Abstract
The low solubility of carotenoids is one of the main challenges for the food industry. Nanoemulsions may be a favorable approach for expanding the application of bioactive compounds with hydrophobic capacity such as β-carotene. The objective of this work was to develop two distinct food matrices (yoghurt and gelato-type ice cream) added with β-carotene nanoemulsions to determine the influence of nanostructures on technological parameters and product stability. The synthesis of nanoemulsions took place with corn oil and 0.2 mg mL−1 of β-carotene by high pressure homogenization. Parameters were evaluated for 28 days of storage. The addition of nanoemulsion increased the gelato yield, by increasing the overrun, without affecting the stability parameters. In yogurt, the nanoemulsions increased the syneresis index and reduced the parameters of firmness, consistency, cohesiveness and viscosity index in the formulation. The nanoemulsions protected the carotenoid during storage, leading to only a slight color variation. The results show that the use of nanoemulsions can improve the dispersion and stability of these compounds in the developed products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural colored pigments have been widely used industrially, mainly by the pharmaceutical and food industry, due to their medicinal and nutritional values (Zhang et al., 2022a, b). Carotenoids are present in some plants, fruits, vegetables, algae and photosynthetic prokaryotes, presenting pigments in the yellow, orange and red scale (Jalili-Jivan et al., 2022). Among the carotenoids, β-carotene stands out, as it is a precursor of vitamin A, important in the benefit of the immune system, mucous membranes and eyes, in addition to being a potent antioxidant, acting in the prevention of cardiovascular and degenerative diseases (Xie et al., 2021; Cardoso et al., 2017).
Foods enriched with active components, such as carotenoids, provide, in addition to basic food, health benefits, helping to prevent diseases, thus being of great interest to the food industry (Zardini et al., 2018). However, the direct application of β-carotene is restricted, since it has low solubility in aqueous media, high sensitivity to extrinsic factors such as pH and light, in addition to low bioaccessibility (Yi et al., 2020).
Nanotechnology has been widely used in order to overcome these limitations, mainly through lipid-based nanodelivery systems, such as nanoemulsions, which enable chemical stability, water solubility and bioavailability (Yi et al., 2021). The use of nanoemulsions can be designed to increase the bioavailability and release of vitamins, stability and appearance of emulsified products, among others (Ozogul et al., 2022). These nanodelivery systems allow the solubilization of non-polar bioactive agents in hydrophobic particles that have a hydrophilic external surface, allowing dispersion in aqueous media and expanding the application of bioactive compounds with hydrophobic capacity (Chen et al., 2020). Nanoemulsions are synthesized by economically viable means suitable for use in food, using technologies already widely used in the sector, such as sonication, microfluidization and homogenization (Rezaei et al., 2019).
Sensory characteristics are important indicators for food formulations that use emulsions, especially considering the interactions between matrices (Xie et al., 2021). However, the addition of nanostructures can change the appearance, texture and stability of products. In this sense, this study stands out for the investigation of the technological parameters and stability of the applied product of nanoemulsions. Unlike the studies published so far in the journal Food and Bioprocess Technology, which aim at the development, characterization and investigation of nanoemulsions themselves (Choi et al., 2011; Pascual-Pineda et al., 2014; Pinilla et al., 2020; Silva et al., 2012), and not the effect of their direct application in food products. Based on this, the objective of this work was to apply β-carotene nanoemulsions in different food matrices such as yogurt and gelato-type ice cream, in order to determine the influence of these nanostructures on the technological parameters and stability of the products.
Materials and Methods
Materials
Corn oil (Cargill, Mato Grosso, Brazil), Tween 20 (LabSynth, São Paulo, Brazil), Span 80 (Sigma–Aldrich, Hessen, Germany), β-carotene (Sigma–Aldrich), and ultrapure water, obtained from a Millipore Milli-Q filtration system (Merck, Hessen, Germany), were used for the preparation of all emulsions. All other reagents were analytical grade.
Preparation of Nanoemulsions
For nanoemulsion preparation, the oil phase was composed of 70% (w w−1) corn oil, 30% (w w−1) Span 80, and β-carotene (0.2 mg mL−1). The aqueous phase consisted of 10 mL of water and 1% (w v−1) Tween 20. The oil and aqueous phases were heated under magnetic stirring, at 60 and 80 °C, respectively. After the complete solubilization of both phases, the aqueous phase was added to the oil phase under constant magnetic stirring (700 rpm). The formed suspension was pre-homogenized (Ultra-Turrax® T10 basic, IKA, Baden-Württemberg, Germany) at 14,500 rpm for 2 min, and then passed through a high-pressure homogenizer (EmulsiFlex-C3, Avestin, Ontario, Canada) at 10,000 psi for six cycles (20 s each) (Borba et al., 2019).
Mean particle size of the nanoemulsions were determined in triplicate by dynamic light scattering, using a Zetasizer (Nano-ZS90, Malvern Instruments, Worcestershire, UK) and its morphology using Transmission Electron Microscope (120 keV, Jeol, JEM-1400, coupled with EDS microprobe).
Product Formulation
Yogurt
In the preparation of the yogurt, instant whole milk powder was used, kindly provided by the company CCGL (Cruz Alta, Brazil), reconstituted according to the manufacturer’s instructions. The milk was heated to 45 °C and BioRich® dairy mix provided by Chr. Hansen (Hoersholm, Denmark) was added. During the fermentation process, the temperature was maintained at 45 °C, the pH was determined to reach 4.7, when fermentation was interrupted by rapid cooling to 8 °C (Quitanilha et al., 2021).
Three yogurt formulations were developed (Table 1): YC (corresponds to the control sample, without addition of dye and nanoemulsion), YβN (sample with addition of β-carotene nanoemulsion) and YFβ (sample with addition of non-encapsulated β-carotene). The β-carotene was kindly provided by Duas Rodas (Jaguará do Sul, Brazil). Samples were packed in sterile plastic bottles and stored at 4 °C for 28 days.
