Abstract
Comparative study of functional properties, radical scavenging and antimicrobial activities of dromedary whey protein and casein hydrolysates was investigated. Dromedary protein hydrolysates were prepared by treatment with digestive proteases (pepsin and pancreatin) and by the proteolytic system of two lactic acid bacteria (Streptococcus thermophilus and Lactobacillus bulgaricus). Solubility and interfacial properties like emulsifying capacity are improved after enzymatic hydrolysis of both whey protein and casein. Whereas, foam capacity and stability are more important in whey protein hydrolysates than casein hydrolysates and are widely influenced by the method of hydrolysis. All hydrolysates showed radical scavenging activities. The highest antioxidant activity is exhibited by WPHE (whey protein hydrolysated by gastro-intestinal enzymes “pepsin and pancreatin”) for DPPH (2,2-diphenyl-1-picrylhydrazyl) test and CNHE (casein hydrolysated by pepsin and pancreatin) for ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) assay. Further, whey protein hydrolysates displayed antibacterial activity and WPHE was the most effective, particularly against Escherichia coli and Staphylococcus epidermidis. Based on the results, we conclude that WPHE has great technological applicability in food ingredients, as a promising source of functional hydrolysate with antioxidant and antimicrobial activities. For this reason, WPHE was added to the dromedary milk based beverage and chemical, microbiological and sensory properties of the resulting product were investigated. Formulated beverage flavored with strawberry or banana possessed a good microbiological quality. The sensory analysis demonstrated a good acceptance mainly in taste and consistency of the beverage samples. There is only significant difference in the color of the different formulated beverages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, consumers have more concerns and recommendations to use natural antioxidants and antimicrobials from food sources rather than synthetic ones. Synthetic antioxidants are used to preserve food quality by retarding oxidation and lipid peroxidation, but their use has been restricted because their toxic and carcinogenic effects [1]. There is also a great concern about natural antimicrobials in order to reduce the need for antibiotics, control microbial spoilage process and limit the occurrence of new food-borne disease outbreaks caused by pathogenic bacteria [2]. Natural antioxidants and antimicrobials are derived from animal, plant and microbial sources. Dromedary milk is one of the most interesting and promising foods with regard to their potential antioxidant and antimicrobial activities derived from caseins and whey proteins [3,4,5,6]. For despite the similar content to cow’s milk regarding proteins, there are differences between them in term of whey protein and casein profiles. For instance, unlike to cow’s milk, α-lactalbumine and β-casein are the major whey protein and casein respectively in dromedary milk [7]. Furthermore, dromedary milk stands out by the presence of certain proteins such as camel whey basic protein, whey acidic protein, lactophorin, immunoglobulins (IgG) lacking a light chain and peptidoglycan-recognition protein [8, 9]. The lack of β-lactoglobulin in this milk make it a suitable diet for people with cow's milk proteins allergy [10].
In addition, the absence of β-lactoglobulin—a thermal aggregated protein- is a techno-functional advantage of dromedary milk such as thermal stability during drying, heat induced aggregation and adherence to fouling properties [11]. However, data concerning techno-functional properties of dromedary milk are still scarce in literature. The heat stability and foaming properties [12], the effect of pH and heat treatment on foaming properties of whey proteins [13] and α-lactalbumin [14] have already been studied and resulted that at neutral pH, heat treatment was found to improve foam ability, whereas at acid pH this property decreased. Recently Momen et al. [15] compared the heat stability of dromedary and bovine milk whey proteins in aqueous solution and protein-rich emulsion systems and observed that emulsions made with dromedary whey proteins were more stable against thermal treatment. In spite of these studies, detailed investigations on technological properties of dromedary proteins should be explore further for the improvement of dromedary milk utilization for formulation of functional foods. Functionality has been defined as any property of a food ingredient that influences its utilisation, except its nutritional value [16]. The functional properties of ingredients directly or indirectly impact the processing, food quality and ultimately their acceptance and incorporation in food formulations [17]. Most of processed food products are multicomponent colloidal systems, enclosing proteins, polysaccharides, food bio-polymers and particles like oil droplets, lipid crystals, starch granules and gas bubbles. Hence, the overall system properties is arbitrated by the nature and strength of interactions amongst them [18]. Solubility, foam and emulsion capacity and stability as well as water and oil absorption ability, are the most interest techno-functional properties in food processing. One possible approach to improve these functional properties is enzymatic hydrolysis since it disrupt the protein tertiary structure and change the peptide profile and size [19].
