Abstract
Drying method is one of the most important steps in the preparation of plant materials for phytochemical analysis and biological evaluation. In this sense, this research endeavoured to evaluate the effects three drying methods (oven, freeze, and shade drying) on phytochemical component, antioxidant, and enzyme inhibitory effects of Salvia absconditiflora Greuter & Burdet. Antioxidant activities were screened by phosphomolybdenum, DPPH, ABTS, FRAP, CUPRAC, and metal chelating activity. Enzyme inhibitory effects were assessed against cholinesterases (AChE, BChE), tyrosinase, α-amylase, and α-glucosidase. Drying methods were found to affect the chemical component and biological properties. Shade drying showed the highest TPC (99.33 mgGAE/g) and TFC (46.88 mgRE/g) followed by oven (58.15 mgGAE/g for TPC, 40.65 for TFC), and freeze drying (43.73 mgGAE/g for TPC, 36.68 mgRE/g for TFC). The main phenolic compound characterized by HPLC was rosmarinic acid, which was observed to be highest following shade drying. Shade drying contained highest total bioactive compounds and exhibited the strongest antioxidant properties. The enzyme inhibitory effects of S. absconditiflora performed using different depends were dependent on the drying methods. Our results tend to suggest that shade drying was most suitable for S. absconditiflora because of possessing the highest rosmarinic acid and biological properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The genus Salvia, commonly known as ‘sage,’ is one of the widest-spread and most significant medicinal members of the Lamiaceae family. This genus comprises of nearly 900 species in the world. In Turkey, the genus is represented by 95 species [1, 2]. Several Salvia species have important medicinal uses including treatment for colds, aches, microbial infections, malaria, cancer, Alzheimer’s and cardiovascular disease all over the world [3,4,5,6,7]. Besides, Salvia species exhibited a wide spectrum of biological activities such as antibacterial, antioxidant, anti-inflammatory, and antiplatelet [8]. Many studies presented that the biological activities of Salvia species linked to their chemical compositions [8, 9]. Furthermore, the genus Salvia have great commercial potential for the food, perfume, and cosmetics industries [10]. For example, essential oils of S. sclarea are utilized as flavoring in cosmetic industries [11]. As for Turkey, dried Salvia leaves, known by local name ‘adaçayı’, are traditionally used as herbal tea in Anatolia [12]. The tea prepared with the aerial parts of Salvia absconditiflora is used for stomach disorder and the plant is also utilized as a colorant [13, 14].
Drying method is an important step to preserve the compounds with biological activity in the post-harvest processes [15, 16]. Different drying methods are widely used for post-harvest of plants. These methods have some advantages and limitations. For example shade drying is the most conventional methods due to lower costs, but the drying period is slow [17, 18]. Freeze drying provide advantages, preserve the flavor and shape. On the other hand, this method is costly [19]. Generally, the drying process affects bioactive components and pharmacological properties [20]. A considerable amount of literature has been published on drying methods of biological effects of fruits and vegetables [21,22,23,24]. However, knowledge of drying methods of medicinal plants is largely based on very limited data. There is currently a dearth of information about the effects of drying methods on S. absconditiflora. The aim of the research was thus to provide more insights of three drying methods (oven, freeze, and shade drying) on the phenolic profile and biological activity (antioxidant and enzyme inhibitory effects) of S. absconditiflora Greuter & Burdet.
Materials and methods
Plant materials and preparation of extracts
The aerial part of S. absconditiflora was collected from Selcuk University, Alaaddin Keykubad Campus, Konya. The plant identification was performed by botanist Dr. Murad Aydin Sanda. Drying methods used are as given below;
Oven drying
Fresh samples were spread on tray and dried in thermostatic oven at 65 C° for 24 h [25].
Freeze drying
Fresh samples were frozen at − 80 C°. Then, samples were rapidly placed in a lyophilizer and dried at 1 Pa pressure [26].
Shade drying
Fresh aerial parts were dried in the dark at room temperature.
After drying, samples were ground to powder using a laboratory mill. The samples (10 g) were stirred with MeOH at room temperature for 24 h. Then, the extracts were concentrated to dryness using a rotary evaporator.
