Abstract
Components of the same structure or characters of the same individual might respond differently to natural and sexual selective pressures, showing complex morphological patterns. Besides, studying interactions between species plays a crucial role in understanding the diversification of sex-linked phenotypes. Specifically, when two closely related species coexist and exhibit interspecific sexual interactions (reproductive interference—IR), key traits for mating can diverge in sympatric areas to prevent interbreeding and ensure reproductive isolation (reproductive character displacement—RCD). RCD is primarily driven by natural selection, although sexual selection pressures can alter the pattern of phenotypic variation. Additionally, to gain a comprehensive understanding of the patterns of morphological diversification, it is essential to consider changes related to phenotypic plasticity across environmental gradients. To date, there are no studies evaluating this topic in scorpions, and two sympatric species (Urophonius brachycentrus and U. achalensis) with RI, provide an ideal model for evaluating phenotypic variation across environmental gradients and the presence of RCD. In this study, we compared intra-specific variation, as well as the size and shape of multiple characters involved in courtship and sperm transfer, between individuals from sympatric and allopatric populations using geometric morphometrics. Our findings revealed an increase in the size of various characters at lower temperatures (higher altitudes) for U. brachycentrus, making them more similar to heterospecifics in sympatric areas, resulting in a pattern of morphological convergence between these species. Increased similarity between species combined with a scramble competition mating system could intensify sexual selection pressures on particular characters. Furthermore, we identified asymmetric RCD in the shape of several sexual characters crucial for mating success (grasping structures) and sperm transfer (genital characters), which could potentially be significant for mechanical isolation during interspecific interactions. Our results highlight significant morphological variability in the size and shape of somatic and genital characters in two scorpion species. This variability may reflect different evolutionary responses, driven in part by natural selection pressures associated with geographic and environmental variations and species recognition mechanisms, and in part by sexual selection pressures at both the intra- and interspecific levels. This comprehensive study reveals the complexity of evolving multifunctional traits in an understudied model and offers valuable insights into traits subject to multiple selective pressures in animal systems experiencing RI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Determining the factors underlying phenotypic variation in natural populations is important for comprehending the evolution of species and their biological diversity and is a fundamental task of evolutionary biology (Coyne & Orr, 2004). The morphology of organisms is shaped by multiple selective pressures, particularly those involved in various components of the life history of organisms. A noteworthy aspect of this process involves the relatively fast evolutionary divergence of secondary sexual characters due to the combined forces of sexual and natural selection (Svensson & Gosden, 2007). Natural selection acts upon morphological traits associated with growth, reproduction, and survival, thus promoting greater reproductive success in specific environments. In contrast, sexual selection underpins the morphological changes that favor reproductive success through mechanisms such as intra-sexual competition, inter-sexual mate choice, or post-copulatory processes (Kraaijeveld et al., 2011; Maan & Seehausen, 2011; Safran et al., 2013).
The study of phenotype variation and its causes may be complicated, primarily because adaptation operates as a multivariate process affecting sets of characters (Blows, 2007; Lande & Arnold, 1983; Schluter & Nychka, 1994). Organisms can be interpreted as composite entities, with characters that are not necessarily independent of one another responding in intricate and diverse ways to different selective pressures (Klingenberg, 2009). This presence of multiple, varied selective regimes can lead to a phenomenon known as “mosaic evolution”, where different components of the same structure exhibit mixed responses to synergistic or antagonistic selective pressures, driven by the multifaceted nature of these forces. Moreover, even the shape and size of the same structure can diverge disparately in response to these pressures (House & Simmons, 2005; Song & Wenzel, 2008; Werner & Simmons, 2008).
Examining interspecific interactions plays a pivotal role in understanding sex-linked phenotypic diversification (Cothran, 2015). Specifically, one intriguing facet of these interactions is reproductive interference (henceforth referred as ‘RI’), a phenomenon defined as any form of interspecific interaction among sympatric species associated with their mating systems stemming from incomplete recognition between them (Burdfield-Steel & Shuker, 2011; Gröning & Hochkirch, 2008). This process can have detrimental effects on the reproductive success of at least one of the involved species (Hochkirch et al., 2007). RI between species can lead to the displacement of key characters in reproductive interactions (i.e., reproductive character displacement—henceforth referred as ‘RCD’) (Howard, 1993). RCD results in a divergence of these characters, which serves to alleviate RI and consequently reinforces reproductive isolation (Coyne & Orr, 2004; Kyogoku, 2015; Servedio & Noor, 2003), and is therefore considered a natural selection mechanism. In sympatry, where these species share the same geographical area, characters of coexisting species should exhibit greater divergence compared to their allopatric populations. The more similar the characters of interacting species are in sympatry, the stronger the consequences of RI on reproductive success (Konuma & Chiba, 2012; Pfennig & Pfennig, 2010).