Since the high nutritional capacity and bio-/technofunctional properties of whey proteins were understood in detail, it has been evolving into a value-added product [20]. In fact, Whey has a long history of use in dairy-based beverage productions which has become attracting both researchers’ and dairy industry’s attention. Indeed, a wide range of whey-based beverage has already studied and today are available in the global markets. These products include fermented or non-fermented beverages, such as whey-fruit (i.e. strawberry, [21]) or vegetable juice [22], probiotic [23]/prebiotic [24]/symbiotic [25] whey beverages. As far as the authors know, there are no studies developed beverage products based on dromedary whey. In addition, dromedary whey is recognized as a source of essential amino acids and comprise 20% of total milk protein having highly prized nutraceuticals ingredients such as α-lactalbumine, lactoferrin, which possesses biological properties in native form and enhanced upon their degradation into bioactive peptides on fermentation or hydrolysis [26, 27]. So judicious use of dromedary whey is became a necessary especially that consumers are today caring about their health through the consumption of healthy foods. Therefore, the incorporation of dromedary whey hydrolysates, to produce whey-based beverage could result in a functional food, serving as a new alternative for the dairy industry and for consumers interested in a healthy, nutritious diet; it also a new sensorial characteristics.
Accordingly, the present study was undertaken to compare the techno-functional, antioxidant and antimicrobial properties of native dromedary whey proteins, caseins and their hydrolysates released by two digestive enzymes and microbial proteases of two lactic acid bacteria. In addition, the objective of this study was to formulate a beverage mix containing the enzymatic dromedary whey protein hydrolysates as an ingredient and evaluate the sensory and keeping quality of this beverage.
Materials and methods
Materials
Fresh dromedary milk was supplied from Livestock and Wildlife laboratory (Arid Land Institute, Medenine, Tunisia). Pepsin (EC 3.4.23.1) [from porcine stomach mucosa; specific activity of 3260 units (U)/mg protein; and pancreatin (from bovine pancreas, activity equivalent to 8 U.S.P. specifications according to the supplier) were purchased from Sigma-Aldrich (Co., St. Louis, MO, USA). Maize oil was supplied from a local supermarket and used without further purification. Deionized water was used for the preparation of all solutions.
Casein and whey protein separation
After the collection, the fat was removed by centrifugation (5000×g, 30 min, 4 °C). The whey was separated from the casein by precipitation at pH 4.2 by the addition of HCl (1 M) followed by centrifugation at 1500×g for 20 min and neutralization by NaOH (1 M). The pellet containing casein was washed three times, its pH was adjusted at pH 4.2 again before centrifugation (1500×g, 20 min, 20 °C).
The whey proteins and casein were dialyzed against dionised water (SpectraPor, cut-off, 100–500 Da), followed by freeze drying and frozen at − 20 °C until use [3].
Protein hydrolysis by S. thermophilus and L. bulgaricus strains
Bacterial strains and growth conditions
Streptococcus thermophilus and L. bulgaricus were isolated in the laboratory from yogurt. S. thermophilus strains were inoculated in M17 medium and incubated overnight at 42 °C. While strains of L. bulgaricus were grown in MRS medium and incubated at 37 °C overnight before use. Precultures were then inoculated in M17 or MRS broths and incubated at 42 °C or 37 °C until OD650nm reached 1 [28].
Protein hydrolysis
Dromedary whey proteins and caseins were hydrolyzed according to the method described by Miclo et al. [28]. Briefly, pre-cultures were harvested by centrifugation at (4000×g, 5 min, 20 °C). The cell pellet was washed in sodium phosphate buffer (50 mM, pH 7.5), heated at 42 °C (for S. thermophilus) or 37 °C (for L. bulgaricus) and was resuspended at 1.5 ml of the same buffer. A volume of five hundred microliters of the cell suspension was incubated with 15 ml of sodium phosphate buffer at 42 °C or 37 °C containing 1 mg/ml of powder of dromedary whey proteins or caseins until obtain OD650 nm of 1.
Enzymatic digestion
Enzymatic digestion was assessed by the successive action of pepsin and pancreatin according to the protocol described by Jrad et al. [3].
Determination of hydrolysis degree, SDS-PAGE and molecular weight distribution
The degree of hydrolysis and SDS-PAGE were determined as described by Jrad et al. [3, 4]. Molecular weight distribution of casein and whey protein before and after hydrolysis were carried out by gel filtration chromatographic procedure using the same materials, methods, and condition as described by Dupas et al. [29].
Determination of functional properties
Protein solubility
The solubility of protein at pH 4 and 7 was determined in triplicate using the method described by Sammartin et al. [30]. A quantity of 500 mg of lyophilized whey proteins, caseins and their hydrolysates were dissolved in 40 ml of NaCl (0.1 M) until triplicate dispersion, the pH of dispersion was adjusted to 4 or 7 with HCl (0.1 M) or NaCl (0.1 M) solutions and left stirred for 1 h. The dispersion was then diluted with NaCl (0.1 M) solution, followed by centrifugation (2260×g, 15 min, 4 °C). The resulting supernatant fraction was filtered through Whatman No. 1 filter paper. Protein content of the filtrate and the original dispersion was determined by Bradford method [31]. The solubility of the products was calculated as shown in Eq. 1
where, PS: Protein solubility, P1 and P2 were the protein concentration (mg of protein/g of sample) in the supernatant and in the sample respectively.