Gelato
The semi-ready gelato base (Specialitá Gelato Aqua®) was used, being gently kindly supplied by Duas Rodas Company (Jaguará do Sul, Brazil). Gelato with the formulations GC (control, without addition of dye and nanoemulsion), GβN (with 15% v v−1 of β-carotene nanoemulsion) and GFβ (with non-encapsulated β-carotene, same concentration the YβN 30 mg L−1) were produced using the ingredients shown in Table 1.
Initially, all the ingredients were dispersed in water using a planetary mixer (Arno Bpa model), and the syrups obtained were then frozen at − 5 °C. Afterwards, the syrups were homogenized for 7 min (Borrin et al., 2018) using the planetary mixer. The gelato formulations were packed in 2 L plastic containers and stored in a freezer at − 18 °C (Kaminska-Dworznicka et al., 2022) for 28 days.
Proximal Composition
Moisture, ash, protein and lipid contents were determined (AOAC, 2000), and carbohydrate content obtained by the difference method.
β-Carotene Content
The carotenoid content of yogurt and gelato was monitored by a methodology described by Mezquita et al. (2014) with modifications. For this, 4 g of sample was mixed with 2 mL of dimethylsulfoxide (DMSO), 3 mL of acetone, 2 mL of 20% NaCl solution (w v−1) and 2 mL of hexane. The mixture was vortexed for 15 s and centrifuged at 2147 × g for 5 min. The hexane phase containing the carotenoids was removed and stored. Successive extractions of the mixture were performed with 2 mL of hexane until no staining was observed. Determination of the total carotenoid concentration in hexane was performed using a spectrophotometer at 450 nm (Mattos et al., 2022) using Eq. (1):
where TC = total carotenoid concentration (μg g−1); A = absorbance; V = volume (mL); m = sample mass (g); and \({A}_{1\%}^{1 {cm}^{-1}}\) = specific absorbance of β-carotene in hexane (2592).
Color
The color variation of the yogurt and gelato was analyzed using a Minolta colorimeter (Chroma Meter Model CR-400/410, Konica Minolta, Osaka, Japan) in the CIELab system (illuminant D65), where a* indicates the region of red (+ a*) to green (− a*), b* indicates the area of yellow (+ b*) to blue (− b*), and L* is luminosity. From these parameters, the color difference (ΔE) was calculated through Eq. (2) (Yuan et al., 2018), chroma through Eq. (3) (Tupuna et al., 2018), and hue angle through Eq. (4) (Tupuna et al., 2018).
where \({L}_{0}^{*}\), \({a}_{0}^{*}\) and \({b}_{0}^{*}\) are the parameters at the initial time (t = 0), and \({L}^{*}\), \({a}^{*}\) and \({b}^{*}\) are the parameters at the time at which the analysis is performed.
Texture Analysis
Penetration assays were used to determine the texture of the products (TA.XT Plus, Stable Micro Systems Ltd., Surrey, UK). The operational conditions described in the equipment for yogurt were applied: 35-mm-diameter disc-shaped probe, 1.0 mm s–1 penetration velocity, and 30 mm penetration distance. With the gelato tests were carried out under the conditions a 2 mm diameter probe penetrated the sample to a depth of 10 mm. The analysis used 250 N load cells and probe speeds of 25 mm min−1 during penetration and 400 mm min−1 (Alfaifi & Stathopoulos, 2010). For each test, 100 g of sample were used, and four parameters were determined: firmness (g), consistency (g s−1), cohesiveness (g), and viscosity index (g s−1).
pH, Titratable Acidity, Syneresis and Viscosity in Yogurt
The pH of the samples was determined throughout the lactic fermentation and in the product, using a digital pH meter (MEDBIO,Vitória, Brazil) (AOAC, 2000). For analysis of the titratable acidity, 10 g of the sample was diluted with 10 mL of CO2-free water, and 4–5 drops of the phenolphthalein indicator were added. The mixture was titrated with a standard solution of NaOH (0.1 N) under stirring until the endpoint detectable by the pink appearance of phenolphthalein (AOAC, 2000).
For the syneresis analysis, approximately 10 g of each yogurt sample was homogenized using a glass rod, and shaken 20 times clockwise and 20 counterclockwise. The homogenization samples were left to stabilize at 4 °C for 2 h and were then centrifuged at 10 °C, 3237 × g for 12 min. The weight of the separated whey was used to calculate the syneresis, expressed by Eq. (5) (Gilbert et al., 2021).
For the viscosity analysis, a Brookfield digital rheometer (AMETEK Brookfield, Middleboro, USA) was used, coupled with a water bath at 25 °C, with an adapter for small samples. The readings were recorded at 15 s intervals, and the shear rate ranged from 5 to 100 s−1 (Trindade et al., 2018). From the apparent viscosity curves (cP) as a function of the shear rate (s−1), the Ostwald–de Waele model (Eq. (6)) was tested:
where η is the viscosity (mPa s–1), K is the consistency index (Pa s−1), and n is the behavior index (dimensionless).
pH, Total Soluble Solids (°Brix), Overrun and Melting in Gelato
The pH of the samples was determined using a digital pH meter (AOAC, 2000) and the total soluble solids content was measured in triplicate using a handheld refractometer. The overrun calculated according to Eq. (7) (Liu et al., 2022).
The melting behavior of the GC and GβN samples was evaluated according to Rinaldi et al. (2014) with modifications. Gelato samples (75 ± 2.0 g at − 18 °C) were placed on a mesh (around 1 mm2) attached to a beaker and maintained in a controlled temperature room at 20 °C. The dripped mass was measured for 120 min, and the recorded data expressed as the percentage of melted volume relative to the initial one. The melting rate (g min−1) was calculated according to Amador et al. (2017) from the slope of the linear portion of the curve of the weight of the dripped portion (grams) plotted against time (minutes).
Statistical Analysis
All experiments were carried out in triplicate (n = 3). The analysis of variance (ANOVA), followed by the Tukey’s test, was used for finding significant differences at 95% confidence level (p ≤ 0.05). In order to evaluate statistical significance between two treatments, the t test was applied (p ≤ 0.05). Data were treated by Statistica 5.0 (StartSoft Inc., Tulsa, OK, USA).
