alternative with antimicrobial properties and also induces defense mechanisms in plant tissues. In this study, Colletotrichum was reactivated and Rhizopus was identified using morphological features and molecular tools. In vitro, the application of 0.5 and 1.0% of Chi alone or in combination with SA and MJ decreased mycelial growth and sporulation, a complete inhibition of spore germination was obtained. Thus, the application of Chi in combination with SA and MJ could be a smart strategy to inhibit the development of pathogens that attack soursop fruit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Mexican state of Nayarit is currently the country’s largest producer of soursop [1]. Soursop (Annona muricata) is a perishable tropical fruit, susceptible to fungus infection. Colletotrichum gloeosporioides and Rhizopus stolonifer are the two main fungi that cause infection. Infection can take place at harvest and during storage and is due to physiological changes and climatic conditions (high relative humidity and temperature). A high rate of postharvest losses is caused mainly by fungal pathogens [2]. Traditionally, the application of synthetic fungicides is the main postharvest disease control method used. However, their application has harmful effects on human health and the environment. For this reason, new strategies have been developed such as the application of Chi, SA and MJ for the control of postharvest pathogens. Chitosan (Chi) (poly-β-(1,4)N-acetyl-d-glucosamine) derived from the outer shell of crustaceans, has become a promising alternative treatment due to its natural character, antifungal activity, and elicitation of defense responses in plant tissue [3]. Chitosan is a polysaccharide of high molecular weight, biodegradable and non-toxic. Several studies have evaluated the chitosan’s potential at different concentrations in controlling postharvest fungal pathogens [4,5,6]. Salicylic acid (SA) is a natural phenolic compound present in many plants. It is a signal-transducing molecule that activates the defense mechanisms when under attack by pathogens. Salicylic acid has been shown to interact synergistically with ethylene or jasmonic acid to activate the expression of pathogenesis-related (PR) proteins in Arabidopsis [7], and plays an important role in the resistance against pathogens [8]. Jasmonic acid and its volatile methyl ester, methyl jasmonate (MJ), are a class of cyclopentanone compounds, regarded as endogenous regulators that play an important role in regulating stress response, plant growth and development [9]. In a previous study, the application of MJ effectively suppressed gray mold rot caused by Botrytis cinerea in strawberry [10]. Cao et al. [11] showed that applying MJ inhibited the mycelial growth of Colletotrichum acutatum in loquat fruit. The objectives of this research were (1) to study the effect of antifungal systems on postharvest control of pathogens, and (2) to observe microstructural alterations on the mycelium and spores of pathogens.
Materials and methods
Isolation and identification of Rhizopus stolonifer
Soursop fruits were harvested at physiological maturity from orchards in the municipality of Compostela, Nayarit, Mexico. Fruits were placed in chambers with high relative humidity (90–95%) at 25 °C to stimulate disease development. Thereafter, tissues with symptoms were cut and disinfected with 2% sodium hypochlorite (NaClO) and placed on PDA (Potato dextrose agar) (Sigma-Aldrich, St. Louis, MO, USA) Petri dishes and incubated at 25 °C. The fungus isolated from damaged tissues was purified. The pathogenicity test based on Koch’s postulates was applied in order to discard saprophytic fungi from phytopathogens. Briefly, healthy fruits were artificially infected with 40 μL of spore’s suspension (106 spores/mL) using an ultra-fine syringe (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Thereafter, the fruits were placed in plastic chambers with elevated relative humidity (90–95%, placing glasses with sterile distilled water), at 25 °C. For fungus re-isolation, cuts (1 × 1 cm) were made that isolated a surface that was 50% affected and 50% healthy from fruits with severe disease symptoms. Tissue sections were superficially disinfected for 1 min (2% sodium hypochlorite), then rinsed with sterile distilled water and placed in the middle of PDA agar plates and incubated at 28 °C for 2 days. Control fruits were inoculated with sterile distilled water.