Emulsifying capacity
Emulsion capacity was determined by the methods described by Beuchat et al. [32] and Beuchat [33]. To a known amount of sample (3 g), 50 ml of distilled water was added. The slurry was transferred to a blender and blended for 30 s at low speed (500 rpm). Refined maize oil was slowly added from a burette while the blending continued. The addition of oil was continued until there was a phase separation. Emulsion capacity was expressed as the amount of oil required to emulsify 1 g of protein.
Foam capacity and foam stability
Foaming capacity and foam stability of the samples were determined in triplicate according to the method of Phillips et al. [34], with minor modification. Dispersions of 5% (w/v) proteins (whey and casein) and their hydrolysates were whipped for 15 min at ambient temperature in a Braum mixer mod at maximum speed (10,000 rpm) using the whipping accessory. The volume of the foam was directly read in the measuring cylinder.
The foam stability was measured by monitoring volume of liquid drained from the resulting foam at ambient temperature for 160 min.
Biological activities
Measurement of 2,2-diphenyl-1-picrylhydrazyl (DPPH+) free radical scavenging capacity
Antioxidant activity was determined by a modified method of Bersuder et al. [35] as the ability to scavenge of DPPH (2,2-diphenyl-1-picrylhydrazyl) free radicals in an aqueous solution of proteins and their hydrolysates. A volume of 0.5 ml of different samples (6 mg/ml) were added to 0.5 ml of ethanol and 0.125 ml (0.02%, w/v) of DPPH in 99.5% ethanol prepared freshly.
After thorough mixing, the solutions were kept at room temperature for 60 min in the dark. The absorbance was monitored at 517 nm using a spectrophotometer (Cecil CE 2041, Cambridge, UK). The ability to scavenge the DPPH radical (% inhibition) was calculated as given in Eq. 2:
where, A0 and AS are the absorbance of the control and the sample, respectively. Butylated hydroxyanisole (BHA) is used as positive control.
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) assay
The ABTS assay was conducted by the method of Re et al. [36], with a slight modification. Briefly, a cation solution of ABTS+ (7 mM dissolved in water) and mixed with potassium persulfate (2.45 mM). Next, 600 μl of diluted ABTS+ was added to 200 μL of each protein (at 6 mg/ml)/ hydrolysate fraction (at 19 µM eq. NH2). After 6 min of reaction, the absorbance was recorded at 734 nm (Cecil CE 2041, Cambridge, UK). Trolox (20–80 μM) was used as the standard, and the ABTS+ antioxidant activity (%) was calculated as given in Eq. 2.
Determination of antimicrobial activity
Microbial strains
Antibacterial activities of dromedary whey proteins, caseins and their hydrolysates were tested against 3 strains of bacteria: 2 Gram-negative (Escherichia coli ATCC 25922, Salmonella Typhimurium ATCC 14028) and 1 Gram-positive (Staphylococcus epidermidis CIP 106510). Pre-cultures were prepared as described by Jrad et al. [37].
Disc diffusion method
Wells with 4.5 mm of diameter were performed and filled by culture suspension (80 µl) of the tested microorganisms (106 colony forming units (CFU)/ml of bacteria cells on the Mueller–Hinton medium (MH). Before incubation, all plates were stored at 4 °C for 2 h, to allow the diffusion of the inhibitor agents. At the end of incubation time (24 h at 37 °C for bacteria strains) antibacterial activity was established by the presence of measurable zones of inhibition. The antimicrobial activity was recorded as the width (in mm, diameter of the well included) of the inhibition zones after incubation. All tests were carried out for three sample replications and the results were averaged [37].
Beverage formulation
The beverage was developed according to the method described by Sinha et al. [38]. The mix formulation consisted on lyophilized WPHE (45%), sugar (2%) and vegetable oil (5%). Strawberry/banana (2%) was added for desirable flavor and color. Citric acid was added at a level of 1%. After that, heated dromedary skim milk (45% at 60 °C) was added. The ingredients were homogenized in a commercial food processor (Moulinex, Paris, France). The beverage, thus obtained, was stored under refrigeration. Microbiological analysis was carried out every 10 days. Chemical analysis (proteins, fat and dry matter) and sensorial evaluations were performed on the 1st day of storage. The sensory characteristics of coded samples of beverages were judged by 30 panel members and asked to evaluate the products on a scale of 1–5 (representing excellent—5, very good—4, good—3, fair—2 and poor—1) for taste, texture, color and overall acceptability.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 10.0 (Chicago, Illinois, USA). The Tukey’s-test and P value < 0.05 were used for statistical evaluation.