However, due to the complex interplay of multiple selective pressures acting on sexual characters, as discussed earlier, predicting the direction of their morphological evolution is sometimes not so straightforward. In sympatric areas, intraspecific sexual selection pressures may combine with interspecific interactions creating a mosaic of selective pressures with different outcomes in terms of morphological variation (Grether et al., 2009). Secondary sexual characters may play a role in specific recognition, and thus their divergence can be attributed to natural selection (Bennet-Clark & Ewing, 1970; Mayr, 1963). However, it has been postulated that mate choice and specific recognition are part of a continuum, and that sexual selection may also lead to reinforcement or RCD (Boake et al., 1997; Liou & Price, 1994; Mendelson & Shaw, 2012; Ryan & Rand, 1993). For example, female choice can promote isolation resulting in the divergence of male sexual characters to prevent RI or hybridization (Butlin, 1987; Gröning & Hochkirch, 2008; Hoskin & Higgie, 2010). Alternatively, in a not mutually exclusive scenario, males may engage in a constant “race” to mate by competing with conspecific and heterospecific males, leading to more frequent discrimination errors in an extended scramble competition style (Takakura et al., 2015). This promiscuous behavior may be adaptive if the costs of the mistakes are outweighed by a higher reproductive success for these not very discriminative males. In these cases, it is expected a convergence of sexual characters (Drury et al., 2015; Grant, 1972; Grether et al., 2009; Sobroza et al., 2021; Tobias et al., 2014) with consequent maintenance or intensification of RI (Takakura et al., 2015; Wheatcroft, 2015; Yamaguchi & Iwasa, 2015). In turn, the degree and direction of divergence of sexual characters may differ according to their function, the moment of the reproductive event in which RI occurs and the evolutionary interests of the sexes (Gröning & Hochkirch, 2008).
Animal genitalia, especially in the male, can display complex morphologies and undergo rapid and divergent evolutionary changes compared to other body parts (Eberhard, 1985; Leonard & Córdoba-Aguilar, 2010; Tuxen, 1970). Sexual selection is widely recognized as a key driver in the evolution of genitalia (Eberhard, 1985, 2010; Hosken & Stockley, 2004; Simmons, 2014). Conversely, the divergence of genitalia can also be attributed to natural selection, as it contributes to reproductive isolation between species, thereby promoting speciation (Eberhard, 1985, 2010; House et al., 2013; Masly, 2012; Wojcieszek & Simmons, 2012). Phenomena such as RCD may contribute to differences in genitalia between species in sympatric regions, where mechanical or interlocking incompatibilities between male and female genitalia may frequently occur (Masly, 2012). Similarly, other non-genital characters used in contact during pre-copulatory or copulatory behavior (e.g., grasping structures, claspers that require morphological complementarity between males and females) may exhibit the same trajectories of rapid and disparate change as genital characters (Eberhard, 1985, 2004, 2010; Robson & Richards, 1936). The relative importance of natural and sexual selection in genitalia and contact character evolution continues under debate (Brennan & Prum, 2015; Eberhard, 1985, 2010; Eberhard & Lehmann, 2019; Jennions & Kelly, 2002; Simmons, 2014; Sloan & Simmons, 2019), although there is compelling evidence suggesting that multiple selective pressures may be important to shape their morphological evolution (Frazee & Masly, 2015; House et al., 2013; Langerhans et al., 2005; McPeek et al., 2009; Simmons, 2014; Simmons et al., 2009; Song & Wenzel, 2008).
Furthermore, phenotypic plasticity refers to the capacity of organisms to change their morphology, behavior, or physiology in response to environmental fluctuations (Stearns, 1989; West-Eberhard, 2003; Whitman & Agrawal, 2009). When characters express some degree of phenotypic plasticity, differences in phenotype resulting from environmental variation among species and populations can give rise to patterns of morphological variation (Garnier et al., 2005; Jennions & Kelly, 2002; Song & Wenzel, 2008). It is important to acknowledge that the environment can directly or indirectly influence both genetic and phenotypic variation, leading to geographic variation among different populations (Kosuda et al., 2016; Sota et al., 2000; Wilson et al., 2021), especially along environmental clines (Goldberg & Lande, 2006). Consequently, one of the prerequisites for testing RCD is to disentangle the effects of allopatric/sympatric contexts from other ecological factors. To identify patterns of divergence that might otherwise go unnoticed, it is important to control correlations between phenotype and environmental or gradients (Goldberg & Lande, 2006).