Results and Discussion
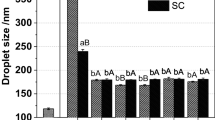
In this study, two refrigerated products were developed: yogurt and gelato, applying nanoemulsions of β-carotene and corn oil, developed and characterized by Borba et al., 2019. The nanoemulsions presented droplets with a size in the range of 300 nm (Fig. 1). The use of products with distinct characteristics such as pH, proximate composition, storage temperature, and physical structure provided important information about the stability of β-carotene nanoemulsions when inserted in complex matrices.
The micrograph shows a smooth, spherical surface and a thin outer coating layer, indicating that the oil has encapsulated the surface of the β-carotene droplets. The average particle size is within the range of nanoemulsions used in industrial preparations, which include sizes between 50 and 500 nm (Chaari et al., 2018).
Both products, gelato and yogurt with nanoemulsion, were visually homogeneous, the yogurt showed a small oily halo on the top of the samples that disappeared with agitation, after 21 days of storage. When non-encapsulated β-carotene was added to the (GFβ) and (YFβ) products, a heterogeneous appearance was observed, with non-solubilized carotenoid particles. The same behavior occurred in the orange drink fortified with unencapsulated lycopene instead of lycopene nanocapsules, where encapsulation overcame the low solubility of lycopene and eliminated the unpleasant taste of the carotenoid (Zardini et al., 2018).
The difference in homogeneity between the sample added with nanoemulsion and those with the unencapsulated carotenoid demonstrates an important advantage of using nanoencapsulation to produce carriers of lipophilic compounds. This process can help and simplify the manipulation, application and solubilization of these compounds in different food matrices (Ghayour et al., 2018). The YFβ sample showed greater coloration (Fig. 2a) than YβN (p < 0.05), presenting ΔE of 7.59 ± 1.77, and 1.52 ± 0.55, respectively. However, the gelato samples did not differ statistically, showing a color variation of ΔE of 2.61 ± 0.82 (GβN) and 3.68 ± 1.98 (GFβ). Color is an essential attribute in foods and often influences consumer preference (Balthazar et al., 2018; Çakmakçı et al., 2016). Color, appearance and homogeneity are essential parameters, linked to the acceptability of products by the consumer, as well as the perception of sensory characteristics (Santagiuliana et al., 2019).
The color tone of the samples indicated by the shade angle (Tupuna et al., 2018) was close to 90° (Fig. 2b), showing a yellowish color, even after 28 days of storage. Despite having a similar hue angle, the color saturation indicated by the chroma values (Fig. 2c) was significantly different (p < 0.05). GβN saturation was higher than GFβ. Rinaldi et al. (2014) also described an increase in the color saturation of gelato samples formulated with four surfactants (mono and diglycerides of saturated fatty acids, soy, milk and rice phospholipids) after 4 weeks of storage, and these changes were described as related to initial oxidation phenomena of fat. The opposite occurred in the yogurt samples, YFβ presented greater tonality than YβN. In addition, the storage period had different effects on the chroma of the formulations. GβN, YβN and YFβ had a significant increase (p < 0.05) while GFβ had a significant decrease (p < 0.05). The same was described by Lima et al. (2016) in ice cream with β-carotene, which according to the authors may indicate that carotenoid oxidation may have occurred. The higher the chroma value, the greater the intensity of the color perceived by the human eye, so GβN presented a more intense color than GFβ (Balthazar et al., 2018). Thus, the addition of β-carotene encapsulated in nanoemulsions allows the color of the product to become more intense without the need to increase the concentration of the dye.
The higher ΔE of the samples with unencapsulated carotenoid can be explained by the hydrophobicity of the bioactive, which makes it difficult to solubilize in the sample, which can be resolved with encapsulation. This process can reduce oxidative degradation associated with acidic pH, as found in yogurt (Feng et al., 2018). The improved performance of nanoencapsulated β-carotene compared to its non-encapsulated form was also described by Assis et al. (2018). The authors applied carotenoid as an antioxidant in biodegradable films, noting that in addition to better dispersion homogeneity, the use of nanoencapsulated β-carotene increased the color intensity of the films and the elongation at break, decreased light transmission and provided greater protection to the sunflower oil, with less formation of oxidation products.
According to Ba et al. (2020), the lower the ΔE, it is indicative that there was less difference in color, that is, less degradation of β-carotene. The low ΔE values were confirmed by the low reduction in the β-carotene content when encapsulated in the nanoemulsion (Fig. 3), evidenced by the C/C0 ratio (final concentration/initial concentration) for the products enriched with nanoemulsion during 28 days of storage. After this period, the products (gelato and yogurt) showed a C/C0 ratio around 0.8 ± 0.1, meaning that the final concentration of β-carotene was not statistically different (p > 0.05) from the initial. Due to the high heterogeneity of samples incorporated with unencapsulated β-carotene, the C/C0 ratios of these samples were not monitored.
The protection of β-carotene by nanoemulsions was also studied by López-Monterrubio et al. (2021). During 30 days of storage, nanoemulsions stabilized by soluble complexes of hydrolyzed whey protein and pectin showed no significant difference (p > 0.05) when compared to fresh nanoemulsions. Where it was established that, as in the present study, the nanoemulsions were able to protect the integrity of the encapsulated β-carotene.
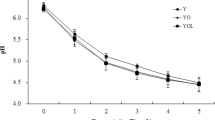
Figure 4 shows the monitoring of titratable acidity, pH and syneresis of formulations developed during yogurt storage. In general, no significant changes (p > 0.05) were observed in the titratable acidity of yogurts throughout the storage period, remaining in the range of 0.6–0.7%. The pH values ranged from 4.2 to 4.4, and at the beginning, the YFβ formulation presented a significantly higher pH value (p < 0.05) than the other formulations. After 28 days of storage, the YC and YFβ samples showed a low pH and significantly (p < 0.05) higher (4.39 ± 0.01) than the YβN sample (4.33 ± 0.02).