Results and discussion
Effect of the hydrolysis on functional properties of proteins
Solubility
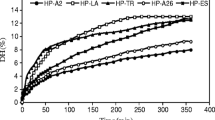
The solubility of intact dromedary milk proteins and their hydrolysates were measured in pH 4 and 7 (Fig. 1). WP had a good solubility at both pH values tested, whereas CN had minimum solubility at pH 4 and thus because the solubility of these proteins is lower at pH around the isoelectric point (4.2). At this pH, less iso-electrostatic repulsion between proteins, therefore resulting in a loss of solubility. The dependence of dromedary whey proteins solubility on pH has been studied by Laleye et al. [12] and reported that solubility is less at iso-electric point (pH 4.5) within the temperature range 80 to 100 °C.
SDS–PAGE profiles of dromedary milk whey proteins (WP), casein (CN) and and their hydrolysates. CN casein; CNHE: casein hydrolysated by enzyme, CNHL casein hydrolysated by Lactobacillus bulgaricus, CNHS casein hydrolysated by Streptococcus thermophilus, WP whey protein, WPHS whey protein hydrolysated by Streptococcus thermophilus, WPHL whey protein hydrolysated by Lactobacillus bulgaricus,M molecular standards
The present study showed that enzymatic hydrolysis increased protein solubility, both for WP and CN at different pH values. This result may be due to the decrease in molecular size of the protein creating small peptides which confirmed by molecular weight distribution analysis (The rate of low MW peptides bellow 1 kDa is 92.5% and 95.7% for CNHE and WPHE, respectively). The presence of small peptides unfolding the protein molecule leading to the exposition of more polar and ionizable groups on the protein surface, which could improve the ability of the protein molecule to form hydrogen bonds with water, thereby augmenting solubility. However, the solubility of CN and WP decreased after microbial hydrolysis. These hydrolysates (WPHS, WPHL, CNHL and CNHS) showed a lower solubility due to a lower degree of hydrolysis (Table 1; Fig. 2) and its impact on the relative reduction of solubility.
Solubility (%) of dromedary whey protein, casein and their hydrolysates at pH 4 and 7. WPHL whey protein hydrolysed by L. bulgaricus, WPHS whey protein hydrolysed by S. thermophilus, WPHE whey protein hydrolysed by digestive enzyme pepsin and pancreatin, CNHL casein hydrolysed by L. bulgaricus, CNHS casein hydrolysed by S.thermophilus, CNHE casein hydrolysed by digestive enzymes pepsin and pancreatin
Emulsion capacity
Figure 3b showed the emulsifying properties of WP, CN and their hydrolysates. Sample of CN showed higher emulsifying capacity than WP, this is may be due to the richness of CN fraction on β-CN. It should be noted that the amphiphilic nature of β-casein allows for non-polar residues to adsorb at hydrophobic surfaces, resulting in good emulsifying properties [39]. As expected, hydrolysis improved significantly (p < 0.05) the emulsion capacity of proteins compared to intact ones. The hydrolysates with better emulsion capacity (CNHE) contained higher amount of medium molecular-weight peptides (2.4% of peptides with molecular weight 5–10 kDa for CNHE). In fact, peptides with medium molecular weight possess the capacity to improve the flexibility of the hydrolysate at the oil/water interface, resulting in a greater emulsion formation.
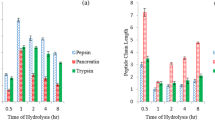
a Faom volume (ml/g) of dromedary whey protein, casein and their hydrolysates. WPHL, whey protein hydrolysed by L. bulgaricus, WPHS, whey protein hydrolysed by S. thermophilus; WPHE, whey protein hydrolysed by digestive enzyme pepsin and pancreatin; CNHL, casein hydrolysed by L. bulgaricus; CNHS, casein hydrolysed by S. thermophilus; CNHE, casein hydrolysed by digestive enzymes pepsin and pancreatin. b Emulsion capacity (ml oil/g protein) of dromedary whey protein, casein and their hydrolysates. WPHL whey protein hydrolysed by L. bulgaricus, WPHS whey protein hydrolysed by S. thermophilus, WPHE whey protein hydrolysed by digestive enzyme pepsin and pancreatin, CNHL casein hydrolysed by L. bulgaricus, CNHS casein hydrolysed by S. thermophilus, CNHE casein hydrolysed by digestive enzymes pepsin and pancreatin. c Foam stability (ml/g) of dromedary whey protein and their hydrolysates. WPHL whey protein hydrolysed by L. bulgaricus, WPHS whey protein hydrolysed by S. thermophilus, WPHE whey protein hydrolysed by digestive enzyme pepsin and pancreatin, CNHL casein hydrolysed by L. bulgaricus, d Foam stability (ml/g) of dromedary whey protein, casein and their hydrolysates. CNHS casein hydrolysed by S. thermophilus, CNHE casein hydrolysed by digestive enzymes pepsin and pancreatin
Foaming properties
Foam capacity and stability of dromedary whey protein, casein and their different hydrolysates are presented in Fig. 3a, c, d. Substances which have the ability to reduce the interfacial tension at the air–water interface form foams. In addition, results showed that undigested dromedary casein (CN) exhibited a higher foaming capacity and stability than those of whey proteins. No data in literature were available for CN foaming ability. Even so, similar trends were found by Lajnaf et al. [13] for foaming capacity of native sweet dromedary whey.