Quantification of phenolic compounds by RP-HPLC
Phenolic compounds were evaluated by reversed-phase high performance liquid chromatography (RP-HPLC, Shimadzu Scientific Instruments, Kyoto, Japan). Detection and quantification were carried out with an LC-10ADvp pump, a diode array detector, a CTO-10Avp column heater, SCL-10Avp system controller, DGU-14A degasser and SIL-10ADvp auto sampler (Shimadzu Scientific Instruments, Columbia, MD, USA). Separation was conducted at 30 °C on Eclipse XDB C-18 reversed phase column (250 mm × 4.6 mm length, 5 µm particle size, Agilent, Santa Clara, CA, USA). The eluates were detected at 278 nm. The mobile phases were A: 3.0% acetic acid in distilled water and B: methanol. For analysis, the samples were dissolved in methanol, and 20 µL of this solution was injected into the system. The elution gradient applied at a flow rate of 0.8 mL/min was 93% A/7% B for 0.1 min, 72% A/28% B for 20 min, 75% A/25% B for 8 min, 70% A/30% B for 7 min and same gradient for 15 min, 67% A/33% B for 10 min, 58% A/42% B for 2 min, 50% A/50% B for 8 min, 30% A/70% B for 3 min, 20% A/80% B for 2 min, and 100%B for 5 min until the end of the run. The chromatographic conditions set for this present study were as described previously [27]. Gallic acid, protocatechic acid, catechin, p-hydroxybenzoic acid, chlorogenic acid, caffeic acid, epicatechin, syringic acid, vanilin, p-coumaric acid, ferulic acid, benzoic acid, o-coumaric acid, rutin, hesperidin, rosmarinic acid, eriodictiol, cinnamic acid, quercetin, luteolin, kaempferol and apigenin were used as standards. Identification and quantitative analysis were done by comparison with standards in the same chromatographic conditions. The amount of each compound was expressed as milligrams per gram of extract using external calibration curves, which were obtained for each phenolic standard.
Total phenolic and flavonoid content
The total phenolic and flavonoids content of the S. absconditiflora were evaluated by Folin–Ciocalteu and AlCl3 methods, respectively [28].
For total phenolic content (TPC): sample solution (2 mg/mL; 0.25 mL) was mixed with diluted Folin–Ciocalteu reagent (1 mL, 1:9, v/v) and shaked vigorously. After 3 min, Na2CO3 solution (0.75 mL, 1%) was added and the sample absorbance was read at 760 nm after a 2 h incubation at room temperature. Results were expressed as milligrams of gallic acid equivalents (mg GAE/g extract).
For the total flavonoids content: sample solution (2 mg/mL; 1 mL) was mixed with the same volume of aluminum trichloride (2%) in methanol. Similarly, a blank was prepared by adding sample solution (1 mL) to methanol (1 mL) without AlCl3. The sample and blank absorbances were read at 415 nm after a 10 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. Rutin was used as a reference standard, and the total flavonoid content (TFC) was expressed as milligrams of rutin equivalents (mgRE/g extract).
Biological activities of S. absconditiflora
Antioxidant activities and enzyme inhibitory effects were evaluated for the biological activities of S. absconditiflora. Antioxidant activity was assessment by using DPPH, ABTS, FRAP, CUPRAC, phosphomolybdenum, and metal chelating assays. Furthermore, enzyme inhibitory effects were tested against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), tyrosinase, α-amylase, and α-glucosidase. All experimental procedures were assessed as reported by Zengin et al. [29] and they are summarized below.
For the DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging assay: sample solution (2 mg/mL; 1 mL) was added to 4 mL of a 0.004% methanol solution of DPPH. The sample absorbance was read at 517 nm after a 30 min incubation at room temperature in the dark. DPPH radical scavenging activity was expressed as milligrams of trolox equivalents (mg TE/g extract).
For ABTS [2,2′-azino-bis (3-ethylbenzothiazoline) 6-sulfonic acid] radical scavenging assay: briefly, ABTS+ was produced directly by reacting 7 mM ABTS solution with 2.45 mM potassium persulfate and allowing the mixture to incubate for 12–16 h in the dark, at room temperature. Prior to beginning the assay, ABTS+ solution was diluted with methanol to an absorbance of 0.700 ± 0.02 at 734 nm. Sample solution (2 mg/mL; 1 mL) was added to ABTS solution (2 mL) and mixed. The sample absorbance was read at 734 nm after a 30 min incubation at room temperature. The ABTS radical scavenging activity was expressed as milligrams of trolox equivalents (mg TE/g extract).