One of the defects found in yogurts is the separation of whey, called syneresis, which results from the rearrangement of casein particles (Baba et al., 2018). In the syneresis analysis, no significant differences (p > 0.05) were observed between the YC and YFβ samples at 0 and 28 days. However, the sample added with nanoemulsion showed syneresis approximately 11.7% and 10.9% higher (p < 0.05) than the other samples at times 0 and 28 days, respectively.
The percentage of syneresis was evaluated by Campo et al. (2019) in yogurts added with zeaxanthin nanoparticles, and also found an increase in samples added with nanoemulsion. The behavior was attributed to the greater amount of water in these samples, which is in agreement with this study. The formulations containing the nanoemulsions contained the same amount as the other samples, plus 250 mL of nanoemulsion.
Yogurts with and without nanoemulsions showed a good fit to the Waele Ostwald model (R2 0.98 and 0.87, respectively) (Fig. 4(d)). The addition of nanoemulsion significantly decreased (p < 0.05) the consistency coefficient (K), from 6.93 ± 1.14 Pa s−1 (control) to 3.68 ± 0.009 Pa s−1 (with nanoemulsion). However, the values are still higher than those reported for this food category (1.312 Pa.s−1), indicating that they have a good consistency (Aguayo-Mendoza et al., 2019). The K value demonstrated the shear strength of the material (Oliveira et al., 2018), which means that the strength of the sample without nanoemulsions is higher than samples with nanoemulsion.
Nanoemulsion yogurts showed 43% reductions in force required during the “first bite”, indicative of firmness (Zhang et al., 2022a, b). This observation may be due to the reduction in the protein content and the increase in lipids observed in the nanoemulsion sample (Table 2), since the protein network recovers the fat globules, which improves the protein–protein interaction and consequently increases the firmness (Marques et al., 2021). In addition, this sample showed a 55% reduction in consistency, 39% reduction in the second compression compared to the first compression (cohesiveness) (Zhang et al., 2022a, b), and a 66% lower viscosity index.
The increase in whey expulsion and its consequent influence on the rheology and texture of the product is probably related to the water added to the product via nanoemulsion. This water cannot be retained by the gel matrix, resulting in increased syneresis and reduced solids content (Vareltzis et al., 2016). This did not happen in the gelato, because in the formulation the amount of water was reduced, (760 mL of water + 240 mL of nanoemulsion) (Table 1). Another cause of increased syneresis may be the damage to the gel structure induced during mixing of the nanoemulsion, reducing its retention capacity (Zhong et al., 2018). Such interferences in syneresis, rheology and texture parameters can be overcome by raising total solids, for example (Krstonosic et al., 2021).
The addition of nanoemulsions significantly increased (p < 0.05) the flow behavior index (n), designating the deviation from the Newtonian flow (Oliveira et al., 2018), from 0.07 ± 0.01 (control) to 0.20 ± 0.007 (with nanoemulsion). This parameter, with values lower than 1, showed that both yogurt formulations studied showed behavior of pseudoplastic fluids (Kadiya & Ghosh, 2022).
The monitoring of soluble solids and pH of gelato formulations (Fig. 5) during the 28 days of storage, showed constant pH, around 6.5, close to the value considered normal (6.3) for ice cream (Goktas et al., 2022). The total soluble solids content, formed mainly by sugars, stabilizers and emulsifiers (Lima et al., 2016), remained around 25 °Brix for all formulations, in the follow-up time.
Gremski et al. (2019) also found a little variable pH in herbal ice creams (Ilex paraguariensis, Melissa officinalis and Cymbopogon citratus), in 72 days of storage. Yeboah et al. (2022) carried out the physical–chemical monitoring of ice cream made with brown sugar, and also identified the stability of the pH of the products around 6.5, noting that its properties did not change when replacing components of the formulation.
During the analysis of the melting behavior (Fig. 5d), the first drop of the two formulations was observed between 35 and 40 min; 50% melting was observed at 88.3 ± 2.8 and 83.3 ± 7.6 min for GC and GβN, respectively, with no significant difference (p > 0.05) between samples. The melting rate of the samples was around 1 g mL − 1, similar to that described by the other authors (Amador et al., 2017; Balthazar et al., 2018; Warren & Hartel, 2018).
When exposed to hot air, heat transferred from the air around the gelato to the product initiates the melting process (Warren & Hartel, 2018). This process can destabilize the product and affect its structure, a process that varies according to the composition of the product. In a study with ice cream containing Fortunella margarita, the first drops occurred between 14 and 18 min, a value that increased with increasing fruit addition. This shorter time compared to that found in the present study may be related to the lower fat content (Lima et al., 2016). The extensive fat network formed between ice crystals and air bubbles by the addition of nanoemulsion stabilized the gelato structure (Amador et al., 2017).
Another technological parameter is the overrun, corresponding to the industrial calculation of the air added to frozen desserts. This quantification and control of air incorporation in the ice cream is essential for the quality, stability of the product and the most crucial parameter to determine the yield of the process (Bekiroglu et al., 2022). The addition of nanoemulsions to gelato influenced this parameter (Fig. 6), with a significant increase (p < 0.05) of 56% compared to the control samples. GFβ samples also showed a significant increase in overrun (p < 0.05). However, this increment, around 24%, was smaller than for the addition of nanoemulsion. Similar behavior occurred in ice creams containing curcumin nanoemulsions and unencapsulated curcumin, with an overrun increase of 8% (Borrin et al., 2018).
Gelato generally shows an overrun value ≤ 30%, and traditional ice cream values are between 50 and 100% (Liu et al., 2022). The overrun values found in samples with nanoemulsions were close to those of ice cream. These values were likely a consequence of the 15% higher lipid content in samples with nanoemulsions (Table 2), which increases the available coalesced fat droplets to retain air bubbles in the ice cream (Wu et al., 2019). On the other hand, the lower overrun values for GFβ samples can be attributed to the increase in the viscosity of the mixtures (Table 2), according to Rizk et al. (2014). They observed a reduction from 46.32 to 34.62% for ice cream made with different levels (0–5%) of lycopene.
