Abstract
The aim of the study is to discriminate mono-floral honey by the chemical characteristics of non-sugar components (NSC) present in honey based on their botanical origin. The NSC in 50 honey samples including jujube, chaste and locust were determined and compared using HPLC and 1H-NMR combined with chemometrics analysis. The results showed that the content of NSC in jujube honey was the highest, with the average value of 780.77 mg/100 g, followed by locust honey, the lowest was chaste honey, with the average value of 292.43 mg/100 g. HPLC and 1H-NMR fingerprints of mono-floral honey samples were established, in which common chromatographic and spectral peak information were obtained. Principal component analysis and discrimination analysis were performed using selected common peaks as dependent variables and floral origin as independent variables, 44 honey samples and six test samples were correctly classified according to their floral origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Honey is made by honeybees from the secretions of plants or nectar of blossoms [1]. Chemically, honey primarily consists of sugars and other constituents such as proteins, amino acids, vitamins, flavonoids, organic acids and minerals [2, 3]. Sugar accounts for about 60–80% of the total mass of honey, which is no obvious difference between different varieties of honey. However, the content and type of non-sugar components (NSC) vary greatly depending on the variety of floral origin of honey.
Honey is classified as mono-floral and multifloral origin. The taste and nutrition of mono-floral honey are better than multiflora honey [4]. Mono-floral honey is often not only used as a sugar substitute in food but also utilized as a primer and an important component in traditional Chinese medicine due to its desirable pharmacologically active ingredients, sweetness and flavor characteristics [5, 6]. The large market demand and high profit of mono-floral honey lead to serious adulteration of honey. The most common form of honey adulteration is the addition of multifloral honeys or cheap syrups such as corn syrup, cane sugar, or fructose syrup [7,8,9]. Although there is no sufficient studies to show that adulterated honey bring a significant threat to human health currently, it is a serious consumer fraud and has adverse effects on beekeepers’ interests and the entire honey market [10]. Therefore, it is utterly necessary and important to efficiently and rapidly identify mono-floral honey.
Various methods have been introduced to establish quality control parameters and standardization of mono-floral honey, such as near-infrared spectroscopy [11, 12], isotope ratio [13, 14], gas chromatography (GC) [15, 16], mass spectroscopy [17]. The advantages of these methods have also been discussed [18]. However, infrared spectrum is less sensitive in terms of quantification, and GC is not suitable for the analysis of high boiling substances. Because of wide range of separation, HPLC is increasingly becoming the most commonly used analytical method [19, 20]. For example, Sun used HPLC as a tool to discover that abscisic acid could be an ingredient for adulteration identification and quality control of locust honey [21]. Presently, nuclear magnetic resonance spectroscopy (NMR) is gradually to be used to assess the quality of food due to its nondestructive and high resolution [22, 23]. The amount of information provided by NMR at the atomic level is unmatched by other analytical methods [24].
Honey produced from jujube, chaste, and locust occupied a huge market share in China due to their multi-varieties, desirable bio-active constituents and pleasant flavor [25]. However, there are few reports regarding the appropriate mono-floral honey identification of them, which seriously affects the quality control of these honeys and their export market. In this work, we used HPLC and 1H-NMR combined with chemometrics analysis to discriminate mono-floral honey by the chemical characteristics of NSC present in these honeys based on their botanical origin. HPLC was used to analysis the UV absorption of NSC in honey. 1H-NMR was used to analyze the characteristic hydrogen signals of NSC. Chemometrics methods were usually used to reduce the complexity and provide better interpretation and understanding of large data sets [26, 27]. Combining the large amounts of data obtained from HPLC and 1H-NMR fingerprints with chemometrics could be an accurate and efficient solution for differentiating jujube, chaste and locust mono-floral honey, so as to confirm the quality and authenticity of honey.