For CUPRAC (cupric ion reducing activity) assay: sample solution (2 mg/mL; 0.5 mL) was added to premixed reaction mixture containing CuCl2 (1 mL, 10 mM), neocuproine (1 mL, 7.5 mM) and NH4Ac buffer (1 mL, 1 M, pH 7.0). Similarly, a blank was prepared by adding sample solution (0.5 mL) to premixed reaction mixture (3 mL) without CuCl2. Then, the sample and blank absorbances were read at 450 nm after a 30 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. CUPRAC results were expressed as milligrams of trolox equivalents (mg TE/g extract).
For ferric reducing antioxidant power (FRAP) assay: sample solution (2 mg/mL; 0.1 mL) was added to premixed FRAP reagent (2 mL) containing acetate buffer (0.3 M, pH 3.6), 2,4,6-tris (2-pyridyl)-S-triazine(TPTZ) (10 mM) in 40 mM HCl and ferric chloride (20 mM) in a ratio of 10:1:1 (v/v/v). Then, the sample absorbance was read at 593 nm after a 30 min incubation at room temperature. FRAP activity was expressed as milligrams of trolox equivalents (mg TE/g extract).
For phosphomolybdenum method: sample solution (2 mg/mL; 0.3 mL) was combined with 3 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The sample absorbance was read at 695 nm after a 90 min incubation at 95 °C. The total antioxidant capacity was expressed as millimoles of trolox equivalents (mmol TE/g extract).
For metal chelating activity assay: briefly, sample solution (2 mg/mL; 2 mL) was added to FeCl2 solution (0.05 mL, 2 mM). The reaction was initiated by the addition of 5 mM ferrozine (0.2 mL). Similarly, a blank was prepared by adding sample solution (2 mL) to FeCl2 solution (0.05 mL, 2 mM) and water (0.2 mL) without ferrozine. Then, the sample and blank absorbances were read at 562 nm after 10 min of incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. The metal chelating activity was expressed as milligrams of EDTA (disodium edetate) equivalents (mg EDTAE/g extract).
For Cholinesterase (ChE) inhibitory activity assay: sample solution (2 mg/mL; 50 µL) was mixed with DTNB [5,5-dithio-bis (2-nitrobenzoic) acid, Sigma, St. Louis, MO, USA] (125 µL) and AChE [acetylcholinesterase (electric ell acetylcholinesterase, Type-VI-S, EC 3.1.1.7, Sigma)], or BChE [butyrylcholinesterase (horse serum butyrylcholinesterase, EC 3.1.1.8, Sigma)] solution (25 µL) in Tris–HCl buffer (pH 8.0) in a 96-well microplate and incubated for 15 min at 25 °C. The reaction was then initiated with the addition of acetylthiocholine iodide (ATCI, Sigma) or butyrylthiocholine chloride (BTCl, Sigma) (25 µL). Similarly, a blank was prepared by adding sample solution to all reaction reagents without enzyme (AChE or BChE) solution. The sample and blank absorbances were read at 405 nm after 10 min incubation at 25 °C. The absorbance of the blank was subtracted from that of the sample, and the cholinesterase inhibitory activity was expressed as galantamine equivalents (mgGALAE/g extract).
For tyrosinase inhibitory activity assay: sample solution (2 mg/mL; 25 µL) was mixed with tyrosinase solution (40 µL, Sigma) and phosphate buffer (100 µL, pH 6.8) in a 96-well microplate and incubated for 15 min at 25 °C. The reaction was then initiated with the addition of LDOPA (40 µL, Sigma). Similarly, a blank was prepared by adding sample solution to all reaction reagents without enzyme (tyrosinase) solution. The sample and blank absorbances were read at 492 nm after a 10 min incubation at 25 °C. The absorbance of the blank was subtracted from that of the sample, and the tyrosinase inhibitory activity was expressed as kojic acid equivalents (mgKAE/g extract).