Overrun can affect ice cream firmness (Wu et al., 2019), but comparing the two formulations, no significant change (p > 0.05) was observed (Table 2), even with the highest overrun observed in samples with nanoemulsion, probably by other parameters, such as the size of the ice crystals, which can affect probe penetration and viscosity (Amador et al., 2017). Velásquez-Cock et al. (2019) also found no significant difference (p > 0.05) in the firmness of ice cream containing 0.15% and 0.3% (p) of cellulose nanofibrils. Similar values (around 5 N) were observed in samples of probiotic ice cream with 2% apple or oat fiber (Akalin et al., 2018) and in pulsed electric field-treated ice cream with and without zinc supplementation (about 6 N) (Pankiewicz et al., 2019).
Gelato consistency was 45% lower (Table 2) in samples with nanoemulsion. Physically, this means that less work or energy is required to compress these samples compared to control samples (Zhang et al., 2022a, b). Cohesiveness (Table 2) was 27% higher in the GβN samples, so more deformation is required before they rupture (Aguayo-Mendoza et al., 2019). More force was required when collecting samples with nanoemulsions than control samples, as their viscosity index was 47% higher. This was probably caused by the decrease in the oil droplet diameter (as observed in nanoemulsions) leading to a larger contact surface, which generated an important frictional force opposed to the free flow of the emulsion in a shear field, thus increasing its viscosity (Zhang et al., 2022a, b).
These changes in texture parameters may also be related to changes in the crystallization temperature of the samples. Truong et al. (2014) evaluated the effects of emulsion droplet size on milk fat crystallization and observed a tendency to reduce crystallization temperature with droplet size reduction. The authors proposed that this change in crystallization temperature between conventional emulsions and nanoemulsions could be used to modify the physical and functional properties of food products containing fat or fat-based encapsulation and delivery systems made from different types of emulsion.
In short, it was possible to develop two products added with β-carotene nanoemulsion, which showed an increase in solubility and homogeneity when compared to non-encapsulated β-carotene, without changing the properties and characteristics of the products. Resulting in products enriched with a bioactive component, providing, in addition to basic nutrition, a benefit to the health of those who consume them.
Conclusion
This study investigated the effect of adding β-carotene nanoemulsion to two formulations, yogurt and gelato. The application of nanoemulsions as β-carotene carriers increased the syneresis index and flow behavior and reduced the parameters of firmness, consistency, cohesiveness and viscosity index in the yogurt formulation. The gelato applied from nanoemulsions had a significant increase in product yield, as evidenced by the increase in overrun, without affecting stability parameters such as pH, soluble solids, firmness and melting. In addition, the nanoemulsion applied to both products developed increased the carotenoid solubility, making the products more homogeneous than the samples added with non-encapsulated β-carotene. The nanoemulsions protected the carotenoid, so the β-carotene content was largely maintained during storage leading to only slight color variation. The results show that the use of nanoemulsions as carriers of lipophilic compounds, such as carotenoids, can improve the dispersion and stability of these compounds in the developed products.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
References
Aguayo-Mendoza, M. G., Ketel, E. C., Linden, E. V., Forde, C. G., Piqueras-Fiszman, B., & Stieger, M. (2019). Oral processing behavior of drinkable, spoonable and chewable foods is primarily determined by rheological and mechanical food properties. Food Quality and Preference, 71, 87–95. https://doi.org/10.1016/j.foodqual.2018.06.006
Akalin, A. S., Kesenkas, H., Dinkci, N., Unal, G., Ozer, E., & Kinik, O. (2018). Enrichment of probiotic ice cream with different dietary fibers: Structural characteristics and culture viability. Journal of Dairy Science, 101, 37–46. https://doi.org/10.3168/jds.2017-13468
Alfaifi, M. S., & Stathopoulos, C. E. (2010). Effect of egg yolk substitution by sweet whey protein concentrate (WPC), on physical properties of gelato ice cream. International Food Research Journal, 17, 787–793.
Amador, J., Hartel, R., & Rankin, S. (2017). The effects of fat structures and ice cream mix viscosity on physical and sensory properties of ice cream. Journal of Food Science, 82, 1851–1860. https://doi.org/10.1111/1750-3841.13780
AOAC. (2000). Official methods of analysis. (17 Ed). CD-ROM.
