Abstract
Many phenolic compounds serve as natural antioxidants by preventing food oxidation and oxidative stress in the body. In this study, antioxidant compounds were extracted from five peanut cultivars. Samples were evaluated for their total phenolic content, total flavonoid content, antioxidant activities using 2,2′-diphenyl-1-picrylhydrazyl radical (DPPH) assay and ferric reducing antioxidant power (FRAP) assay, antiproliferative activities against two colon cancer cell lines (HCT116 and HT29), and intracellular ROS generation. The peanuts rich in phenolics (185.4–300.9 mg GAE/100 g DW) and flavonoids (62.79–86.27 mg CE/100 g DW), and has relative good antioxidant capability (DPPH, 6.65–9.45 μmol Trolox/g DW and FRAP, 8.80–13.6 μmol Fe (II)/g DW). The peanut extracts exhibited strong antiproliferative effect against HCT116 and HT29 with IC50 value of 1.39–9.33 mg dry extract/ml and 1.56–7.55 mg dry extract/ml, respectively. The antiproliferative effects are partly due to the intracellular reactive oxygen species (ROS) generation. Ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS) was used to characterize the phenolic profiles of peanut cultivar extract and 23 phenolic compounds were tentatively identified, most of which were flavonoids. Peanuts are rich in phenolic compounds and have antioxidant activity and antiproliferative activity, thus, it may serve as viable functional food ingredients with antioxidant potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen and nitrogen species with unpaired electrons are produced in human cells through endogenous metabolic activities and maintain the balance between oxidants and antioxidants under optimal physiological conditions [1]. Excessive amount of free radicals cause damage to biomolecules, such as lipids, proteins, and DNA, leading to a wide range of diseases including cancer, cardiovascular diseases, and inflammatory diseases [2]. Colorectal cancer is the fourth top cause of cancer deaths worldwide. It is estimated that more than 600,000 case of colorectal cancer worldwide per year are expected to death [3]. Increasing evidence shows that consumption of fruits, vegetables, and whole grains is associated with reduced chronic diseases, inducing colorectal cancer, cardiovascular disorders, and ageing [4]. These fruits, vegetables, and whole grains are rich in antioxidant compounds, which can prevent free radical-induced oxidative damage to biomolecules [5].
Peanut (Arachis hypogea L.), belonging to Fabaceae family, is native to South America [6] and is recognized as the fourth largest oilseed crop in the world, producing high nutritional, medical, and commercial values. It has been reported that the consumption of peanuts offers multiple health benefits, such as reducing the risks of cardiovascular diseases [7], neurodegenerative diseases [8], cancer [9], inflammation [10], and osteoporosis [11]. The beneficial effects of peanuts may be associated with a variety of bioactive compounds in peanut seeds, particularly antioxidant phytochemicals such as phenolic acids and flavonoids. After absorption by our body, these compounds can act on the sites or at remote sites to prevent the incidence of colon cancer and other chronic diseases [12]. Previous study reported that peanut seeds contain significant content of phenolic compounds, and flavonoids are the predominant bioactive compounds [13, 14]. Like other phenolic compounds, flavonoids impart multiple health promoting benefit, such as antioxidant and antiproliferative activity [15]. However, the flavonoid content in peanut seeds varies greatly due to genetic variation between peanut genotypes [16], therefore, a comprehensive evaluation of different peanut varieties is needed to provide detailed information about their components.
To the best of our knowledge, despite various peanut cultivars grown in different regions of China, there is no systematic study of phenolic profiles and bioactivity of these peanuts. In this study, we assessed the contents of total phenolics, flavonoids, in vitro antioxidant activities of five China-grown peanut cultivars. In addition, we also investigated the anticancer activity of peanut extracts through its capacity to inhibit cancer proliferation and evaluate the intracellular ROS level. Additionally, phytochemicals were identified, including those rarely reported in peanut seeds but found in other medicinal herbs or vegetables, such as coumarins, flavonoids, and other antioxidants. This study might provide valuable information for the cultivar selection of the tested peanuts as nutraceuticals or functional foods.