For α-amylase inhibitory activity assay: sample solution (2 mg/mL; 25 µL) was mixed with α-amylase solution (ex-porcine pancreas, EC 3.2.1.1, Sigma) (50 µL) in phosphate buffer (pH 6.9 with 6 mM sodium chloride) in a 96-well microplate and incubated for 10 min at 37 °C. After pre-incubation, the reaction was initiated with the addition of starch solution (50 µL, 0.05%). Similarly, a blank was prepared by adding sample solution to all reaction reagents without enzyme (α-amylase) solution. The reaction mixture was incubated 10 min at 37 °C. The reaction was then stopped with the addition of HCl (25 µL, 1 M). This was followed by addition of the iodine-potassium iodide solution (100 µL). The sample and blank absorbances were read at 630 nm. The absorbance of the blank was subtracted from that of the sample, and the α-amylase inhibitory activity was expressed as acarbose equivalents (mmol ACE/g extract).
For α-glucosidase inhibitory activity assay: sample solution (2 mg/mL; 50 µL) was mixed with glutathione (50 µL), α-glucosidase solution (from Saccharomyces cerevisiae, EC 3.2.1.20, Sigma) (50 µL) in phosphate buffer (pH 6.8) and PNPG (4-N-trophenyl-α-D-glucopyranoside, Sigma) (50 µL) in a 96-well microplate and incubated for 15 min at 37 °C. Similarly, a blank was prepared by adding sample solution to all reaction reagents without enzyme (α-glucosidase) solution. The reaction was then stopped with the addition of sodium carbonate (50 µL, 0.2 M). The sample and blank absorbances were read at 400 nm. The absorbance of the blank was subtracted from that of the sample and the α-glucosidase inhibitory activity was expressed as acarbose equivalents (mmol ACE/g extract).
Statistical analysis
The effect of different drying methods was analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test. These analyses were performed using statistical analysis software (XLSTAT 18.0).
Results and discussion
Identification of phenolic compounds
Twenty-two phenolic standards were used for the chromatographic separation of S. absconditiflora, among them 13 were identified. The result indicated that major compound S. absconditiflora extracts was rosmarinic acid (Table 1). Most of earlier research reported rosmarinic acid as predominant compounds in Salvia species. For example, rosmarinic acid was identified for S. blepharochlaena (22 mg/g), S. euphratica var. leiocalycina (10.3 mg/g), and S. verticillata subsp. amasiaca (67 mg/g) [29], but the content of rosmarinic acid was lower than that in the present study. Rahman et al. [30] described the presence of rosmarinic acid in S. hispanica seeds (738.2 µg/g in the free and 31.03 µg/g in esterified fractions). Similarly, rosmarinic acid was found to be major phenolic acid in S. officinalis (8445.52–10768.65 µg/g DW) [31]. Moreover, the phenolic compounds of S. absconditiflora extracts by different drying methods varied significantly. This result match those observed in earlier studies [25, 32]. The maximal rosmarinic acid was found in shade drying (90.96 mg/g) > oven drying (38.50 mg/g) and minimal from freeze drying (12.58 mg/g). Our results were according to the previously studies; Nunes et al. [33] and Hossain et al. [34] found that oven drying showed higher level of rosmarinic acid compared to the freeze drying. This situation could be explained by the fact that duration of the freeze process has generation of ice crystals within tissue matrix, which may result in cell disruption and exposure of phenolic components to the oxidative status [35]. Moreover, Zhang et al. [36] found that rosmarinic acid did not degrade under room temperature with light exposure or dark for 13 day. This data support the highest activity of shade drying in our study. Unlike these studies, some authors have reported that increasing the temperature have adverse effect on the concentrations of the bioactive components [37, 38].
Total bioactive components
TPC and TFC of S. absconditiflora by different drying methods are shown in Table 2. The S. absconditiflora displayed differences in their total bioactive components depending on the drying methods. As shown in Table 2, shade drying showed the highest TPC (99.33 mgGAE/g) and TFC (46.88 mgRE/g) followed by oven drying (58.15 mgGAE/g for TPC, 40.65 for TFC), and freeze drying (43.73 mgGAE/g for TPC, 36.68 mgRE/g for TFC). The lowest total bioactive component was obtained in freeze drying. In contrast, Samoticha et al. [39] revealed that freeze drying of chokeberry contained the highest TPC. Quispe-Fuentes et al. [40] reported that freeze drying contained higher TFC than other drying methods. In another study performed by Horszwald et al. [41], vacuum-oven drying possessed higher TPC and TFC than freeze drying. This result is consistent with our data.