Materials and methods
Sample information
Fifteen jujube honey (Z1–Z15), sixteen chaste honey (J1–J16) and nineteen locust honey (Y1–Y19) samples (a total of 50 honey samples) were provided by Tong Ren Tang Chinese Medicine. Honey samples numbered Z1–Z10, J1–J10, Y1–Y14 were used to establish the identification method, and the remaining honey (Z11, Z12, J11, J12, Y15, Y16) was used to verify the accuracy of this method. All samples were stored at 4 °C for later analysis.
Sample preparation
NSC were obtained by using macroporous resin XAD-2. Honey samples (100 g) were dissolved in 1000 mL hydrochloric acid solution (pH 2), stirred at room temperature for 3 min and filtered with degreasing cotton so as to remove solid particles in the solution. The filtrate was transferred to a glass column (2.6 cm × 50 cm) containing a well-balanced XAD-2 resin (150 g) at a rate of 0.5 mL/min by the constant current pump. Sugar and other polar substances were removed by washing the column with distilled water (3BV). Then methanol (3BV) was used to elute and concentrate the NSC under the reduced pressure (35 °C). The concentrate was reconstituted with the appropriate amount of pure water and methanol. The solutions were passed through the organic filter (0.45 µm) and stored at 4 °C for the further HPLC and 1H-NMR analysis.
HPLC analysis
The HPLC analysis conditions were as follows: Agilent 1260 with a DAD detector. Agilent Zorbax SB-C18 column (250 mm × 4.6 mm, 5 µm). The column was operated at 30 °C and the flow rate was maintained at 1.0 mL/min. The mobile phase was: (A) Ultra-pure water (1% acetic acid, v/v), (B) Methanol. Elution conditions: the time program of gradient elution was: 0–15–35–36–37–45–47–50–52–60–64 min, corresponding to the volume fraction of methanol (B): 5%–10%–43%–42%–50%–51%–51%–56%–57%–85%–88%.
1H-NMR analysis
With 600 MHz nuclear magnetic resonance spectrometer, the experiment was carried out at 30 °C. The field was locked through D2O. In order to restrain the resonance signal of the water in the sample, adopt the way of pre-saturation to suppress the water peak. All samples were analyzed through noesyprld pulse sequence. The specific parameters of MestReNova were set as follows: PULPROG = noesypr1d, AQ = 3.27 s, TD = 65536, NA = 64, DS = 4, TD0 = 1, SWH = 10,000 Hz, D1 = 3 s, D8 = 0.1 s, D11 = 0.03 s, D12 = 0.00002s, DE = 6.5 µs, DW = 50 µs. All samples were randomly injected to avoid instrumental errors during the analysis.
Method validation
The International Conference on Harmonization (ICH) Guidelines was followed to explore the precision, repeatability, and stability of this method [28]. A sample was randomly selected for HPLC and 1H-NMR analysis. HPLC sample was measured twice a day to observe the change within 3 months. The sample was measured for 1H-NMR every 2 h within 14 days, and its variation was observed.
Data analysis
The similarity Evaluation System for HPLC Chromatographic of TCM (2004A) was used to establish honey fingerprinting. 1H-NMR spectrum of honey was analyzed by MestReNova 6.1.1. Due to the complex composition of honey, chemometrics might be useful to achieve a multiplier effect. The chemometrics analysis was performed using SPSS 17.0 including principal component analysis (PCA) and discrimination analysis (DA). PCA was conducted to examine the correlation between multiple variables and derive a handful of principal components from the original variable so that they retain as much information as possible of the original variable, which was not related to each other. DA was based on certain criteria to establish one or more discriminant functions, which was used to evaluate the potential index of identifying monofloral honeys.
Result and discussion
The contents of NSC in different mono-floral honeys
The NSC content in jujube, chaste and locust honey samples were shown in Table 1. Jujube honey had the highest NSC, which ranged from 738.91 to 881.34 mg/100 g, with the average of 780.77 ± 8.98 mg/100 g, while chaste honey was the lowest, from 282.60 to 333.41 mg/100 g, with the average of 292.43 mg/100 g. The NSC content of locust honey was lying between these two honeys. It is found that the contents of NSC, especially phenolics and flavonoids in dark honey are higher than that of light-colored honey, and the antioxidant capacity is also stronger [29]. The appearance of jujube honey was darker and more aromatic, while the color and taste of chaste honey was comparatively lighter, which was consistent with the content of NSC.