Materials and methods
Chemicals and reagents
All the reagents and solvents used in this study were of analytical or HPLC grade. Ethanol, HCl, NaOH, Folin–Ciocalteu phenol reagent, NaNO2, AlCl3·6H2O, CH3COONa, FeCl3·H2O, and K2S2O8 were purchased from Titan Corp. (Shanghai, China). Gallic acid, and catechin were purchased from Chengdu Derick Biotechnology Ltd. Co. (Chengdu, Sichuan, China). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2, 4, 6-Tri(2-pyridyl)-s-triazine (TPTZ), 6-hydroxy-2, 5, 7, 8-tetramethylchromane-2-carboxylic acid (Trolox), Dulbecco’s minimal essential medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Sigma Aldrich Co. (St. Louis, MO, USA). 2′, 7′-dichlorofluorescein di-acetate (DCFH-DA) probe was purchased from Yeasen Biotech. Ltd. Co. (Shanghai, China). Deionized water was used in all the experiments.
Sample collection and preparation
Five dry peanut cultivars were purchased from online shops of the Taobao Mall, China. The peanut cultivars were authenticated by Dr. Harold Corke from Department of Food Science & Technology, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China, Voucher specimen: PN2017010801 (Haihua), PN2017010802 (Luhua-NO.11), PN2017010803 (Sanhua-NO.11), PN2017010804 (Xiaosuangli), PN2017010805 (Xiaoshilihong). The thick hulls were shelled, and peanut seeds together with red coats were ground into fine powder, and stored at 4 °C for further analysis.
Extraction procedure
0.5 g of each peanut sample was soaked in 10 ml of 70% ethanol and then shaken at 50 °C for 24 h at 130 rpm with a shaker (HerryTech Ltd., Shanghai, China). The resulting slurry was centrifuged for 15 min at 3000×g (Shanghai Lu Xiangyi Centrifuge Instrument Ltd., Shanghai, China), and the supernatant was collected and stored at − 20 °C, which was directly applied to evaluate total phenolic content, total flavonoid content, antioxidant activity, and HPLC–MS analysis.
For intracellular antioxidant and antiproliferative activity evaluation, the supernatant was evaporated and freeze dried. The freeze dried samples were dissolved in deionized water to prepare a stock solution (100 mg/ml), which was used within 2 h.
Determination of total phenolic content (TPC)
The Folin–Ciocalteu method was carried out as previously described [17, 18] to determine TPC. Briefly, 2.0 ml Folin–Ciocalteu solution agents were added to 400 μl properly diluted sample and, 4 min later, 1.6 ml Na2CO3 solution (75 g/l) was added to the mixture for a further 2 h reaction. The absorbance of the reactants was measured at 760 nm using a UV–visible spectrophotometer (UV1800, Jinghua Instrument Ltd., Shanghai, China). Gallic acid was used as standard and was dissolved in 70% ethanol. The results were expressed as milligrams of gallic acid equivalent (mg GAE) /100 g dry weight (DW) of samples.
Determination of total flavonoid content (TFC)
The AlCl3-based colorimetric method was carried out as previously described [17, 18] to determine the TFC. Briefly, 500 μl sample was added to 3.5 ml distilled water and mixed well. NaNO2 solution (150 μl, 0.5 M) was added to the mixture, mixed well, and reacted for 6 min. After that, AlCl3 solution (150 μl, 0.3 M) was added to the reaction system and further reacted for 5 min. Finally, 1.0 ml NaOH solution (1.0 M) was added to the system and the absorbance was measured at 510 nm. Catechin was used as standard and was dissolved in 70% ethanol. The results were expressed as mg catechin equivalent (mg CE/100 g DW).
Determination of antioxidant activity
The antioxidant activity of peanut extracts was determined using DPPH free radical scavenging assay and ferric-reducing antioxidant power (FRAP) assay as previously described [19, 20].
For DPPH assay, DPPH working solution was prepared by adjusting the absorbance of DPPH stock solution (100 μM) at 515 nm to 0.70 ± 0.05. Thereafter, DPPH working solution (3.9 ml) was added to properly diluted sample solution (100 μl) and the mixture was reacted at room temperature in dark for 2 h. The absorbance of the reactants was detected at 515 nm. Trolox was used as standard, which was dissolved in 80% methanol. The results were expressed as μmol Trolox/g DW.