Examples of RI exist in many animal and plant groups (e.g., Armbruster & Herzig, 1984; Dame & Petren, 2006; Gröning & Hochkirch, 2008; Hettyey & Pearman, 2003; Levin, 1970; Matsumoto et al., 2010), and among them, arthropods have provided interesting models for studying this phenomenon (Shuker & Burdfield-Steel, 2017). Although some cases of ecological character displacement have been described in insects and arachnids, there are fewer examples of RCD in these taxa due to the difficulty of empirically evidencing this process (Waage, 1979). However, in arthropods, evidence of RCD was found in pre-copulatory characters used during courtship (Dyer et al., 2014; Jang & Gerhardt, 2006; Kronforst et al., 2007; Marshall & Cooley, 2000; Rundle & Dyer, 2015; Yukilevich, 2021) and there are also examples of RCD in genital characters (Kawakami & Tatsuta, 2010; Kawano, 2002; Kosuda et al., 2016; Nishimura et al., 2022). In arachnids, there are some suggestions that RCD might occur between species in sympatry (Agnarsson et al., 2016; Barth, 1990; Muster & Michalik, 2020; Stratton, 1997), as is the case of genital characters between Paratrechalea spider species with RI (Costa-Schmidt & de Araújo, 2010). Nevertheless, RCD has not yet been investigated in scorpions and only one case of IR was recently reported among species of the Family Bothriuridae (Oviedo-Diego, 2022; Oviedo-Diego et al., 2021). However, there are several records of interspecific mating in scorpions (Auber, 1963; Le Pape & Goyffon, 1975; Matthiesen, 1968; Peretti, 1993; Peretti et al., 2000; Probst, 1972). Furthermore, the coexistence of species in the same spatial area appears to be a common among scorpions (Acosta, 1995; Dionisio-da-Silva et al., 2018; Goodman & Esposito, 2020; Graham et al., 2012; Nime et al., 2014; Polis & McCormick, 1986; Vignoli et al., 2005).
Additionally, scorpions offer an interesting model for investigating these topics because, in certain species, we possess substantial knowledge regarding the functional significance of numerous courtship behaviors (Peretti, 2010; Polis & Sissom, 1990). Throughout sexual interactions, individuals engage in signal exchange and various behaviors involve traits to stimulate or appease the female (Carrera et al., 2009; Chantall-Rocha & Japyassú, 2017; Lira et al., 2018; Olivero et al., 2015, 2019; Peretti, 2013; Peretti et al., 2001). Several of these traits, in addition to the complex genitalia have been the subjects of morphological analysis suggesting that in many cases they are found under various selective regimes (Carrera et al., 2009; Fox et al., 2015; Mattoni et al., 2012; Monod et al., 2017; Peretti, 2003; Peretti et al., 2001; Sánchez-Quirós et al., 2012; Santibáñez-López et al., 2017; Visser & Geerts, 2021). Scorpions present indirect sperm transfer via a sclerotized spermatophore deposited in the substrate (that is regenerated each time the male mates from two chitinous halves—i.e., hemispermatophores produced in internal glandular structures called paraxial organs) (Polis & Sissom, 1990; Proctor, 1998; Weygoldt, 1990). These genital characters are incredibly complex and can be divided into subunits offering interesting opportunities for studying the evolution of genitalia (Mattoni et al., 2012; Monod et al., 2017; Peretti, 2003, 2010; Peretti et al., 2001). In particular, these characters were extensively studied in the family Bothriuridae in the evolutionary framework of sexual selection (Carrera et al., 2009; Olivero et al., 2014, 2015, 2019; Oviedo-Diego et al., 2020; Peretti, 2003, 2010; Peretti et al., 2001).
Here, we examined the occurrence of RCD in two closely related scorpion species of the genus Urophonius Pocock, 1893 (U. brachycentrus and U. achalensis, Bothriuridae) (Ojanguren-Affilastro et al., 2020) that have partially sympatric ranges in mountainous regions of central Argentina with overlapping reproductive seasons and share the same habitat (Acosta, 1985; Maury, 1969; Ojanguren-Affilastro et al., 2020). These scorpions exhibit winter habits and adaptations for this lifestyle, which is rather peculiar among scorpions (Garcia et al., 2021; Ojanguren-Affilastro et al., 2020, 2023). These species lack specific recognition through chemical signals, which, coupled with a promiscuous mating system in the sympatric area, leads to an asymmetric RI scenario with heterospecific mating (Oviedo-Diego, 2022; Oviedo-Diego et al., 2021). Therefore, we expect greater morphological differentiation in at least one of the analyzed Urophonius species in sympatric populations regarding allopatric ones (RCD pattern), which would hinder or prevent heterospecific mating, given the costs they may entail in terms of gamete loss, female plugging (Oviedo-Diego et al., 2019, 2020; Romero-Lebrón et al., 2019) or potential hybridization. For test this we conducted a comprehensive morphometric study of genital and somatic characters (utilized during courtship) comparing males and females of sympatric and allopatric populations of these species. Additionally, we analyzed the effect of the environmental cline linked to these mountainous areas, in order to take into account the role of phenotypic plasticity in the morphological evolution of the analyzed characters. Our results, incorporating multiple lines of evidence, underscore the intrinsic complexity of sexual characters in scorpions and provide valuable insights in the possible selective pressures driving the evolution of these traits.