Stability test of analysis method
As can be seen from Fig. 1a, the sample was homogeneous and the spectrum was highly stable. The similarity of the HPLC profile at 280 nm was 0.998, which proved that the stability of sample was good. In 1H-NMR stability verification, the abscissa represents the chemical shift of hydrogen signal, and the ordinate represents the signal intensity. The stability of 1H-NMR was shown in Fig. 1b. Within 14 days, the relative standard deviations of the common peak areas and chemical shift are 1.04% and 0.49%, respectively. The chemical shift within δ0.5–8.0 was relatively stable. However, in 14th day, there was a slight fluctuation at δ0.00 (marked with a red box), it may due to the fatty acid oxidized.
HPLC fingerprint analysis
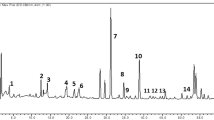
The HPLC chromatography was processed by the Similarity Evaluation System chromatographic fingerprint of TCM (2004A) to obtain the fingerprints of 34 honeys, which was shown in Fig. 2. Ten jujube honey samples had a total of 23 common peaks at 280 nm (peak 1–peak 23), shown in Fig. 2a, which suggested that jujube honey samples clearly exhibit high similarities, and the similarities of these jujube honeys ranged from 0.7921 to 0.9811. Ten chaste honey samples were selected from five different areas of Beijing, Shandong and Xinjiang province and their chromatographic fingerprints (Fig. 2b) also showed 24 common peaks at 280 nm, and the similarities of them were ranged from 0.8434 to 0.9336, except for J5 (< 0.684). Fourteen locust honey samples were selected from Beijing, Hebei and Gansu province, and 33 common peaks were detected in chromatographic fingerprints, shown in Fig. 2c. The similarities of 14 locust honey samples were ranged from 0.8182 to 0.9154. Upon comparing the HPLC fingerprints of the three types of mono-floral honey samples, there are distinct differences in the distribution of the chromatographic peaks, which could be used in the research of floral origin identification of honey.
Based on the established HPLC fingerprints of jujube, locust and chaste honeys, there were 17 common peaks in them. PCA was applied for the common peaks to decrease the dimension to facilitate subsequent submissions and ensure the 17 common peaks were all significant for the determination. The results were shown in Fig. 3a. The first two principal components had the cumulative contribution rate up to 89.1%. The variance contribution rates of principal components 1 (PC1) and 2 (PC2) were 79.2% and 9.9%, respectively. The first principal component (PC1) was mainly composed of v2, v10, v14 and v15. PC2 were formed by v6, v8, v12 and v13. It can be seen from the loading map that the peak v13, v14, v15 have the greatest impact on identification result, so the potential floral markers were determined.
Using PCA, it was determined that the common peaks were significant for the classification of mono-floral honey samples. These common peaks were taken as dependent variables and the floral origins as independent variables for DA. The results indicated that two significant discriminate functions were formed (Wilks lambda = 0.000, X2 = 239.366, df = 18, p < 0.05 for the first function, and Wilks lambda = 0.051, X2 = 68.248, df = 8, p < 0.05 for the second function). The first function accounted for 88.9% of the total variance and the second function accounted for 11.1% of the total variance. Both functions accounted for 100% of the total variance, which demonstrating a favourable result. As can be seen from Fig. 3b, the three types of mono-floral honeys were obviously classified, and the overall correct classification rate for floral origin was 100%, and the rate was also 100% for the cross validation method.