Antioxidant capacity
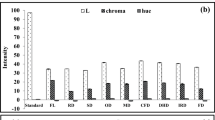
In order to provide a more complete evaluation of the antioxidant capacity, several in vitro methods including, DPPH, ABTS, CUPRAC, FRAP, phosphomolybdenum and metal chelating assays were used. The results are shown in Table 2. The radical scavenging activities were performed by DPPH and ABTS assays. In these assays, shade drying (198.26 and 223.63 mgTE/g for DPPH and ABTS, respectively) exhibited the highest free radical scavenging activity followed by oven drying and freeze drying. In order to assess the reducing power activity, CUPRAC and FRAP were employed. Similar to the radical scavenging results, the best reducing abilities were obtained in shade drying. The total antioxidant capacity was measured by phosphomolybdenum assay. Oven, freeze, and shade drying showed no significant (p > 0.05) differences in phosphomolybdenum assay. The highest metal chelating activity was obtained by shade drying (8.31 mgEDTA/g) and its lowest activity was observed in freeze drying (5.73 mgEDTA/g). The observed activity could be explained by the presence of higher level of rosmarinic acid in shade drying. Our result is also in agreement with results obtained by Hossain et al. [37], who reported vacuum-oven drying has a stronger antioxidant activity than freeze drying and a high concentration obtained for rosmarinic acid by oven drying.
Enzyme inhibitory effects
Alzheimer’s disease (AD) is one of the most common neurodegenerative disorder worldwide [42, 43]. Similarly, diabetes has become a serious disease and World health organization (WHO) estimates that diabetes will be the 7th biggest killer in the world by 2030 [44,45,46]. As for tyrosinase enzyme, the enzyme is the key enzyme of melanin biosynthesis and it is linked to generally disorders associated with melanin [47]. The use of inhibitors of enzymes associated with these diseases (AChE and BChE for Alzheimer’s; α-amylase and α-glucosidase for diabetes; tyrosinase for hyperpigmentation) is effective approach in the treatment of non-communicable diseases. Numerous synthetic inhibitors are actually used for treating such diseases [48, 49]. However, plant-derived natural inhibitors are considered as the most interesting agents due to lack of side effects [50,51,52].
In this context, S. absconditiflora was tested for in vitro enzyme inhibitory effects against AChE, BChE, tyrosinase, α-amylase, and α-glucosidase. The results are represented in Table 3. Freeze drying showed strong cholinesterase effects (AChE: 2.27, BChE:1.43 mg GALAE/g). With respect to α-amylase and α-glucosidase assays, shade drying displayed the highest activity. Oven drying (37 93 mgKAE/g) showed maximal effect against tyrosinase, followed by freeze drying (35.69 mgKAE/g), and shade drying (34.13 mgKAE/g). In terms of drying methods, the difference in the results was observed. rosmarinic acid and other compounds present in S. absconditiflora could also affect the enzyme activity. Effect of drying process on enzyme inhibitory activity have not been reported previously in the literature as previous studies have focused on quality, chemical components, and antioxidant of dried plants.
Chemometric analysis
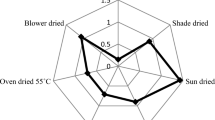
Principal component analysis (PCA) is one of the most commonly used chemometric technique [53]. Drying methods are divided into two groups as listed in Fig. 1a. Freeze and shade drying are classed into one group. Shade drying is separated from each other. Similarly, the dendrogram clearly exhibited that drying methods can be divided in to two groups at truncation point 10 (Fig. 1b).
Conclusion
Three different drying methods were used to compare biological activity and phenolic profile of S. absconditiflora. Our work has led us to conclude that drying methods can considerably influence phytochemical component and biological activities. Shade drying was more effective than oven and freeze drying because of its highest level of rosmarinic acid. Considering that no previous report on drying methods of S. absconditiflora have been produced, the results of the present research is the first time in the literature. However, further research on S. absconditiflora is desirable to extend our knowledge of the in vivo biological activities, cytotoxic property, and to isolate the active components.