However, hydrolysis enhanced significantly the foam capacity of dromedary whey proteins and the highest foaming capacity, which reached 160 ml, was observed for WPHE. This result is attributed to the capacity of enzymatic hydrolysis to liberate peptides with reduced molecular weight (< 1 kDa), which would enhance the flexibility of the whey protein, and then facilitate the formation of interfacial membrane and foam production. However, foam stability of dromedary whey protein decreased after hydrolysis, which was mainly attributed to the presence of smaller peptides that did not have enough structural support to generate stable form.
Biological activities
Radical scavenging activity of intact proteins and their hydrolysates
Table 2 showed the results of DPPH·-scavenging capacity of dromedary milk proteins (WP and CN) and their hydrolysates. From the observed values of DPPH·-scavenging activity, undigested dromedary proteins exhibited lower antioxidant activity than their hydrolysates (27.1% and 30% at 6 mg/ml for whey proteins and caseins, respectively). In addition, results showed that the radical scavenging activity of dromedary milk proteins increased after hydrolysis by microbial proteases as well as enzymatic digestion. Hence, among the six hydrolysates, CNHS sample displayed the highest value of DPPH-radical-scavenging activity (83.12%) followed by WPHE (78.56%) and WPHL (68.15%). The increased free radical scavenging activity after hydrolysis suggests that there is a generation of peptides, encrypted in the sequence of dromedary casein and whey protein, acting as electron donors that could react with free radicals, converting them into more stable molecules and terminating the radical chain reaction. This is confirmed by the studies of Jrad et al. [3] and El-Hatmi et al. [5], who identified several free radical scavenging peptides derived from β-casein after dromedary milk digestion by gastro intestinal enzymes or fermentation of by S. thermophilus LMD-9, respectively.
The second measure of antioxidant potential, the ABTS+ radical inhibition (Table 2), showed that hydrolysates prepared using gastro-intestinal enzymes performed better for both casein and whey proteins. The highest ABTS antioxidant capacity was observed in CNHE fraction (83.47 ± 4.94%). This might be due to the synergetic effect of medium (5–10 kDa) and low (< 1 kDa) molecular-weight peptides, which were present in high rate in CNHE. These peptides with their amphilic characteristics seem to be important for the observed cationic radical scavenging activity of ABTS+, most likely as they participate in protons exchange with radical species. These findings were in accordance with the findings of Kumar et al. [40]. This study also supports the higher activity of the casein hydrolyzed by S. thermophilus (CNHS). This might also be due to the enzyme specificity to the particular site in the peptide chain. In fact, antioxidant activity depends on the nature of hydroxyl groups, molecular weight, the size and sequence of their amino acid of peptides [41].
Antibacterial activity
The ability of dromedary WP, CN and their hydrolysates to inhibit the growth of three pathogenic bacteria was investigated. The results are shown in Table 2 by evaluating the inhibition zones. As can be seen in this table, tested samples showed varying degrees of antimicrobial activity against all strains screened. WPHE displayed the highest inhibition zone against the strains of E. coli and S. epidermidis. This suggests that peptides with higher antibacterial activity than origin proteins were liberated during enzymatic proteolysis of dromedary whey protein. This result was consistent with the observation of Salami et al. [42] who reported that limited hydrolysis by proteinase K of dromedary whey proteins enhanced markedly their inhibition of E. coli growth. Dromedary milk casein and its hydrolysates exhibited antibacterial activities against only Gram-negative bacteria. Similar trends are observed by Jrad et al. [43] for dromedary casein before and after hydrolysis by pepsin and pancreatin.
Chemical composition, sensory properties and microbiological quality of formulated beverage
Since WPHE displayed a considerable techno-functional, radical scavenging and antibacterial activities, it has been incorporated as an ingredient in a dromedary-milk based beverage.
The study of the composition of whey beverages is fundamental for knowing the nutritional value of these products. Chemical composition of the two dromedary whey beverage flavored with Strawberry (mix a) or banana (mix b) were presented in Table 3. The strawberry/banana-flavored whey beverages presented the following proximate composition (% dry basis): proteins (58 ± 0.04 and 56.5 ± 0.05), fat (16 ± 0.02 and 14 ± 0.03) and minerals (12 ± 0.2 and 11.8 ± 0.6), corroborating with a previous studies on beverages made using whey protein hydrolysates [38], reconstituted skim milk and various fruit pulps, such as apple, banana, and mango [44]. There is no significant difference in chemical composition between the beverage mix a and b. Formulated beverage mix a and b could be considered as product with high nutritional value since are rich in protein and minerals.