The identification at genus level of the pathogen was based on a microscopic analysis of conidia and mycelium, using a Motic BA300 optical microscope (Scientific Instrument Company, Inc, Hamilton, CA, USA); based on its characteristics, it was identified using dichotomous keys [12]. The identification at species level of the pathogen was based on molecular tests. DNA was extracted from the pathogen mycelium according to Álvarez et al. [13], with some modifications. The obtained DNA was quantified and its quality determined. PCR was performed under the following conditions: initial denaturation at 95 °C for 5 min, denaturation at 95 °C for 1 min, annealing at 48 °C for 30 s, extension at 72 °C for 1 min and final extension at 72 °C for 10 min with 30 cycles. Sequencing was provided by Macrogen Humanizing Genomics (Seoul, Korea) and the conserved region was analyzed in the NCBI (National Center for Biotechnology Information) database.
Reactivation of Colletotrichum gloeosporioides
The culture of C. gloeosporioides was activated by adding 40 μL of a spore suspension (1 × 106 spores/mL) in Petri dishes with PDA, incubated at 27 ± 2 °C for 8 days. Confirmation of the pathogen was based on a microscopic analysis of conidia and mycelium, using a Motic BA300 optical microscope.
Preparation of chitosan, salicylic acid and methyl jasmonate
A stock solution was made with 1.5 g of Chi (medium molecular weight) (cp = 590, 75–85% deacetylation) (Sigma-Aldrich, St. Louis, MO, USA), which was dissolved in 100 mL of distilled water with 2 mL of acetic acid with constant agitation for 24 h at room temperature. The appropriate dilutions were made to adjust the concentrations to 0.1, 0.5, 1.0 and 1.5%. The solutions were adjusted to pH 5.6 with a sodium hydroxide solution (1 N), and 0.1 mL of Tween 80 was added to improve wetting properties of the solution.
To prepare the different concentrations of salicylic acid (SA) (Mw: 138.12 g/mol) (Sigma-Aldrich, St. Louis, MO, USA), a stock solution of 10 mM was prepared and dissolved in 100 mL of sterile distilled water and 0.5 mL of glycerin was added. The dilutions were made to adjust the concentrations to 2, 4 and 6 mM. The solutions were adjusted to pH 5.5 with potassium hydroxide solution (10%).
A stock solution of 5 mM methyl jasmonate (MJ) was made (Mw: 224.3 g/mol) (Sigma-Aldrich, St. Louis, MO, USA) and dissolved in 50 mL of methanol. The appropriate dilutions were made to adjust the concentrations to 0.01, 0.05 and 0.1 mM [14].
Interactions were prepared using concentrations of 0.5 and 1.0% Chi, 2 and 6 mM SA and 0.01 and 0.1 mM MJ. The treatments combinations (Table 1) were done as follows: Chi-SA, Chi-MJ, SA-MJ, Chi-SA-MJ, which were dissolved in PDA. The Chi, SA, MJ and PDA solutions were sterilized separately, then mixed after cooling PDA to about 45 °C and dispersed in Petri dishes (85 mm).
In vitro tests
In order to evaluate the effects of Chi, SA and MJ on mycelial growth, plugs (5 mm in diameter) were cut from 8-day-old PDA cultures of C. gloeosporioides and 2-day-old PDA cultures of R. stolonifer. The plugs were then replaced in Petri dishes with PDA medium containing different concentrations of the inducers. Plates with treatments were incubated at 25 ± 1 °C for 8 (for C. gloeosporioides) and 2 days (for R. stolonifer), the colony diameter was registered daily. The results were reported as percentage of mycelial growth inhibition as compared to control.