References
N. Tan, S. Yazici-Tutunis, Y. Yesil, B. Demirci, E. Tan, Rec. Nat. Prod. 11, 456–461 (2017)
J.B. Walker, K.J. Sytsma, J. Treutlein, M. Wink, Am. J. Bot. 91(7), 1115–1125 (2004)
G. Kamatou, N. Makunga, W. Ramogola, A. Viljoen, J. Ethnopharmacol. 119(3), 664–672 (2008)
G. Kamatou, R. Van Zyl, S. Van Vuuren, A. Figueiredo, J. Barroso, L. Pedro, A. Viljoen, S. Afr. J. Bot. 74(2), 230–237 (2008)
M.R. Loizzo, M. Abouali, P. Salehi, A. Sonboli, M. Kanani, F. Menichini, R. Tundis, Nat. Prod. Res. 28(24), 2278–2285 (2014)
M. Mohammadhosseini, J Essent. Oil Bear. PL. 18(2), 464–476 (2015)
Y. Xu, R. Wan, Y. Lin, L. Yang, Y. Chen, C. Liu, Asian J. Pharmacodyn. Pharmacokinet. 7(2), 99–130 (2007)
Y.-B. Wu, Z.-Y. Ni, Q.-W. Shi, M. Dong, H. Kiyota, Y.-C. Gu, B. Cong, Chem. Rev. 112(11), 5967–6026 (2012)
I. Cvetkovikj, G. Stefkov, J. Acevska, J.P. Stanoeva, M. Karapandzova, M. Stefova, A. Dimitrovska, S. Kulevanova, J. Chromatogr. A 1282, 38–45 (2013)
M.B. Bahadori, H. Valizadeh, B. Asghari, L. Dinparast, M.M. Farimani, S. Bahadori, J. Funct. Food 18, 727–736 (2015)
P. Salehi, A. Sonboli, M. Dayeni, F. Eftekhar, M. Yousefzadi, Chem. Nat. Compd. 44(1), 102–105 (2008)
T. Baytop, Türkiyede bitkiler ile tedavi (geçmişte ve bugün), 2nd edn. (İstanbul Üniversitesi, Istanbul, 1984), pp. 142–143
T. Baytop, Türkçe bitki adları sözlüğü, 2nd edn. (Turk Dil Kurumu, Ankara, 1994), pp. 20–21
G. Honda, E. Yeşilada, M. Tabata, E. Sezik, T. Fujita, Y. Takeda, Y. Takaishi, T. Tanaka, J. Ethnopharmacol. 53(2), 75–87 (1996)
J. Yuan, L.-J. Hao, G. Wu, S. Wang, J. Duan, G.-Y. Xie, M.-J. Qin, J. Funct. Foods. 19 786–795 (2015)
L. Zhang, T. Liu, Y. Xue, C. Liu, H. Ru, M. Dong, Z. Yu, J. Food Process Eng. 39(2), 107–120 (2016)
P. Juyan, G. Yuehua, W. Junru, L. Yan, L. Zongsuo, Acta Bot. Boreal.-Occident. Sin. 26(10), 2044–2050 (2006)
Y. Soysal, S. Öztekin, J. Agric. Eng. Res. 78(2), 159–166 (2001)
J. Huang, M. Zhang, Dry. Technol. 34(8), 900–911 (2016)
Y. Zhu, B.-Q. Pu, G.-Y. Xie, M. Tian, F.-Y. Xu, M.-J. Qin, Molecules 19(7), 10440–10454 (2014)
Y. Chen, A. Martynenko, LWT-Food Sci. Technol. 87 470–477 (2018)
S.M. Jafari, D. Azizi, H. Mirzaei, D. Dehnad, J. Food Process. Preserv. 40(3), 362–372 (2016)
X. Jin, T. Oliviero, R. van der Sman, R. Verkerk, M. Dekker, A. van Boxtel, LWT-Food Sci. Technol. 59(1), 189–195 (2014)
M.C. Karam, J. Petit, D. Zimmer, E.B. Djantou, J. Scher, J. Food Eng. 188, 32–49 (2016)
X.-F. Shi, J.-Z. Chu, Y.-F. Zhang, C.-Q. Liu, X.-Q. Yao, Ind. Crops Prod. 104, 45–51 (2017)
N. Jiang, C. Liu, D. Li, Z. Zhang, C. Liu, D. Wang, L. Niu, M. Zhang, LWT-Food Sci. Technol. 82 216–226 (2017)
S. Uysal, G. Zengin, A. Aktumsek, S. Karatas, J. Funct. Foods. 22, 518–532 (2016)