Also, assessment of microbiological quality of food facilitates risk assessment and ensures the marketing of safe food to consumers. The microbiological quality of whey-beverage mix a and b are followed for twenty days of storage at 4 °C and the results are given in Table 3. The total flora of different beverages were ranging from 1.30 × 102 cfu/g at the first day of storage and reached 2.10 × 103 cfu/g at the end of storage period. This suggest that both beverages (mix a and b) were of good hygiene quality, during storage, being in accordance with European specific legislation for milk-based products [45]. In addition, the lactic acid bacteria count obtained in the present study increased at the end of storage period and was in the range between 3 and 4 104 CFU/g respectively for beverage mix a and b. This result showed that dromedary whey hydrolysate is a good medium for growth of lactic acid bacteria. Similar findings are reported by Kumar et al. [46], whose showed that cow whey has gained recognition from a dairy waste to an excellent medium for growth of probiotic lactobacilli.
Sensory attributes of dromedary beverage samples flavored with strawberry (mix a) and banana (mix b) are profiled in Fig. 4. The strawberry-flavored dromedary beverages received scores in the range of 3.74 and 4.82 in a 5-point scale, indicating that the consumers liked very well some attributes of the beverage (mix a) and liked moderately others. Thus, it is important to emphasize that despite the highest scores of the strawberry-flavored beverage, the variation with respect to banana-flavored beverage is not statistically significant for the overall acceptance, taste and texture sensory attributes. However, a significant difference was observed for in the appearance (color) of the two products, with higher color liking of strawberry-flavored beverage with score of 4.82. In fact, the pink color of the beverage flavored by the strawberry (beverage mix a) was more appreciated by the consumer.
Conclusion
The hydrolysis by pepsin and pancreatin enhanced the antioxidant, antimicrobial activities and techno-functional properties of dromedary whey proteins. Hence, the WP hydrolysated by pepsin and pancreatin (WPHE) can be used as new bioactive additive, emulsifying or foaming agent, in functional foods.
Therefore, these results encourage us to introduce WPHE as an ingredient of the beverage mix product and evaluate its storage stability. From sensory evaluation view, panelists appreciated more the beverage mix flavored by strawberry. In this study, incorporating successfully the WPHE with antioxidant and antimicrobial activities into a beverage appears to create an exciting link between food science and therapeutic nutrition. However, further research, including evaluation of health promoting effects of on food systems containing WPHE is recommended.
References
M.A. Alenisan, H.H. Alqattan, L.S. Tolbah, A.B. Shori, Antioxidant properties of dairy products fortified with natural additives: a review. J. Assoc. Arab. Univ. Basic Appl. Sci. 24, 101–106 (2017)
M.M. Tajkarimi, S.A. Ibrahim, D.O. Cliver, Antimicrobial herb and spice compounds in food. Food Control 21, 1199–1218 (2010)
Z. Jrad, H. El Hatmi, I. Adt, J.M. Girardet, C. Cakir-Kiefer, J. Jardin, P. Degraeve, T. Khorchani, N. Oulahal, Effect of digestive enzymes on antimicrobial, radical scavenging and angiotensin I-converting enzyme inhibitory activities of camel colostrum and milk proteins. Dairy Sci. Technol. 94, 205–224 (2014)
Z. Jrad, J.M. Girardet, I. Adt, N. Oulahal, P. Degraeve, T. Khorchani, H. El Hatmi, Antioxidant activity of camel milk casein before and after in vitro simulated enzymatic digestion. Mljekarstvo 64, 287–294 (2014)
H. El-Hatmi, Z. Jrad, T. Khorchani, J. Jardin, C. Poirson, C. Perrin, C. Cakir-Kiefer, J.M. Girardet, Identification of bioactive peptides derived from caseins, glycosylation-dependent cell adhesion molecule-1 (GlyCAM-1), and peptidoglycan recognition protein-1 (PGRP-1) in fermented camel milk. Int. Dairy J. 56, 159–168 (2016)