1H-NMR fingerprint analysis
According to the formant of 1H-NMR, the honey samples were analyzed by MestReNova 6.1.1, and the characteristic hydrogen signal of three types of honey was determined. In order to show the information more clearly, only the resonance area in common peaks was intercepted. The abscissa represents the chemical shift of the format, and the ordinate represents the signal intensity of the 1H-NMR peak. The chemical shift of the hydrogen resonance signals of three types of honey samples were mainly distributed from δ 0 to 8 ppm. The NSC content of jujube honey was higher, its 1H-NMR spectrum was correspondingly more complicated. Three types of mono-floral honeys have obvious common formants at δ0.93 ppm, 1.83 ppm, 1.99 ppm, 2.10 ppm, 3.37 ppm, 3.82 ppm, 5.82 ppm, 6.23 ppm, 6.36 ppm, 7.72 ppm, 7.75 ppm, shown in Fig. 4. The chemical shift at δ 7.75 ppm, 7.72 ppm, 5.82 ppm, 1.99 ppm and 0.97 ppm belongs to the hydrogen signal of abscisic acid. The chemical shift of the hydrogen signal of the methyl syringate was located on δ7.22 ppm, 3.82 ppm and 3.37 ppm. This is consistent with the data reported in the literature [30]. The corresponding hydrogen signals of abscisic acid and methyl syringate may be regarded as common peaks.
The common 1H-NMR peaks of the 36 honey samples were analyzed by PCA, and the result was shown in Fig. 5a. The first two principal components were chosen to examine the dataset and they had a cumulative contribution rate of 85.6% in the total variance. The first principle component (PC1) represented 71.2% of the variance and the second principle component (PC2) represented 14.4%. PC1 was primarily associated with peaks v1, v3 and v4, the dominant variables in PC2 were peaks v2, v5, v6. The common peaks v4, v5 and v6 had the greatest effect on the differentiation of the three types of honeys.
DA was performed on the common peaks in 1H-NMR analysis. The results indicated that two significant discriminate functions were formed (Wilks lambda = 0.000, X2 = 205.035, df = 12, p < 0.05 for the first function, and Wilks lambda = 0.133, X2 = 49.404, df = 8, p < 0.05 for the second function). The first two principal components had a cumulative contribution rate of 79.8%. The first function accounted for 64.5% of the total variance and the second function accounted for 15.3%. It can be seen from Fig. 5b, in which 44 honey samples were properly classified, and the rate of correct classification for the floral origin was 100%.
Test samples analysis
Six honey samples (Z11–Z12, J11–J12, Y15–Y16) were randomly selected to be used as test samples. The corresponding fingerprints were established after HPLC analysis. The common peaks of NSC were taken as dependent variables and the floral origins as independent variables for DA. The results showed two significant discrimination functions (Wilks lambda = 0.002, X2 = 213.266, df = 14, p < 0.05 for the first function, and Wilks lambda = 0.091, X2 = 70.248, df = 6, p < 0.05 for the second function). The first function accounted for 78.9% of the total variance and the second function accounted for 21.1% of the total variance. Both functions accounted for 100% of the total variance, showing a favourable result. As shown in Fig. 6a, in which all samples were successfully identified, and the overall correct classification rate for floral origin was 100%.
Same to 1H-NMR method, it can be seen from the map of DA that honey samples were well separated into distinct groups. Two significant discriminate functions were formed (Wilks lambda = 0.000, X2 = 239.366, df = 18, p < 0.05 for the first function, and Wilks lambda = 0.051, X2 = 68.248, df = 8, p < 0.05 for the second function). The first function accounted for 88.9% of the total variance and the second function accounted for 11.1% of the total variance. The result was shown in Fig. 6b, in which six honey samples were 100% properly classified.
The verification results showed that three types of honey samples were successfully identified. Not only 44 honey samples but also six test samples achieved convincing classification results based on the common peaks extracted from chromatography-spectral spectroscopy combined with chemometric analysis, which is effective and accurate for the identification of honey samples according their floral origin.