Assis, R. Q., Hickmann, S., Pagno, C. H., Maria, T. & Costa, H. (2018). Synthesis of biodegradable films based on cassava starch containing free and nanoencapsulated β‐carotene. Packaging Technology and Science, 31, 8–10. https://doi.org/10.1002/pts.2364
Ba, C., Fu, Y., Niu, F., Wang, M., Jin, B., Li, Z., Chen, G., Zhang, H., & Li, X. (2020). Effects of environmental stresses on physiochemical stability of β-carotene in zein-carboxymethyl chitosan-tea polyphenols ternary delivery system. Food Chemistry, 311, 125878. https://doi.org/10.1016/j.foodchem.2019.125878
Baba, W. N., Jan, K., Punoo, H. A., Wani, T. A., Dar, M. M., & Masoodi, F. A. (2018). Techno-functional properties of yoghurts fortified with walnut and flaxseed oil emulsions in guar gum. LWT, 92, 242–249. https://doi.org/10.1016/j.lwt.2018.02.007
Balthazar, H. L., Silva, A., Esmerino, E. A., Rocha, R. S., Moraes, J., Carmo, M. A. V., Azevedo, L., Camps, I., Abud, Y. K. D., Sant’Anna, C, Franco, R. M., Freitas, M. Q., Silva, M. C., Raices, R. S. L., Escher, G. B., Granato, D., Ranadheera, C. S., Nazarro, F., & Cruz, A. G. (2018). The addition of inulin and Lactobacillus casei 01 in sheep milk ice cream. Food Chemistry, 246, 464–472. https://doi.org/10.1016/j.foodchem.2017.12.002
Bekiroglu, H., Goktas, H., Karaibrahim, D., Bozkurt, F., & Sagdic, O. (2022). Determination of rheological, melting and sensorial properties and volatile compounds of vegan ice cream produced with fresh and dried walnut milk. International Journal of Gastronomy and Food Science, 28, 100521. https://doi.org/10.1016/j.ijgfs.2022.100521
Borba, C. M., Tavares, M. N., Macedo, L. P., Araújo, G. S., Furlong, E. B., Dora, C. L., & Burkert, J. F. M. (2019). Physical and chemical stability of β-carotene nanoemulsions during storage and thermal process. Food Research International, 121, 229–237. https://doi.org/10.1016/j.foodres.2019.03.045
Borrin, T. R., Georges, E. L., Brito-Oliveira, T. C., Moraes, I. C. F., & Pinho, S. C. (2018). Technological and sensory evaluation of pineapple ice creams incorporating curcumin-loaded nanoemulsions obtained by the emulsion inversion point method. International Journal of Dairy Technology, 71, 491–500. https://doi.org/10.1111/1471-0307.12451
Çakmakçı, S., Topdas, E. F., Çakir, Y., & Kalın, P. (2016). Functionality of kumquat (Fortunella margarita) in the production of fruity ice cream. Journal of the Science of Food and Agriculture, 96, 1451–1458. https://doi.org/10.1002/jsfa.7241
Campo, C., Assis, R. Q., Silva, M. M., Costa, T. M. H., Paese, K., Guterres, S. S., Rios, A. O., & Flores, S. H. (2019). Incorporation of zeaxanthin nanoparticles in yogurt: Influence on physicochemical properties, carotenoid stability and sensory analysis. Food Chemistry, 301, 125230. https://doi.org/10.1016/j.foodchem.2019.125230
Cardoso, L. A., Karp, G., Vendruscolo, F., Kanno, Y. F., & Carvalho, J. C. (2017). Biotechnological production of carotenoids and their applications in food and pharmaceutical products. (Cvetkovic, DG & Nikolic, GS, Eds.). https://doi.org/10.5772/65523
Chaari, M., Theochari, I., Papadimitriou, V., Xenakis, A., & Ammar, E. (2018). Encapsulation of carotenoids extracted from halophilic Archaea in oil-in-water (O/W) micro- and nano-emulsions. Colloids and Surfaces B: Biointerfaces, 161, 219–227. https://doi.org/10.1016/j.colsurfb.2017.10.042
Chen, Y., Zhang, R., Xie, B., Sun, Z., & McClements, C. J. (2020). Lotus seedpod proanthocyanidin-whey protein complexes: Impact on physical and chemical stability of β-carotene-nanoemulsions. Food Research International, 127, 108738. https://doi.org/10.1016/j.foodres.2019.108738
Choi, A. J., Kim, C. J., Cho, Y. J., Hwang, J. -K., Lim, C. T. (2011). Characterization of capsaicin-loaded nanoemulsions stabilized with alginate and chitosan by self-assembly. Food and Bioprocess Technology, 4, 1119–1126. https://doi.org/10.1007/s11947-011-0568-9
Feng, Z. Z., Li, M. Y., Wang, Y. T., & Zhu, M. J. (2018). Astaxanthin from Phaffia rhodozyma: Microencapsulation with carboxymethyl cellulose sodium and microcrystalline cellulose and effects of microencapsulated astaxanthin on yogurt properties. LWT - Food Science and Technology, 96, 152–160. https://doi.org/10.1016/j.lwt.2018.04.084
Ghayour, N., Hosseini, S. M. H., Eskandari, M. H., Esteghlal, S., Nekoei, A. R., Gahruie, H. H., Tatar, M., & Naghibalhossaini, F. (2018). Nanoencapsulation of quercetin and curcumin in casein-based delivery systems. Food Hydrocolloids, 87, 394–403. https://doi.org/10.1016/j.foodhyd.2018.08.031
Gilbert, A., Rioux, L. E., St-Gelais, D., & Turgeon, S. L. (2021). Smoothing temperature and ratio of casein to whey protein: Two tools to improve nonfat stirred yogurt properties. Journal of Dairy Science, 104, 10485–10499. https://doi.org/10.3168/jds.2020-20040
Goktas, H., Dikmen, H., Bekiroglu, H., Cebi, N., Dertli, E., & Sagdic, O. (2022). Characteristics of functional ice cream produced with probiotic Saccharomyces boulardii in combination with Lactobacillus rhamnosus GG. LWT, 153, 112489. https://doi.org/10.1016/j.lwt.2021.112489
Gremski, L. A., Coelho, A. L. K., Santos, J. S., Daguer, H., Malognoni, L., Prado-Silva, L., Sant’Ana, A. S., Rocha, R. S., Silva, M. C., Cruz, A. G., Azevedo, L., Carmo, M. A. V., When, M., Zhang, L., & Granato, D. (2019). Antioxidants-rich ice cream containing herbal extracts and fructooligossaccharides: Manufacture, functional and sensory properties. Food Chemistry, 298, 125098. https://doi.org/10.1016/j.foodchem.2019.125098
Jalili-Jivan, M., Rostamabadi, H., Assadpour, E., Tomas, M., Capanoglu, E., Alizadeh-Sani, M., Kharazmi, M. S. & Jafari, S. M. (2022). Recent progresses in the delivery of β-carotene: From nano/microencapsulation to bioaccessibility. Advances in Colloid and Interface Science, 307, 102750. https://doi.org/10.1016/j.cis.2022.102750