D. Almi-Sebbane, I. Adt, P. Degraeve, J. Jardin, E. Bettler, R. Terreux, N. Oulahal, A. Mati, Casesidin-like anti-bacterial peptides in peptic hydrolysate of camel milk β-casein. Int. Dairy J. 86, 49–56 (2018)
O.A. Al haj, H.A. Alkanhal, Compositional, technological and nutritional aspects of dromedary camel milk. Int. Dairy J. 20, 811–821 (2010)
O.U. Beg, H.V. Bahr-Lindstrom, Z.H. Zaidi, H. Jornvall, A camel milk whey protein rich in half-cystine: primary structure, assessment of variations, internal repeat patterns, and relationships with neurophysin and other active polypeptides. Eur. J. Biochem. 159, 195–201 (1986)
H. El-Hatmi, J.M. Girardet, J.L. Gaillard, M.H. Yahyaoui, H. Attia, Characterisation of whey proteins of camel (Camelus dromedarius) milk and colostrum. Small Rumin. Res. 70, 267–271 (2007)
E. El-Agamy, M. Nawar, S.M. Shamsia, S.F.W. Awad, G. Haenlein, Are camel milk proteins convenient to the nutrition of cow milk allergic children? Small Rumin. Res. 82, 16 (2009)
Y. Hailu, E.B. Hansan, E. Seifu, M. Eshetu, R. Ipsen, S. Kappeler, Functional and technological properties of camel milk proteins: a review. J. Dairy Res. 83, 422–429 (2016)
L.C. Laleye, B. Jobe, A.A.H. Wasesa, Comparative study on heat stability and functionality of camel and bovine whey proteins. J. Dairy Sci. 91, 4527–4534 (2008)
R. Lajnaf, L. Picart-Palmade, E. Cases, H. Attia, S. Marchesseau, M.A. Ayadi, The foaming properties of camel and bovine whey: the impact of pH and heat treatment. Food Chem. 240, 295–303 (2018)
R. Lajnaf, L. Picart-Palmade, H. Attia, S. Marchesseau, M.A. Ayadi, The effect of pH and heat treatments on the foaming properties of purified α-lactalbumin from camel milk. Colloids Surf. B 156, 55–61 (2017)
S. Momen, M. Salami, F. Alavi, Z. Emam-Djomeh, A.A. Moosavi-Movahedi, The techno-functional properties of camel whey protein compared to bovine whey protein for fabrication a model high protein emulsion. LWT Food Sci. Technol. 101, 543–550 (2019)
J.E. Kinsella, Functional properties of protein foods. Crit. Rev. Food Sci. Nutr. 1, 219–229 (1976)
A. Mahajan, S. Dua, Salts and pH induced changes in functional properties of amaranth (Amaranthus tricolor L.) seed meal. Cereal Chem. 79, 834–837 (2002)
E. Dickinson, M. Golding, Influence of calcium ions on creaming and rheology of emulsions containing sodium caseinate. Colloids Surf. A 144, 167–177 (1998)
F. Tamm, S. Herbst, A. Brodkorb, S. Drusch, Functional properties of pea protein hydrolysates in emulsions and spray-dried microcapsules. Food Hydrocoll. 58, 204–214 (2016)
C.I. Onwulata, P. Tomasula, Whey texturization: a way forward. Food Technol. 58, 50–55 (2004)
D.R. Janiaski, T.C. Pimentel, A.G. Cruz, S.H. Prudencio, Strawberry-flavored yogurts and whey beverages: what is the sensory profile of the ideal product? J. Dairy Sci. 99, 5273–5283 (2016)
A.G. Cruz, A.S. de Sant’Ana, M.M. Macchione, Â.M. Teixeira, F.L. Schmidt, Milk drink using whey butter cheese (queijo manteiga) and acerola juice as a potential source of vitamin C. Food Bioprocess. Technol. 2, 368–373 (2009)
P. Saha, P.R. Ray, P.K. Ghatak, S.K. Bag, T. Hazra, Physico-chemical quality and storage stability of fermented Chhana whey beverages. Indian J. Dairy Sci. 70, 398–403 (2017)
J.T. Guimarães, E.K. Silva, V.O. Alvarenga, A.L.R. Costa, R.L. Cunha, A.S. Sant'Anna, M.Q. Freitas, M.A.A. Meireles, A.G. Cruz, Physicochemical changes and microbial inactivation after high-intensity ultrasound processing of prebiotic whey beverage applying different ultrasonic power levels. Ultrason. Sonochem. 44, 251–260 (2018)
F.P. Souza, C.F. Balthazar, J.T. Guimarães, T.C. Pimentel, E.A. Esmerino, M.Q. Freitas, R.S.L. Raices, M.C. Silva, A.G. Cruz, The addition of xyloligoosaccharide in strawberry-flavored whey beverage. LWT-Food Sci. Technol. 109, 118–122 (2019)
M. Salami, R. Yousefi, M.R. Ehsani, S.H. Razavi, J.M. Chobert, T. Haertlé, A.A. Saboury, M.S. Atri, A. Niasari-Naslaji, F. Ahmad, A.A. Moosavi-Movahedi, Enzymatic digestion and antioxidant activity of the native and molten globule states of camel α-lactalbumin: possible significance for use in infant formula. Int. Dairy J. 19, 518–523 (2009)