For FRAP assay, FRAP reagent were prepared by mixing sodium acetate buffer (300 mM, pH 3.6), TPTZ solution (10 mM TPTZ, 40 mM HCl), and FeCl3 solution (20 mM) in a volume ratio of 10:1:1. Thereafter, FRAP reagent (3 ml) was reacted with properly diluted sample solution (100 μl) for 4 min at room temperature. The absorbance was detected at 593 nm and the results were expressed as μmol Fe (II)/g DW.
Determination of antiproliferative activity
Human colon cancer cell lines (HCT116 and HT29) were provided by Dr. Yueliang Zhao (Shanghai Ocean University). All cells were cultured with RMPI medium supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37 °C in a humidified atmosphere with 5% CO2. The antiproliferative activity of peanut extracts was evaluated by cell counting kit 8 assay (CCK8). Briefly, cancer cells (5000 cells/well) in 96-well plates were treated with different concentrations of peanut extracts for 48 h. CCK 8 solution (10 μl) was added to each well and the cells were further cultured 1 h and the absorbance was recorded at 450 nm and the IC50 values were calculated.
Evaluation of the intracellular reactive oxygen species (ROS) generation
Intracellular ROS levels were measured using 2′,7′-dichlorofluorescein di-acetate (DCFH-DA) probe. Cancer cell lines (5 × 103 cells/well) were seeded into 96-well black plates and cultured for 48 h. The medium was removed and replaced with reduced-serum medium containing different concentrations of peanut extracts for 4 h. After that, the medium was replaced with reduced-serum medium containing DCFH-DA (10 μM) and cultured for 30 min at 37 °C. The medium was removed and the cells were washed twice with PBS and then immediately detected the fluorescence at 485/535 nm using a multi-function microplate reader SpectraMax® iD3 (Molecular Devices, San Jose, CA).
Identification of antioxidant compounds by ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS) analysis
Peanut extracts were analyzed with a primer UPLC-QTOF mass spectrometer (Waters, Milford, MA) equipped with an electrospray ionization source. A BEH C18 column (2.1 mm × 100 mm, 1.7 μm) was applied to separate the sample with following settings: column temperature, 45 °C; mobile phase A, ultrapure water; mobile phase B, acetonitrile; flow rate, 0.4 ml/min; injection volume, 1 μl. The gradient conditions were: 0 min, 5% B; 3 min, 20% B; 10 min, 100% B. Mass spectrometric analysis was performed in negative ion mode with following settings: capillary, 2 kV, sample cone voltage, 40 V; desolvation gas temperature, 450 °C; source temperature, 115 °C; flow rate of cone gas, 50 l/h; flow rate of desolvation gas, 900 l/h; acquisition range, 50–1000 m/z; collision energy, and 6 eV/20–45 eV; scan rate, 0.2 s. The mass data were processed with MassLynx 4.1 software. The exact elemental composition of each parent and generated fragments were calculated with molecular formula calculator.
Statistical analysis
All measurements were carried out in triplicate, and the results were expressed as mean values ± standard deviation (SD). One-way ANOVA was performed, and the statistical significance analysis was performed by Tukey’s HSD test (p < 0.05) using statistical software GraphPad Prism (Graphpad Software, Inc, San Diego, CA).
Results and discussion
Phenolic profiles in peanut cultivars
As Table 1 shows, 23 compounds were tentatively identified as the main phenolics in peanuts by UPLC-QTOF-MS in negative ion mode. The major coumarins and flavonoids were elucidated in detail by comparing retention time, and m/z values with values reported in literature.
Coumarins
Compounds 1 and 11 shared the same molecular ion at m/z 147.0447 were tentatively identified as dihydrocummarin isomers according to published data [21]. According to the published data [22], Compound 23 was tentatively identified as hydroxy-methoxy-psoralen isomer.
Flavonoids
Two flavanols, catechin-O-hexoside (3) and procyanidin A2 (16), were tentatively identified according to published literature [23].