Kadiya, K., & Ghosh, S. (2022). Pectin degree of esterification influences rheology and digestibility of whey protein isolate-pectin stabilized bilayer oil-in-water nanoemulsions. Food Hydrocolloids, 131, 107789. https://doi.org/10.1016/j.foodhyd.2022.107789
Kaminska-Dworznicka, A., Laba, S., & Jakubczyk, E. (2022). The effects of selected stabilizers addition on physical properties and changes in crystal structure of whey ice cream. LWT, 154, 112841. https://doi.org/10.1016/j.lwt.2021.112841
Krstonosic, V., Jovicic-Bata, J., Maravic, N., Nikolic, I., & Dokic, L. (2021). Chapter 2 - Rheology, structure, and sensory perception of hydrocolloids. Food Structure and Functionality, 23–47. https://doi.org/10.1016/B978-0-12-821453-4.00005-3
Lima, J. G., Brito-Oliveira, T. C., & Pinho, S. C. (2016). Characterization and evaluation of sensory acceptability of ice creams incorporated with beta-carotene encapsulated in solid lipid microparticles. Food Science and Technology, 36, 664–671. https://doi.org/10.1590/1678-457x.13416
Liu, X., Sala, G., & Scholten, E. (2022). Effect of fat aggregate size and percentage on the melting properties of ice cream. Food Research International, 160, 111709. https://doi.org/10.1016/j.foodres.2022.111709
López-Monterrubio, D. I., Lobato-Calleros, C., Vernon-Carter, E. J., & Alvarez-Ramirez, J. (2021). Influence of β-carotene concentration on the physicochemical properties, degradation and antioxidant activity of nanoemulsions stabilized by whey protein hydrolyzate-pectin soluble complexes. LWT, 143, 111148. https://doi.org/10.1016/j.lwt.2021.111148
Marques, L. T. O., Vasconcelos, F. R., Alves, J. P. M., Montenegro, A. R., Fernandes, C. C. L., Oliveira, F. B. B., Silva, C. P., Nagao, C. S., Figueiredo, S. C., Beserra, F. J., Moura, A. A., & Rondina, D. (2021). Proteome of milk fat globule membrane and mammary gland tissue in goat fed different lipid supplementation. Small Ruminant Research, 199, 106378. https://doi.org/10.1016/j.smallrumres.2021.106378
Mattos, M. V. C. V., Michelon, M., & Burkert, J. F. M. (2022). Production and stability of food-grade liposomes containing microbial carotenoids from Rhodotorula mucilaginosa. Food Structure, 33, 100282. https://doi.org/10.1016/j.foostr.2022.100282
Mezquita, P. C., Barragán-Huerta, B. E., Ramirez, J. P., & Hinojosa, C. O. (2014). Stability of astaxanthin in yogurt used to simulate apricot color, under refrigeration. Food Science Technology, 34. https://doi.org/10.1590/1678-457x.6386
Oliveira, J. M., Amaral, S. A., & Burkert, C. A. V. (2018). Rheological, textural and emulsifying properties of an exopolysaccharide produced by Mesorhizobium loti grown on a crude glycerol-based medium. International Journal of Biological Macromolecules, 120, 2180–2187. https://doi.org/10.1016/j.ijbiomac.2018.06.158
Ozogul, Y., Karsli, G. T., Durmus, M., Yazgan, H., Oztop, H. M., McClements, D. J., & Ozogul, F. (2022). Recent developments in industrial applications of nanoemulsions. Advances in Colloid and Interface Science, 304, 102685. https://doi.org/10.1016/j.cis.2022.102685
Pankiewicz, U., Goral, M., Kozlowicz, K., & Goral, D. (2019). Novel method of zinc ions supplementing with fermented and unfermented ice cream with using PEF. International Journal of Food Science & Technology, 54, 2035–2044. https://doi.org/10.1111/ijfs.14103
Pascual-Pineda, L. A., Flores-Andrade, E., Alamilla-Beltrán, L., et al. (2014). Micropores and their relationship with carotenoids stability: A new tool to study preservation of solid foods. Food and Bioprocess Technology, 7, 1160–1170. https://doi.org/10.1007/s11947-013-1162-0
Pinilla, C. M. B., Reque, P. M., & Brandelli, A. (2020). Effect of oleic acid, cholesterol, and octadecylamine on membrane stability of freeze-dried liposomes encapsulating natural antimicrobials. Food and Bioprocess Technology, 13, 599–610. https://doi.org/10.1007/s11947-020-02419-8
Quitanilha, G. E. O., Baptista, A. T. A., Gomes, R. G., & Vieira, A. M. S. (2021). Yogurt production added ultrafiltered seed extract of Moringa oleifera Lam. Biocatalysis and Agricultural Biotechnology, 37, 102159. https://doi.org/10.1016/j.bcab.2021.102159
Rezaei, A., Fathi, M., & Jafari, S. M. (2019). Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocolloids, 88, 146–162. https://doi.org/10.1016/j.foodhyd.2018.10.003
Rinaldi, M., Dall’Astra, C., Paciulli, M., Guizzetti, S., Barbanti, D., & Chiavaro, E. (2014). Innovation in the Italian ice cream production: effect of different phospholipid emulsifiers. Dairy Science & Technology, 94, 33–49. https://doi.org/10.1007/s13594-013-0146-1
Rizk, E. M., El-KAdy, A. T., & El-Bialy, A. R. (2014). Characterization of carotenoids (lyco-red) extracted from tomato peels and its uses as natural colorants and antioxidants of ice cream. Annals of Agricultural Sciences, 59, 53–61. https://doi.org/10.1016/j.aoas.2014.06.008
Santagiuliana, M., Bhaskaran, V., Scholten, E., Piqueras-Fiszman, B., & Stieger, M. (2019). Don’t judge new foods by their appearance! How visual and oral sensory cues affect sensory perception and liking of novel, heterogeneous foods. Food Quality and Preference, 77, 64–77. https://doi.org/10.1016/j.foodqual.2019.05.005
Silva, H. D., Cerqueira, M. Â., & Vicente, A. A. (2012). Nanoemulsions for food applications: Development and characterization. Food and Bioprocess Technology, 5, 854–867. https://doi.org/10.1007/s11947-011-0683-7