Z. Jrad, H. El-Hatmi, I. Adt, S. Gouin, J. Jardin, O. Oussaief, M. Dbara, S. Arroum, T. Khorchani, P. Degraeve, N. Oulahal, Antilisterial activity of dromedary lactoferrin peptic hydrolysates. J. Dairy Sci. 102, 4844–4856 (2019)
L. Miclo, E. Roux, M. Genay, E. Brusseaux, C. Poirson, N. Jameh, C. Perrin, A. Dary, Variability of hydrolysis of β-, αs1-, and αs2-caseins by 10 strains of Streptococcus thermophilus and resulting bioactive peptides. J. Agric. Food Chem. 60, 554–565 (2012)
C. Dupas, I. Adt, A. Cottaz, R. Boutrou, D. Molle, J. Jardin, T. Jouvet, P. Degraeve, A chromatographic procedure for semi-quantitative evaluation of casein phosphor peptides in cheese. Dairy Sci. Technol. 89, 519–529 (2009)
B. Sammartin, O. Diaz, L. Rodriguez-Turienzo, A. Cobos, Functional properties of caprine whey protein concentrates obtained from clarified cheese whey. Small Rumin. Res. 110, 52–56 (2013)
M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 249–254 (1976)
L.R. Beuchat, J.P. Cherry, M.R. Quinn, Physicochemical properties of peanut flour as affected by proteolysis. J. Agric. Food Chem. 23, 616–620 (1975)
L.R. Beuchat, Functional and electrophoretic characteristics of succinylated peanut flour protein. J. Agric. Food Chem. 25, 258–261 (1977)
L.G. Phillips, J.B. German, T.E. Oneill, E.A. Foegeding, V.R. Harwalkar, A. Kilara, B.A. Lewis, M.E. Mangino, C.V. Morr, J.M. Regenstein, D.M. Smith, J.E. Kinsella, Standardized procedure for measuring foaming properties of three proteins, a collaborative study. J. Food Sci. 55, 1441–1453 (1990)
P. Bersuder, M. Hole, G. Smith, Antioxidants from a heated histidine glucose model system I: investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J. Am. Oil Chem. Soc. 75, 181–187 (1998)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237 (1999)
Z. Jrad, H. El Hatmi, I. Fguiri, S. Arroum, M. Assadi, T. Khorchani, Antibacterial activity of lactic acid bacteria isolated from Tunisian camel milk. Afr. J. Microbiol. Res. 7, 1002–1008 (2013)
R. Sinha, C. Radha, J. Prakash, P. Kaul, Whey protein hydrolysate: functional properties, nutritional quality and utilization in beverage formulation. Food Chem. 101, 1481–1491 (2007)
E. Dickinson, Stabilising emulsion-based colloidal structures with mixed food ingredients. J. Sci. Food Agric. 93, 710–721 (2013)
D. Kumar, M.K. Chatli, R. Singh, N. Mehta, P. Kumar, Antioxidant and antimicrobial activity of camel milk casein hydrolysates and its fractions. Small Rumin. Res. 139, 20–25 (2016)
M. Salami, R. Yousefi, M.R. Ehsani, M. Dalgalarrondo, J.M. Chobert, T. Haertlé, S.H. Razavi, A.A. Saboury, A. Niasari-Naslaji, A.A. Moosavi-Movahedi, Kenitic characterization of hydrolysis of camel and bovine milk proteins by pancreatic enzymes. Int. Dairy J. 18, 1097–1102 (2008)
Z. Jrad, H. El Hatmi, I. Adt, T. Khorchani, P. Degraeve, N. Oulahal, Anti-microbial activity of camel milk casein and its hydrolysates. Acta Alim. 44, 609–616 (2015)
A.E. Hangerman, K.M. Riedl, G.A. Jones, K.N. Sovik, N.T. Ritchard, P.W. Hartzfeld, T.L. Riechel, High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 46, 1887–1892 (1998)
M. Shukla, Y. Jha, S. Admassu, Development of probiotic beverage from whey and pineapple juice. J. Food Process. Technol. 4, 2–4 (2013)
U. Council Directive, Directive laying down the health rules for the production and placing on the market of raw milk, heat-treated milk and milk-based products. (1992).
S.M.H. Kumar, D. Saxena, L. Sabikhi, Developments in whey based beverages. Indian J. Dairy Sci. 66, 281–287 (2013)
Acknowledgements
The authors thank Dr Isabelle ADT (University of Claude Bernard Lyon 1, ISARA Lyon, BioDyMIA (Bioingénierie et Dynamique Microbienne aux Interfaces Alimentaires), Equipe Mixte d’Accueiln°3733), IUT Lyon 1, 01000 Bourg en Bresse, France) for his kind help with gel filtration analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jrad, Z., Oussaief, O., Khorchani, T. et al. Microbial and enzymatic hydrolysis of dromedary whey proteins and caseins: techno-functional, radical scavenging, antimicrobial properties and incorporation in beverage formulation. Food Measure 14, 1–10 (2020). https://doi.org/10.1007/s11694-019-00261-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00261-x