Flavone derivatives show absolute richness among all assigned compounds. Compound 6 was tentatively identified as vaccarin, a flavonoid in the hexoside form, because it gave [M+HCOO]– at m/z 771.1986 and an MS2 signal at m/z 591.13535, which was consistent with the loss of deoxyhexosyl (m/z 132.04504). This compound was also analyzed with HPLC–MS/MS [24]. Isoetin-hexosyl-O-deoxyhexoside (10) with the deprotonated molecular ion at m/z 595.1301 was tentatively identified as it showed the loss of hexosyl and deoxyhexosyl and gave an MS2 signal at m/z 300.02714, which matched with the molecular weight of isoetin [25]. Compounds 12 and 13 were tentatively identified as kaempferol-di-O-hexoside isomers, because they gave the same [M–H]– at m/z 609.1464 and MS2 signal at m/z 285.04048, which was consistent with the loss of two hexosyl moieties, thus releasing the kaempferol moiety [26]. Like kaempferol-di-O-hexoside, Compounds 18 and 19 showed kaempferol MS2 signal at m/z 285.04007, however, they showed [M+HCOO]– at 623.1613, which matched the molecular weight of kaempferitrin, therefore they were tentatively identified as kaempferitrin isomers. Compound 14 was tentatively identified as hydroxykaempferol-O-hexoside with deprotonated molecular ion [M–H]– at m/z 463.0881 and MS2 product ion at m/z 299.01949, the latter reflecting the loss of hexoside moiety. Similarly, methoxykaempferol-O-hexoside isomers (20 and 21) were tentatively identified due to their deprotonated molecular ion [M–H]– at m/z 477.1033 and MS2 signal at m/z 313.03511 and m/z 297.04000, the latter two reflecting the loss of hexoside moiety and O-hexoside moiety, respectively. Quercetin-O-(-O-acetyl)-hexoside (17) with the deprotonated molecular ion [M–H]– at m/z 505.0982 was tentatively identified as it showed the loss of (O-acetyl)-hexoside moiety and gave an MS2 signal at m/z 300.02737, which matched the molecular weight of quercetin [27]. Apigenin-Ο-hexoside (22) with the deprotonated molecular ion [M–H]– at m/z 443.0981 was tentatively identified as it showed the loss of hexoside moiety and gave an MS2 signal at m/z 269.04500, which matched the molecular weight of apigenin [28].
Three flavanone derivatives were assigned in this study, including eriocitrin, viscumneoside V, and narirutin. Compound 5 was tentatively identified as eriocitrin by comparing with the literature data [29]. Viscumneoside V (7) with the deprotonated molecular ion at m/z 727.2090 was tentatively identified as it showed the loss of deoxyhexosyl-O-deoxyhexosyl and methyl moiety giving m/z 445.07986. Narirutin (15) was tentatively identified according to its deprotonated molecular ion [M–H]– at m/z 579.1734 [23].
Total phenolic content (TPC) and total flavonoid content (TFC) of different peanut cultivars
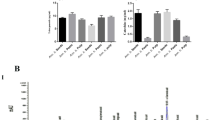
The peanut seeds were compared with respect to TPC, TFC, and antioxidant activity (Fig. 1). TPC of peanut cultivars varied from 185.4 to 300.9 mg GAE/100 g DW (Fig. 1a), among which, Xiaoshilihong peanut seeds had the highest TPC, followed by Sanhua NO.11. The TPC observed for the cultivars here were higher than those in Korea-grown peanut seeds (15.33–52.81 mg GAE/100 g DW) [30], which might be due to different growing conditions, genotypes, and cultivars. On the other hand, the TFC values ranged from 62.79 to 86.27 mg CE/100 g DW and Xiaoshilihong cultivar had the highest TFC (Fig. 1b). An earlier study revealed that the TFC of Indian peanuts were 24–51 mg CE/g [31], which is lower than that of China-grown peanut cultivars in this study. Previous study also investigated the TFC of 57 peanut seeds from province of Hebei, China, ranging from 0.39 to 4.53 mg rutin equivalent/g fresh weight [16]. Correlation analysis (Table 2) revealed that TFC has low correlation with TPC (r = 0.601), indicating that flavonoids are only contributed to parts of polyphenols.