Discussion
Both HPLC and 1H-NMR have successfully identified monofloral honey from different botanical origins. HPLC-based detection of adulteration is favored for less expensive and relatively easy to operate. HPLC has been used for the evaluation of honey quality, and the profile of phenolic compounds are mostly used as floral markers. Wang et al. [31] identified jujube, longan and chaste honey using HPLC–ECD at different levels (5–50%, w/w) using ellagic acid and chlorogenia acid as potential markers. Zhao et al. [32] used HPLC to detect monofloral honeys, 36 honeys could be correctly identified according to their phenolic markers. However, honey also contains other characteristic components besides phenolics. The main difference between our research and the reported studies is that we focused on NSC in different types of monofloral honey such as phenolics, fat acids, amino acids, vitamins and so on. In our study, solid-phase extraction, combined with HPLC fingerprints of NSC, jujube, chaste and locust honey were successfully identified according to their botanical origins.
1H-NMR can provide better understanding of the complex structures in complicates systems, the analysis time is short and there is no damage to the sample. At present, 1H-NMR has been used to distinguish the floral origin of honey by identifying characteristic hydrogen signals of specific marker compounds. Spiteri et al. [23] differentiated manuka honey from other floral honeys using characteristic hydrogen signals of the high amounts of methylglyoxal (MGO). Another NMR-based profiling for discrimination of chestnut honey from other floral honeys was used by Cho et al. [33]. Their study was based on the identification of the protons and the carbons of two quinolinone alkaloids (kynurenic acid and 4-quinolone-2-carboxylic acid) in 1H and 13C-NMR spectra. The two quinolinone alkaloids were found to be present in all samples of chestnut honey, while they were absent in other honeys. In our research, the 1H NMR spectrum fingerprints of honeys from jujube, longan and chaste produced in China were established. Furthermore, using the Similarity Evalution System combined with chemometic analysis for spectrum fingerprint to analyze the spectral data, differentiation of honeys from jujube, longan and chaste was achieved without the need to confirm every spectral peak.
Conclusion
In our research, 50 honey samples from three selected botanical sources in China (jujube, longan and chaste) were processed. The three types of mono-floral honey samples were characterized using HPLC and 1H-NMR, and the corresponding chromatogramic and spectral fingerprints were established. The results showed that the common peaks of NSC extracted from HPLC and/or 1H-NMR fingerprints were sufficient to achieve accurate discrimination of the three different mono-floral honey samples. Furthermore, this method does not require the confirmation of every chromatographic or spectral peak. In addition, we demonstrate that other typical botanical origins from China can also be identified using this method. Therefore, HPLC and/or 1H-NMR profiling appear to be rapid, accurate and convenient analytical techniques for a comprehensive and efficient test to control the integrity and safety of mono-floral honeys in China.
References
P.M. Kuś, F. Congiu, D. Teper, Z. Sroka, I. Jerković, C.I.G. Tuberoso, LWT-Food Sci. Technol. 55, 124–130 (2014)
Y.H. Li, X.B. Zou, T.T. Shen, J.Y. Shi, J.W. Zhao, M. Holmes, Food Anal. Methods 10, 1034–1044 (2017)