To determine sporulation, Petri dishes where mycelial growth was measured were used. Ten mL of sterile distilled water were added to the Petri dishes, the fungal lawn was then rubbed with a sterile glass rod. The suspension was filtered through a sterile cheesecloth to retain the mycelium. Finally, spore concentration was determined by microscopic counting using a hemocytometer. A total of 100 observations per treatment using a Motic BA300 optical microscope (Motic Instruments Inc., Canada) were performed. Values were expressed as number of spores/mL.
The effectiveness of treatments on conidial germination was evaluated as follows: 50 μL of the spore suspension were placed on PDA discs (20 mm in diameter) prepared with the different concentrations of inducers. The samples were observed microscopically after 8 h for C. gloeosporioides and 4 h for R. stolonifer to quantify the germinated spores. The germination process was stopped by adding one drop of lactophenol-safranin. Spores were considered germinated when the length of the germinative tube was at least twice the spore diameter.
Scanning electron microscopy
Treatments with best performance during in vitro tests were used for SEM analysis. Petri dishes (PDA) were prepared with the inducers and the combination of treatments. Then, the Petri dishes were inoculated with 100 μL of the spore suspensions (1 × 106 spores/mL) of each pathogen and incubated until growth. Plugs (8 mm diameter) of each treatment (PDA + pathogen) were cut, thereafter, they were replaced into glass vials with 3 mL of 2.5% glutaraldehyde for 20 h at 4 °C. The plugs were rinsed with phosphate buffer for 5 min (three times). The plug samples were dehydrated with ethanol at gradual concentrations (30–90% v/v) for 40 min at each concentration and three times at 100% for 20 min. The samples were dried to critical point with CO2 for 40 min (Quorum dryer K850, Quorum Technologies Ltd, USA). Finally, the samples were mounted on plate slides and covered with gold using a metal ionizer (Quorum Q150RS, Quorum Technologies Ltd, USA) for 15 min [15]. Samples were examined using a scanning electron microscope (Quanta FEG 250, FEI Company Quanta™, USA) operating at 5 kV.
Statistical analysis
The analysis was made using four Petri dishes per treatment with three replicates. Each experiment was repeated at least twice, using a completely randomized block design. Data were subjected to analysis of variance (ANOVA) and a Tukey test (p ≤ 0.05) was used for the comparison of means.
Results and discussion
Isolation, identification of R. stolonifer, and reactivation of C. gloeosporioides
The pathogen that causes soft rot in soursop fruit was isolated and identified. The characteristics of the pathogen are: cottony mycelium and black spherical conidia 8–20 µm. Based on the taxonomic keys used, it was possible to identify that the pathogen in study belongs to the genus Rhizopus sp. [16]. The identification of Rhizopus was performed by PCR using the primers ITS1 (5′-CAACTCCCAAACCCCTGTGA-3′) and ITS4 (5′-GCGACGATTACCAGTAACGA-3′) gave a 600–700 bp fragment (data not shown). Sequences reported at Bold Systems v4 (Barcode of Life Data Systems, http://www.boldsystems.org/), were used to compare the obtained sequence. Thereafter, the tested pathogen was identified with a homology of 71% (accession number AM933546) as Rhizopus stolonifer.
In vitro tests. Mycelial growth of C. gloeosporioides and R. stolonifer
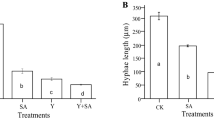
The results of mycelial growth of C. gloeosporioides are shown in Fig. 1(A). A reduction in colony diameter was observed, the percentage of growth inhibition ranged from 81 to 96% applying 1.0% Chi alone or in combination with MJ or SA. For the case of R. stolonifer, the results are shown in Fig. 1(B), a reduction in mycelial growth was obtained applying 0.5 or 1.0% of Chi alone or in combination with MJ or SA, the percentage of mycelial growth inhibition ranged from 81 to 98%. A significant difference (p ≤ 0.05) between the treatments was observed. The effect can be attributed to its cationic character, where the free amino groups, positively charged in acid medium, interact with the negative residues of the macromolecules exposed on fungal cell wall, changing the permeability of the plasma membrane, leading to an alteration of its main functions, such as waste output and nutrient input [17,18,19]. García-Rincón et al. [20] reported the inhibition of mycelial growth of R. stolonifer ranging from 28 to 64% using Chi at concentrations of 1 and 2 mg/mL. Qing et al. [21] reported the inhibition of mycelial growth (87.5%) of S. sclerotiorum using 1.0% Chi. In a recent study, Chen et al. [22], applied 0.1% Chi with 0.5 mM MJ in cherry tomato fruits, obtaining a 56.9% of Alternaria alternata inhibition. Waewthongrak et al. [23] also reported the effectiveness of Chi against Penicillium digitatum applying different concentrations (0.5, 1 and 5 mg/mL).