Evaluation of antioxidant phenolics in peanut extracts. a total phenolic content (TPC), b total flavonoid content (TFC), c DPPH free radical scavenging activity (DPPH) d ferric-reducing antioxidant power (FRAP). Each measurement was determined in triplicate, and the results were expressed as mean ± SD. One-way ANOVA plus post hoc Tukey test was performed to compare means of TPC, TFC, DPPH, and FRAP of five peanut cultivars. Different superscript lowercase numbers indicated statistical significance (p < 0.05)
Antioxidant activities of different peanut cultivars
Polyphenolic compounds largely contributed to the chemopreventive and antioxidant properties of fruits and vegetables, which was supported by several cross-cultural epidemiological studies [32,33,34]. Therefore, we further investigated the antioxidant activity of peanut cultivars by DPPH assay and FRAP assay (Fig. 1c, d). For the sample analyzed, the DPPH value was highest in Xiaoshilihong, followed by Sanhua-NO.11, and lowest in Luhua-NO.11, ranged from 6.65 to 9.45 μmol Trolox/g DW. Correlation analysis (Table 2) revealed that DPPH value was significantly associated with TPC (r = 0.984, p < 0.01). The FRAP value was ranged from 8.80 to 13.61 μmol Fe (II)/g DW. Again the Xiaoshilihong exhibited the highest FRAP value, followed by Xiaosuangli, and lowest in Luhua-NO.11. The FRAP of Xiaoshilihong cultivar (13.61 μmol Fe (II)/g DW) was a little higher than that of common bean (9.87 μmol Fe (II)/g DW) [35], but lower than that of kidney bean seed coats (34.00–1066.46 μmol Fe (II)/g DW) [36]. According to statistical correlation analysis, the FRAP value was closely related to TPC (r = 0.886, p < 0.05). The FRAP value and DPPH value showed a relatively correlation with each other (r = 0.904, p < 0.01). The correlation analysis indicated that phenolics had significant contribution to the in vitro antioxidant activity of peanut extracts.
Antiproliferative activity of different peanut cultivars
Peanut extracts were tested to evaluate their antiproliferative activity on human colon cancer cell lines, HCT116 and HT29. Data are reported in Fig. 2. All extracts showed antiproliferative effects in a dose-dependent manner. The Sanhua-NO.11 extracts were the most active against both HCT116 and HT29 with IC50 values of 1.39 mg dry extract/ml and 1.56 mg dry extract/ml, respectively, which is comparable to red grape (1.5 mg dry extract/ml) [37]. Except for Sanhua-NO.11 extracts, the most promising results against HCT116 and HT29 cell lines were Xiaosuangli extracts with IC50 values of 3.55 mg dry extract/ml and 2.45 mg dry extract/ml, respectively, while Xiaosilihong extracts have the lowest antiproliferative activity against HCT116 and HT29. Our results suggested that despite Xiaosilihong extracts showed the highest in vitro antioxidant activity, it showed the lowest antiproliferative activity, indicating that in vitro antioxidant activity does not directly reflect the antiproliferative activity of peanuts. Correlation results also support this conclusion (r < 0.5). Similar results were also obtained in previous study [38], and even excessive antioxidant supplementation led to the increased mortality in patients with cancer [39].
ROS generation
ROS accumulation has been shown to trigger apoptosis and necrosis [40]. Therefore, we further evaluated the intracellular ROS level and found that peanut extracts induced intracellular ROS generation with a dose-dependent manner (Fig. 3), especially in HCT116. Treatment with Luhua-NO.11 and Sanhua-NO.11 led to a sharp increase of intracellular ROS in both HCT116 and HT29. Many other alcoholic extracts of plants are also reported to induce ROS-dependent apoptosis in cancer cells [40,41,42]. Correlation analysis given in Table 2 revealed that intracellular ROS generation in HCT116 has good association with antiproliferative activity against HCT116 (r = 0.765). Similarly, good association of intracellular ROS level in HT29 with antiproliferative activity against HTT29 (r = 0.901, p < 0.05) was also observed. The intracellular ROS in HCT116 and in HT29 showed strong correlation with each other (r = 0.917, p < 0.05). Thus, these results indicate that peanut extracts have antiproliferative activity and the effect is, at least in part, due to the ROS generation.