S. Uysal, A. Ugurlu, G. Zengin, M.C. Baloglu, Y.C. Altunoglu, A. Mollica, L. Custodio, N.R. Neng, J.M. Nogueira, M.F. Mahomoodally, Food Chem. Toxicol. 111, 525–536 (2018)
G. Zengin, E.J. Llorent-Martínez, M.L. Fernández-de Córdova, M.B. Bahadori, A. Mocan, M. Locatelli, A. Aktumsek, Ind. Crops Prod. 111, 11–21 (2018)
M.J. Rahman, A.C. de Camargo, F. Shahidi, J. Funct. Foods. 35, 622–634 (2017)
M.B. Farhat, R. Chaouch-Hamada, J.A. Sotomayor, A. Landoulsi, M.J. Jordán, Ind. Crops Prod. 54, 78–85 (2014)
R.-L. Qin, C.-N. Lv, Y. Zhao, Y.-D. Zhao, Y. Yu, J.-C. Lu, Ind. Crops Prod. 107, 288–296 (2017)
J.C. Nunes, M.G. Lago, V.N. Castelo-Branco, F.R. Oliveira, A.G. Torres, D. Perrone, M. Monteiro, Food Chem. 197, 881–890 (2016)
M. Hossain, C. Barry-Ryan, A.B. Martin-Diana, N. Brunton, Food Chem. 123(1), 85–91 (2010)
D.S. Sogi, M. Siddiq, K.D. Dolan, LWT-Food Sci. Technol. 62(1), 564–568 (2015)
Y. Zhang, J.P. Smuts, E. Dodbiba, R. Rangarajan, J.C. Lang, D.W. Armstrong, J. Agric. Food Chem. 60(36), 9305–9314 (2012)
R. Bruni, G. Sacchetti, Molecules 14(2), 682–725 (2009)
H. Tanko, D.J. Carrier, L. Duan, E. Clausen, Plant Genet. Resour. 3(2), 304–313 (2005)
J. Samoticha, A. Wojdyło, K. Lech, LWT-Food Sci. Technol. 66, 484–489 (2016)
I. Quispe-Fuentes, A. Vega-Gálvez, M. Aranda, J. Sci. Food Agric. 98(11), 4168–4176 (2018)
A. Horszwald, H. Julien, W. Andlauer. Food Chem. 141(3), 2858–2863 (2013)
S.T. Ngo, M.S. Li, Mol. Simul. 39(4), 279–291 (2013)
A.Y. Sun, Q. Wang, A. Simonyi, G.Y. Sun, Neuromol. Med 10(4), 259–274 (2008)
F. Hiroyuki, Y. Tomohide, O. Kazunori, J. Nutr. Biochem. 12(6), 351–356 (2001)
M.C.N. Picot, O. Bender, A. Atalay, G. Zengin, L. Loffredo, F. Hadji-Minaglou, M.F. Mahomoodally, Biomed. Pharmacother. 89, 342–350 (2017)
M.C.N. Picot, M.F. Mahomoodally, Pharm. Biol. 55(1), 864–872 (2017)
M.T.H. Khan, Curr. Med. Chem. 19(14), 2262–2272 (2012)
W.V. Graham, A. Bonito-Oliva, T.P. Sakmar, Annu. Rev. Med. 68, 413–430 (2017)
U. Wehmeier, W. Piepersberg, Appl. Microbiol. Biotechnol. 63(6), 613–625 (2004)
N. Martins, I.C. Ferreira, in Food Bioactives, ed. by P. Munish (Springer, Cham, 2017), pp. 267–298
P. Pradeep, Y.N. Sreerama, Food Chem. 247, 46–55 (2018)
C. De Monte, B. Bizzarri, M.C. Gidaro, S. Carradori, A. Mollica, G. Luisi, A. Granese, S. Alcaro, G. Costa, N. Basilico, S. Parapini, M.M. Scaltrito, C. Masia, F. Sisto, J. Enzyme. Inhib. Med. Chem. 30(6), 1027–1033 (2015)
A.A. Zielinski, C.W. Haminiuk, C.A. Nunes, E. Schnitzler, S.M. Ruth, D. Granato, Compr. Rev. Food Sci. Food Saf. 13(3), 300–316 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uysal, S. A comparative study of three drying methods on the phenolic profile and biological activities of Salvia absconditiflora. Food Measure 13, 162–168 (2019). https://doi.org/10.1007/s11694-018-9929-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9929-7