Sporulation and conidial germination of C. gloeosporioides and R. stolonifer
In sporulation assessment of C. gloeosporioides, the results showed that the application of Chi 1.0% alone or in combination with SA and MJ, decreased the spore concentration (1.19 × 105–9.25 × 105 spores/mL) significantly compared to control (5.18 × 106 spores/mL). The same behavior was observed for R. stolonifer using Chi at 0.5 and 1.0% alone or in combination with SA and MJ, with a decreased spore concentration (1.88 × 104–8.69 × 105 spores/mL) compared to control (6.59 × 106 spores/mL). The best results were obtained applying Chi at 1.5%, with a total inhibition of sporulation. A significant difference (p ≤ 0.05) between the treatments was observed.
Table 2 shows that the application of Chi at 1.0% alone or in combination with SA and MJ, a total inhibition of germination of C. gloeosporioides was obtained. However, with the application of Chi at 0.5% alone or in combination with the inducers, a reduction of conidia germination (75–93%) was obtained. A total inhibition of conidial germination of R. stolonifer was obtained applying any inducer (Table 2). No significant difference (p ≤ 0.05) between the best treatments was observed.
The present findings are in agreement with the results reported by Berumen-Varela et al. [24]. In their study, a decrease in the spore concentration of Colletotrichum sp. was obtained by applying 3 and 5 mM of SA (1.16 × 106 and 1.18 × 106 spores/mL) compared to control (3.85 × 107 spores/mL). Guerra-Sánchez [25] observed an inhibitory effect on spore concentration of R. stolonifer at 1.5 and 2.0% Chi. Zhu and Tian [26] show that applying 10 mM of MJ can inhibit the spores’ germination process of B. cinerea (75.8%). Qiu et al. [27] and López-Mora et al. [28] observed a total inhibition of germination of Fusarium concentricum and A. alternata by applying 0.05 and 1.0% of Chi. Chen et al. [22] reported that applying a combination of 0.5 mM of MJ and 0.1% of Chi results in a total inhibition in the germination process of A. alternata.
The fungicidal activity of Chi at spore level is related to the inhibition of the synthesis of enzymes (polygalacturonase, pectin methyl esterase, pectate lyase and cellulase), involved in tissue softening in fungi acting as a chelating agent by sequestering the ions metals required for enzymatic reactions [29]. This biopolymer can also bind the metals present in the external structures of microorganisms (Mg+2 and Ca+2), inhibiting the production of toxins. The enzymes’ inhibition, involved in synthesis of germ tube development is another reported action mechanism [30]. In vitro tests showed that the best treatments against C. gloeosporioides were: 1.0% Chi alone, 1.0% Chi–0.01 mM MJ, 1.0% Chi–2 mM SA and 1.0% Chi–0.1 mM MJ–6 mM SA. For R. stolonifer the best treatments were: 0.5% Chi alone, 0.5% Chi–0.1 mM MJ, 0.5% Chi–6 mM SA, and 0.5% Chi–0.01 mM MJ–2 mM SA. These treatments were used for SEM observations.