A. Naila, S.H. Flint, A.Z. Sulaiman, A. Ajit, Z. Weeds, Food Control 90, 152–165 (2018)
Z. Wei, J. Wang, Y. Wang, J. Food Eng. 96, 469–479 (2010)
C. Das, S. Chakraborty, K. Acharya, N.K. Bera, D. Chattopadhyay, A. Karmakar, S. Chattopadhyay, Talanta 171, 327–334 (2017)
I. Revilla, A.M. Vivar-Quintana, I. Gonzalez-Martin, O. Escuredo, C. Seijo, Microchem. J. 134, 211–217 (2017)
H.E. Tahir, X.B. Zou, Z.H. Li, J.Y. Shi, X.D. Zhai, S. Wang, A.A. Mariod, Food Chem. 226, 202–211 (2017)
L.A. Frink, D.W. Armstrong, Food Chem. 205, 23–27 (2016)
H. Dong, K. Xiao, Y. Xian, Y. Wu, Food Chem. 240, 717–724 (2018)
L. Sobrino-Gregorio, R. Bataller, J. Soto, I. Escriche, Food Control 91, 254–260 (2018)
N. Altangerel, J.W. Walker, P.M. González, D.W. Bailey, R.E. Estell, M.O. Scully, Rangel. Ecol. Manag. 70, 781–786 (2017)
D. Rashmi, P. Shree, D.K. Singh, Food Control 79, 169–176 (2017)
O.V. Vetrova, D.A. Kalashnikova, V.N. Melkov, G.V. Simonova, J. Anal. Chem. 72, 756–760 (2017)
C. Nikolett, A. Loránd, C.J.C. Phillips, K. Béla, Eur. Food Res. Technol. 244, 1439–1445 (2018)
M. Tosun, Food Chem. 138, 1629–1632 (2013)
M.V. Russo, P. Avino, I. Notardonato, Food Anal. Methods 10, 2132–2142 (2017)
M.S. Azevedo, S.K.T. Seraglio, G. Rocha, C.B. Balderas, M. Piovezan, L.V. Gonzaga, D.B. Falkenberg, R. Fett, M.A.L. de Oliveira, A.C.O. Costa, Food Control 78, 383–392 (2017)
C. Kumaravelu, A. Gopal, Detection and quantification of adulteration in honey through near infrared spectroscopy. Int. J. Food Prop. 18, 1930–1935 (2015)
S. Ouchemoukh, N. Amessis-Ouchemoukh, M. Gómez-Romero, F. Aboud, A. Giuseppe, A. Fernández-Gutiérrez, LWT-Food Sci. Technol. 85, 460–469 (2017)
C. Sun, H. Tan, Y. Zhang, H. Zhang, J. Food Compos. Anal. 53, 91–101 (2016)
P. Truchado, I. Martos, L. Bortolotti, A.G. Sabatini, F. Ferreres, F.A. Tomasbarberan, J. Agric. Food Chem. 57, 5680–5686 (2009)
T. Takashima, T. Ohnuma, T. Fukamizo, Biomol. NMR Assign. 11, 85–90 (2017)
M. Spiteri, E. Jamin, F. Thomas, A. Rebours, M. Lees, K.M. Rogers, D.N. Rutledge, Food Chem. 189, 60–66 (2015)
D. Bertelli, M. Lolli, G. Papotti, L. Bortolotti, G. Serra, M. Plessi, J. Agric. Food Chem. 58, 8495–8501 (2010)
S. Amiry, M. Esmaiili, M. Alizadeh, Food Chem. 224, 390–397 (2017)
I.N. Pasias, I.K. Kiriakou, C. Proestos, Food Chem. 229, 425–431 (2017)
A.P. Louppis, M. Kontominas, C. Papastephanou, Food Anal. Methods 10, 3385–3397 (2017)
C. Pita-Calvo, M. Vázquez, Trends Food Sci. Technol. 59, 79–87 (2017)
S.M. Priscila, G. Cony, G.V. .Luciano, C.O. Ana, Food Chem. 196, 309–323 (2016)
C.L. Sun, H.B. Tan, Y.H. Zhang, H.C. Zhang, J. Food Compos. Anal. 53, 91–101 (2016)
I. Wang, X. Xue, X. Du, N. Cheng, L. Chen, J. Zhao, Food Anal. Methods 7(10), 2003–2012 (2014)
J. Zhao, X. Du, N. Cheng, L. Chen, X. Xue, J. Zhao, Food Chem. 194, 167–174 (2016)
J.Y. Cho, S.H. Bae, H.K. Kim, M.L. Lee, Y.S. Choi, B.R. Jin, J. Agric. Food Chem. 63(13), 3587–3592 (2015)
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant No. 2016YFD0400802), National High Technology Research and Development Program of China (863 Program) (Grant No. 2011AA060701). 2018 Postgraduate Research Capacity Improvement Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, H., Yue, J., Wang, D. et al. HPLC and 1H-NMR combined with chemometrics analysis for rapid discrimination of floral origin of honey. Food Measure 13, 1195–1204 (2019). https://doi.org/10.1007/s11694-019-00035-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00035-5