Trindade, R. A., Munhoz, A. P., & Burkert, C. A. V. (2018). Impact of a carbon source and stress conditions on some properties of xanthan gum produced by Xanthomonas campestris pv. mangiferaeindicae. Biocatalysis and Agricultural Biotechnology, 15, 167–172. https://doi.org/10.1016/j.bcab.2018.06.003
Truong, T., Bansal, N., Sharma, M., Palmer, M., & Bhandari, B. (2014). Effects of emulsion droplet sizes on the crystallisation of milk fat. Food Chemistry, 145, 725–735. https://doi.org/10.1016/j.foodchem.2013.08.072
Tupuna, D. S., Paese, K., Guterres, S. S., Jablonski, A., Flôres, S. H., & Rios, A. O. (2018). Encapsulation efficiency and thermal stability of norbixin microencapsulated by spray-drying using different combinations of wall materials. Industrial Crops and Products, 111, 846–855. https://doi.org/10.1016/j.indcrop.2017.12.001
Vareltzis, P., Adamopoulos, K., Stavrakakis, E., Stefanakis, A., & Goula, A. M. (2016). Approaches to minimize yoghurt syneresis in simulated tzatziki sauce preparation. International Journal of Dairy Technology, 69, 191–199. https://doi.org/10.1111/1471-0307.12238
Velásquez-Cock, J., Serpa, A., Vélez, L., Ganan, P., Hoyos, C. G., Castro, C., Duizer, L., Goff, H. D., & Zuluaga, R. (2019). Influence of cellulose nanofibrils on the structural elements of ice cream. Food Hydrocolloids, 87, 204–213. https://doi.org/10.1016/j.foodhyd.2018.07.035
Warren, M. M., & Hartel, R. W. (2018). Effects of emulsifier, overrun and dasher speed on ice cream microstructure and melting properties. Journal of Food Science, 83, 639–647. https://doi.org/10.1111/1750-3841.13983
Wu, B., Freire, D. O., & Hartel, R. W. (2019). The effect of overrun, fat destabilization, and ice cream mix viscosity on entire meltdown behavior. Journal of Food Science, 84, 2562–2571. https://doi.org/10.1111/1750-3841.14743
Xie, H., Zhang, Y., Cao, M., Liu, C., Mao, Y., Ren, G., Wu, Z., Fang, S., Tian, S., & Wu, D. (2021). Fabrication of PGFE/CN-stabilized β-carotene-loaded peppermint oil nanoemulsions: Storage stability, rheological behavior and intelligent sensory analyses. LWT, 138, 110688. https://doi.org/10.1016/j.lwt.2020.110688
Yeboah, J., Santoro, A. M., Arrieta-Escobar, J. A., Caballero, I. M., Orjuela, A., Novoa, C. F., Fuenmayor, C. A., & Hamdani, F. E. (2022). Heuristic-based computer-aided design of ice creams and validation by using jaggery as refined sugar substitute. Chemical Engineering Research and Design, 184, 256–266. https://doi.org/10.1016/j.cherd.2022.06.018
Yi, J., Gao, L., Zhong, G., & Fan, Y. (2020). Fabrication of high internal phase Pickering emulsions with calcium-crosslinked whey protein nanoparticles for β-carotene stabilization and delivery. Food & Function, 11, 768–778. https://doi.org/10.1039/C9FO02434D
Yi, J., Huang, H., Wen, Z., & Fan, Y. (2021). Fabrication of chitosan-gallic acid conjugate for improvement of physicochemical stability of β-carotene nanoemulsion: Impact of Mw of chitosan. Food Chemistry, 130218. https://doi.org/10.1016/j.foodchem.2021.130218
Yuan, B., Danao, M. G. C., Lu, M., Weier, S. A., Stratton, J. E., & Weller, C. L. (2018). High pressure processing (HPP) of aronia berry puree: Pilot scale processing and a shelf-life study. Innovative Food Science and Emerging Technologies, 47, 241–248. https://doi.org/10.1016/j.ifset.2018.03.006
Zardini, A. A., Mohebbi, M., Farhoosh, R., & Bolurian, S. (2018). Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. Journal of Food Science and Technology, 55, 287–298. https://doi.org/10.1007/s13197-017-2937-5
Zhang, L., Liao, W., Wang, Y., Tong, Z., Li, Q., & Gao, Y. (2022a). Thermal-induced impact on physicochemical property and bioaccessibility of β-carotene in aqueous suspensions fabricated by wet-milling approach. Food Control, 141, 109155. https://doi.org/10.1016/j.foodcont.2022.109155
Zhang, Y., Pandiselvam, R., Zhu, H., Su, D., Wang, H., Ai, Z., Kothakota, A., Khaneghah, A. M., & Liu, Y. (2022b). Impact of radio frequency treatment on textural properties of food products: An updated review. Trends in Food Science & Technology, 124, 154–166. https://doi.org/10.1016/j.tifs.2022.04.014
Zhong, J., Yang, R., Cao, X., Liu, X., & Qin, X. (2018). Improved physicochemical properties of yogurt fortified with fish oil / γ-oryzanol by nanoemulsion technology. Molecules, 23, 1–11. https://doi.org/10.3390/molecules23010056
Funding
The authors are grateful to FAPERGS (Foundation Research Support in the State of Rio Grande do Sul), CNPq (National Council of Science and Technological Development) and support of the Coordination of Improvement of Higher Level Personnel—Brazil (CAPES) —Financing Code 001.
Author information
Authors and Affiliations
Contributions
Carina Molins Borba: Conceptualization, research, writing, original draft writing, methodology, laboratory practice. Gabriela de Moraes Soares Araújo: Resources, laboratory practice, conceptualization and review. Camila Ramão Contessa: Conceptualization, writing, original draft preparation, proofreading and editing. Cristiana Lima Dora: Project Management, supervision, formal review and review. Janaína Fernandes de Medeiros Burkert: Project management, supervision, writing and edition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors confirm that this is an original research article, and no conflict of interests associated with this publication. All authors have read, approved the MS and are aware of its submission to JFST.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borba, C.M., de Moraes Soares Araújo, G., Contessa, C.R. et al. Influence of β-Carotene Nanoemulsions on Technological Parameters and Stability in Food Matrices. Food Bioprocess Technol 16, 2430–2442 (2023). https://doi.org/10.1007/s11947-023-03060-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03060-x