Scanning electron microscopy
The micrographs of C. gloeosporioides, applying 1% Chi alone [Fig. 2(B)], 1% Chi with 0.01 mM MJ [Fig. 2(C)], 1% Chi with 2 mM SA [Fig. 2(D)] and 1% Chi–MJ 0.1 mM–6 mM SA [Fig. 2(E)] showed distorted hyphae, nodule formation, cell wall thinning as well as a reduction of hyphae diameter. In control treatment [Fig. 2(A)], a mycelium with healthy aspect without deformations was observed. In the conidia of C. gloeosporioides no damage was observed, this is probably due to the spore being more resistant compared to the mycelium, which is more susceptible. The micrographs of R. stolonifer mycelium exposed to 0.5% Chi alone [Fig. 3(B)], 0.5% Chi with 0.1 mM MJ [Fig. 3(C)], 0.5% Chi with 6 mM SA [Fig. 3(D)] and Chi 0.5%–0.01 mM MJ–2 mM SA [Fig. 3(E)] showed distorted, dehydrated and collapsed hyphae. The control treatment [Fig. 3(A)] showed turgid mycelia, without deformations and the sporangium presents a firm structure. The micrographs of R. stolonifer spores, applying 0.5% Chi alone [Fig. 4(B)], 0.5% Chi with 0.1 mM MJ [Fig. 4(C)], 0.5% Chi with 6 mM SA [Fig. 4(D)] and 0.5% Chi–0.01 mM MJ–2 mM SA [Fig. 4(E)] showed deformations, nodule formation and detached cell wall. In the control treatment [Fig. 4(A)] no damage was observed in the morphology of the spores. Previously, Oliveira et al. [31] observed deformations and swelling in hyphae of Aspergillus exposed to Chi (500 mg/L). Bautista-Baños et al. [32], reported an alteration of reproductive structures of R. stolonifer spores (damage of the cell wall) by the application of Chi, beeswax and essential lemon oil. Rodríguez et al. [33] reported deformations and detachment of cellular material in spores of Bipolaris oryzae. These deformations can be attributed to the interaction of Chi with the fungal cell membrane, especially at the apex of hyphae where the membrane is less protected, causing pore formation, inducing changes in membrane conformation, disorder in biosynthesis and finally a degradation of cell wall components [29]. Chi also binds essential metals (Mg+2 and Ca+2) of mycelial structures, inhibiting enzymatic reactions of the pathogen, causing alterations in the morphology of mycelium and spores [30, 34].
The results of this investigation showed the effectiveness of Chi, SA and MJ as antifungal agents in controlling soursop anthracnose by C. gloeosporioides and soft rotting by R. stolonifer. The combination of these inducers increases the effectiveness in the postharvest control of these pathogens and presents an alternative to synthetic fungicides.
References
SIAP/SAGARPA. Cierre de la producción agrícola por estado. Anuario Estadístico de la Producción Agrícola de guanábana en México. Available from: http://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119. Accessed Dec. 10th, 2016
Andrades I, Yender F, Labarca J, Ulacio D, Paredes C, Marín Y. Evaluación de la antracnosis (Colletotrichum sp.) en guanábana (Annona muricata L.) tipo Gigante en el sector Moralito del Estado Zulia, Venezuela. Rev. UDO Agric. 9: 148–157 (2009)
Terry L, Joyce D. Elicitors of induced disease resistance in postharvest horticultural crops: a brief review. Postharvest Biol. Technol. 32: 1–13 (2004)
Berúmen-Varela G, Coronado-Partida L, Ochoa-Jiménez A, Chacón-López M, Gutiérrez-Martínez P. Effect of chitosan on the induction of disease resistance against Colletotrichum sp in mango (Mangifera indica L.) cv Tommy Atkins. Rev. Inv. Ciencia. 23(66): 16–21 (2015a)