Conclusions
In summary, the present study investigated the antioxidant and antiproliferative activity and phenolic profile of five peanut cultivars from China. Xiaosilihong had the highest contents of phenolics and flavonoids and exhibited the strongest antioxidant activities, but exhibited the weakest antiproliferative activity against colon cancer cells (HCT116 and HT29). Sanhua-NO.11 exhibited the strongest antiproliferative activities against colorectal cancer cells (HT29 and HCT116). The antiproliferative activity of peanut extracts was closely related to ROS generation and had low correlation with phenolic compounds and antioxidant activities. Besides, different peanut cultivars differed in their antioxidant contents and components. These data can be used as a reference to prioritize which peanut varieties should be studied in the future to determine their in vivo activity as potential applications in functional foods.
References
A.M. Abbasi, F. Liu, X. Guo, X. Fu, T. Li, R.H. Liu, Int. J. Food Sci. Tech. 52(3), 817–826 (2017)
A.D. Assefa, Y.S. Keum, R.K. Saini, J. Food Meas. Charact. 12(3), 1548–1555 (2018)
H.F. Hetta, A. Elkady, R. Yahia, A.K. Meshaal, M.M. Saad, M.A. Mekky, I.M. Al-Kadmy, J. Immunol. Methods. 13, 112753 (2020)
R.H. Liu, J. Food Sci. 78(S1), A18–25 (2013)
Y.S. Chen, G.Y. Wang, H. Wang, C.H. Cheng, G.G. Zang, X.B. Guo, R.H. Liu, PLoS ONE 9, e108140 (2014)
I.B. de Sousa-Machado, T. Felippe, R. Garcia, G. Pacheco, D. Moreira, E. Mansur, Plant Cell Tiss. Org. 134(3), 491–502 (2018)
A.B. Jafari, E. Daneshzad, L. Azadbakht, Crit. Rev. Food Sci. Nutr. 13, 1–18 (2019)
W. Li, T. Zhao, J. Zhang, C. Wu, M. Zhao, G. Su, J. Chem. (2016). https://doi.org/10.1155/2016/9358285
D. Aune, N. Keum, E. Giovannucci, L.T. Fadnes, P. Boffetta, D.C. Greenwood, S. Tonstad, L.J. Vatten, E. Riboli, T. Norat, BMC Med. 14, 353 (2016)
P. Zandberg, J.L.M. Peters, P.N.M. Demacker, E.G. de Reeder, M.J. Smit, Menopause 8(2), 96–105 (2001)
P. Limmongkon, P. Nopprang, T. Chaikeandee, P. Somboon, Wongshaya. Food Chem. 239, 569–578 (2018)
F.M. Bhat, C.S. Riar, Food Chem. 237, 264–274 (2017)
A.C. de Camargo, M.A.B. Regitano-d’Arce, G.B. Rasera, S.G. Canniatti-Brazaca, L. Do Prado-Silva, V.O. Alvarenga, A.S. Sant’Ana, F. Shahidi, Food Chem. 237, 538–544 (2017)
M.L. Wang, A.G. Gillaspie, J.B. Morris, R.N. Pittman, J. Davis, G.A. Pederson, Plant Genet. Resour C. 6, 62–69 (2008)
Q.Q. Yang, R.Y. Gan, Y.Y. Ge, D. Zhang, H. Corke, Compr. Rev. Food Sci. Food 17(6), 1518–1539 (2018)
M. Hou, G. Mu, Y. Zhang, S. Cui, X. Yang, L. Liu, Crop. Breed. Appl. Biotechnol. 17(3), 221–227 (2017)
E.E.I. Maaiden, Y.E.I. Kharrassi, K. Moustaid, A.K. Essamadi, B. Nasser, J. Food Meas. Charact. 13, 121–130 (2019)
M. Palanisamy, S. Töpfl, R.G. Berger, C. Hertel, Eur. Food Res. Technol. 18, 1–10 (2019)
K. Li, M. Zeng, Q. Li, B. Zhou, J. Food Meas. Charact. 13, 51–60 (2019)
S. Gonçalves, E. Moreira, P.B. Andrade, P. Valentão, A. Romano, Eur. Food Res. Technol. 245(3), 753–762 (2019)
Q.Q. Yang, R.Y. Gan, D. Zhang, Y.Y. Ge, L.Z. Cheng, H. Corke, LWT-Food Sci. Technol. 114, 108321 (2019)
H. Huo, P. Jia, X. Zhang, Z. Zhang, H. Yang, Q. Zhang, H. Shi, L. Zhang, J. Chromatogr. B. 995, 85–92 (2015)
Y. Zheng, X. Zeng, W. Peng, Z. Wu, W. Su, Molecules 23(50), 1235 (2018)
G. Yang, N. Zhang, T. Wang, S. Zhang, R. Xu, Z. Zhu, K. Liu, Biomed. Chromatogr. 28(12), 1789–1794 (2014)
S. Shi, Y. Zhao, H. Zhou, Y. Zhang, X. Jiang, K. Huang, J. Chromatogr. A. 1209(1–2), 145–152 (2008)
X. Ma, J. Moilanen, O. Laaksonen, W. Yang, E. Tenhu, B. Yang, Food Chem. 272, 1–11 (2019)
B.B. Ismail, Y. Pu, M. Guo, X. Ma, D. Liu, Food Chem. 277, 279–288 (2019)
J. Wang, W. Yang, G. Wang, P. Tang, Y. Sai, J. Chromatogr. B 951–952, 78–88 (2014)
Y. He, P. Cheng, W. Wang, S. Yan, Q. Tang, D. Liu, H. Xie, Molecules 23(7), 1700–1706 (2018)
B. Adhikari, S.K. Dhungana, M.W. Ali, A. Adhikari, I.D. Kim, D.H. Shin, Food Sci. Biotechnol. 27(5), 1275–1284 (2018)
S. Salve, J. Arya, Microbiol. Biotechnol. Food. Sci. 8(2), 835–841 (2018)
D. Baci, M. Gallazzi, C. Cascini, M. Tramacere, D. De Stefano, A. Bruno, D.M. Noonan, Int. J. Mol. Sci. (2019). https://doi.org/10.3390/ijms20020307
H.F. Gu, Y.Y. Mao, M. Du, Crit. Rev. Food Sci. Nutr. 24, 1–16 (2019)
E. Riboli, T. Norat, Am. J. Clin. Nutr. 78(3), 559S–569S (2003)
Q.Q. Yang, R.Y. Gan, Y.Y. Ge, D. Zhang, H. Corke, Antioxidants 8(4), 83 (2019)
L. Kan, S. Nie, J. Hu, Z. Liu, M. Xie, J. Funct. Food. 26, 622–631 (2016)
C. Mazewski, K. Liang, E.G. de Mejia, Food Chem. 242, 378–388 (2018)
F.J. Jimenez-Gonzalez, J.M. Vélez-Gómez, J.J. Melchor-Moncada, L.A. Veloza, J.C. Sepúlveda-Arias, Pharmacogn. Mag. 14(55), 25 (2018)
E. Giovannucci, A.T. Chan, J. Clin. Oncol. 28(26), 4081–4085 (2010)
A. Pérez-Sánchez, E. Barrajón-Catalán, V. Ruiz-Torres, L. Agulló-Chazarra, M. Herranz-López, A. Valdés, A. Cifuentes, V. Micol, Micol. Sci. Rep. 9(1), 1–11 (2019)
Y.F. Kuo, Y.Z. Su, Y.H. Tseng, S.Y. Wang, H.M. Wang, P.J. Chueh, B. Flavokawain, Free Radical Bio. Med. 49(2), 214–226 (2010)
K.W. Park, J. Kundu, I.G. Chae, S.C. Bachar, J.W. Bae, K.S. Chun, Asian Pac. J. Cancer Prev. 15(17), 7291–7296 (2014)
Acknowledgements
This study was funded by the Shanghai Basic and Key Program (18JC1410800) and the Shanghai Agricultural Science and Technology Key Program (18391900600).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Qiong-Qiong Yang declares that she has no conflict of interest, Gowoon Kim declares that she has no conflict of interest, Arakkaveettil Kabeer Farha declares that she has no conflict of interest, Qiong Luo declares that she has no conflict of interest, and Harold Corke declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, QQ., Kim, G., Farha, A.K. et al. Phenolic profile, antioxidant and antiproliferative activities of diverse peanut cultivars. Food Measure 14, 2361–2369 (2020). https://doi.org/10.1007/s11694-020-00483-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00483-4