Romanazzi G, Feliziani E, Bautista-Baños S, Sivakumar D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 57: 579–601 (2017)
Sánchez-Domínguez D, Bautista-Baños S, Castillo O. Efecto del quitosano en el desarrollo y morfología de A. alternata (Fr.) Keissl. Anales Biol. 29: 23–32 (2007)
Xu Y, Chang PFL, Liu D, Narasimhan ML, Raghothanma KG, Gasegawa PM, Bressan RA. Plant Defense Genes Are Synergistically Induced by Ethylene and Methyl Jasmonate. Plant Cell. 6(8): 1077–1085 (1994)
Naylor M, Murphy AM, Berry JQ, Carr JP. Salicylic acid can induce resistance to plant virus movement. Mol. Pl. Microbe Interact. 11: 860–868 (1998)
Creelman RA, Mullet JE. Biosynthesis and action of jasmonate in plants. Annu. Rev. Plant Physiol. Mol. Biol. 48: 355–381 (1997)
Moline HE, Buta JG, Saftner RA, Maas JL. Comparison of three volatile natural products for the reduction of postharvest decay in strawberries. Adv. Strawberry Res. 16: 43–48 (1997)
Cao SF, Zheng YH, Yang ZF, Tang SS, Jin P, Wang KT, Wang XM. Effect of methyl jasmonate on the inhibition of Colletotrichum acutatum infection in loquat fruit and the possible mechanisms. Postharvest Biol. Technol. 49: 301–307 (2008)
Suárez-Quiroz M, Mendoza-Bautista I, Monroy-Rivera J, De la Cruz-Medina J, Angulo-Guerrero O, González-Ríos O. Aislamiento, identificación y sensibilidad a antifúngicos de hongos fitopatógenos de papaya cv. maradol (Carica papaya L.). Rev Tecnol. Postcosecha. 14(2): 115–124 (2013)
Álvarez E, Ospina C, Mejía J, Llano G. Caracterización morfológica, patogénica y genética del agente causal de la antracnosis (Colletotrichum gloeosporoides) en guanábana (Annona muricata) en Valle de Cauca. Fitopatol. Colombiana. 28: 1–8 (2011)
Dhandhukia P, Thakkar V. Separation Quantitation of jasmonic acid using HPTLC. J. of Chromatographic Science. 46(4): 320–324 (2008)
Bozzala J, Russell L. Specimen preparation for Scanning Electron Microscopy. Jones and Bartlett. Principles and Techniques for Biologists. Sudbury Massachusetts (1992)
Pitt JI, Hocking AD. Fungi and food spoilage. Springer Science, NY, USA (2009)
Benhamou N. Ultrastructural and cytochemical aspects of chitosan on Fusarium oxysporum f. sp. radicis-lycopersici, agent of tomato crown and root rot. Phytopathology. 82: 1185–1193 (1992)
Song Y, Babiker E, Usui M, Saito A, Kato A. Emulsifying properties and bactericidal action of chitosan-lysozyme conjugates. Food Res. Int. 35: 459–466 (2002)
Bautista-Baños S, Hernández-Lauzardo A, Velázquez-Valle M, Hernández-López M, Ait-Barka E, Bosquez-Molina E, Wilson C. Chitosan as a potential natural compound to control pre-and postharvest diseases of horticultural commodities. Crop Prot. 25: 108–118 (2006)
García-Rincón J, Vega-Pérez J, Guerra-Sánchez M, Hernández-Lauzardo, A, Peña-Díaz A, Velázquez-del Valle M. Effect of chitosan on growth and plasma membrane properties of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Pesticide Biochemistry and Physiology. 97: 275–278 (2010)
Qing W, Jin-hua Z, Qian W, Yang N, Li-pu G. Inhibitory effect of chitosan on growth of the fungal phytopathogen, Sclerotinia sclerotiorum, and sclerotinia rot of carrot. J. of Integrative Agriculture. 14(4): 691–697 (2015)
Chen J, Zou X, Liu Q, Wang F, Feng W, Wan N. Combination effect of chitosan and methyl jasmonate on controlling Alternaria alternata and enhancing activity of cherry tomato fruit defense mechanisms. Crop Protection. 56: 31–36 (2014)
Waewthongrak W, Pisuchpen S, Leelasuphakul W. Effect of Bacillus subtilis and chitosan applications on green mold (Penicillium digitatum Sacc.) decay in citrus fruit. Postharvest Biol. Technol. 99: 44–49 (2015)
Berumen-Varela G, Ochoa-Jiménez A, Báez-Sañudo R, Gutiérrez-Martínez P. Efecto del ácido salicílico en la inducción de resistencia a Colletotrichum sp. en frutos de plátano durante postcosecha. Rev. Iber. Tecnología Postcosecha. 16: 27–34 (2015b)
Guerra-Sánchez M, Sandoval-Escobar L, Amora-Lazcano E, Vásquez-Méndez L, Velázquez del Valle M, Hernández-Lauzardo A. Efecto del quitosano en el desarrollo in vitro de Rhizopus stolonifer (Ehrenb.:Fr.) Vuill en dos medios de cultivo. Revista Colombiana de Biotecnología. 12(2): 214–222 (2010)
Zhu Z, Tian S. Resistant responses of tomato fruit treated with exogenous methyl jasmonate to Botrytis cinerea infection. Scientia Horticulturae. 142: 38–43 (2012)
Qiu M, Wu C, Ren G, Liang X, Wang X, Huang J. Effect of chitosan and its derivatives as antifungal and preservative agents on postharvest green asparagus. Food Chem. 155: 105–111 (2014)
López-Mora L, Gutiérrez-Martínez P, Bautista-Baños S, Jiménez-García L, Zavaleta-Mancera H. Evaluación de la actividad antifúngica del quitosano en Alternaria alternata y en la calidad del mango ‘tommy atkins’ durante el almacenamiento. Rev Chapingo Serie Horticultura. 19(3): 315–331 (2013)
El Ghaouth A, Grenier J, Asselin A. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology. 82: 398–402 (1992)
Ayala G. Efecto antimicrobiano del quitosano: Una revisión de la literatura. Scientia Agroalimentaria. 2: 6–12 (2014)
Oliveira R, Takaki M, Castilho T, Luiz V, Cláudio J, José M, Oliveira V. Synthesis, characterization and antifungal activity of quaternary derivatives of chitosan on Aspergillus flavus. Microb. Res. 168: 50–55 (2013)
Bautista-Baños S, Ramos-García M, Hernández-López M, Córdova-Albores L, López- Mora L, Gutiérrez-Martínez P, Sánchez-Domínguez D. Use of scanning and transmission electron microscopy to identify morphological and cellular damage on phytopathogenic fungi due to natural products application. Current Microscopy Contributions to Advances in Science and Technology. 1: 401–405 (2012)
Rodríguez A, Plascencia M, Bautista-Baños S, Onofre M, Ramírez M. Actividad antifúngica in vitro de quitosanos sobre Bipolaris oryzae patógeno del arroz. Plant and Crop Protection. 65: 98–103 (2016)
Zhang H, Li R, Liu W. Effects of chitin and its derivative chitosan on postharvest decay of fruits: A review. Int. J. of Mol. Sci. 12: 917–934 (2011)
Acknowledgements
The authors are grateful for the financial support from Tecnológico Nacional de México (TecNM) for Project 5214.14-P and CONACYT for the fellowship granted to Anelsy Ramos-Guerrero.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ramos-Guerrero, A., González-Estrada, R.R., Hanako-Rosas, G. et al. Use of inductors in the control of Colletotrichum gloeosporioides and Rhizopus stolonifer isolated from soursop fruits: in vitro tests. Food Sci Biotechnol 27, 755–763 (2018). https://doi.org/10.1007/s10068-018-0305-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-0305-5