Materials and Methods
Study Species and Sampling
Urophonius brachycentrus has a wide geographic range distributed throughout central Argentina, while U. achalensis is endemic to the mountainous regions of Córdoba in central Argentina (Acosta, 1985; Ojanguren-Affilastro et al., 2020). The two species share partially sympatric distribution areas in the Sierras Grandes in Córdoba, Argentina (Acosta, 1985). Urophonius brachycentrus and U. achalensis are closely related species within the brachycentrus species group, but they are not sister species, showing some phylogenetic distance (Ojanguren-Affilastro et al., 2020). This species group is extremely old, around 64 Ma (Ojanguren-Affilastro et al., 2023), but still all the species show a basic common phenotype, sharing many characters (Ojanguren-Affilastro et al., 2020). Adult scorpions (n = 25 per population context and per sex of each species) of U. achalensis and U. brachycentrus were collected during the day during the mating season (May–August) (Acosta, 1985; Maury, 1969; Ojanguren-Affilastro et al., 2020) for 3 consecutive years (2018, 2019, 2020) by turning over rocks. We collected individuals in two allopatric populations of U. brachycentrus (31° 22′ 42.4″ S 64° 35′ 34.0″ W, 876 m.a.s.l..; 31° 31′ 46.3″ S 64° 51′ 52.7″ W, 996 m.a.s.l.), two allopatric populations of U. achalensis (31° 35′ 49.1″ S 64° 44′ 49.3″ W, 2030 m.a.s.l., 31° 21′ 17.3″ S 64° 48′ 21.3″ W, 1927 m.a.s.l.), and in two sympatric populations (31° 23′ 13.5″ S 64° 46′ 10.2″ W, 1796 m.a.s.l.; 31° 34′ 07.6″ S 64° 42′ 43.8″ W, 1610 m.a.s.l.) (Fig. 1).
Maps of the study area. A Map of Urophonius brachycentrus and U. achalensis species collection sites, map in upper right corner indicating Argentina and the approximate study area delimited with a red square. B Limit between one of the sympatric zones with the allopatric population of U. achalensis, separated by the “Río Yuspe” (Yuspe River). C Map of temperature range in the collection sites. Color reference indicated in lower right corner. Black dots: U. achalensis; white dots, U. brachycentrus (Color figure online)
Processing of Individuals and Selected Characters
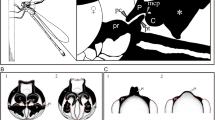
Collected individuals were first identified and sexed (Ojanguren-Affilastro, 2005) with a Nikon SMZ 1500 stereo microscope and preserved in 80% EtOH within glass containers for morphological studies. Our study encompassed both classical and geometric morphometric analyses involving measurements of characters that were compared between sexes and species across different contexts (sympatry versus allopatry) (Table 1; Fig. 2). We selected characters used during feeding, defense, and courtship such as pedipalps, chelicerae and telson vesicle (Table 1; Fig. 2). Also, we considered characters used only in a sexual context, such as those involved in female stimulation (male telson gland) and those facilitating the grasping of the female pedipalps (male pedipalp apophyses) (Table 1; Fig. 2). Finally, we measured genital characters involved in sperm transfer that have also been shown to be under sexual selection pressures (Olivero et al., 2015; Peretti, 2010) (Table 1; Fig. 2). To analyze the selected characters, individuals were dissected, and internal structures were extracted with fine tweezers for photographic treatment. The measurements of individuals were taken from images captured under the stereo microscope with a digital coupled camera (Nikon Digital Sight DS-FI1-U2). Because the internal female genitalia consist of flexible structures that vary in size and shape according to the female mating status (Peretti, 2010), morphometric analysis was not performed. In subsequent analyses, individuals, and characters with damaged or incomplete portions were not considered.
Selected characters for morphological study in Urophonius achalensis and U. brachycentrus. A General diagrams of measured somatic and genital characters. B Prosoma. C Lateral view of the male pedipalp. D Apophysis of the male pedipalp. E Lamella of the hemispermatophore. F Capsular lobe of the hemispermatophore. G Lateral view of male telson. H Dorsal view of male telson with telson gland. ap pedipalp apophysis; ch chelicerae; cl hemispermatophore capsular lobe; e median eye; fc hemispermatophore frontal crest; ff pedipalp fixed finger; la hemispermatophore lamella; mf pedipalp mobile finger; pe pedipalp; pr prosoma; st sting; tg telson gland; tr hemispermatophore trunk; tv telson vesicle. References: Red dots, Landmarks position (details in Tables 1, S1); black dotted line, character analyzed with semilandmarks; red dotted line, character analyzed by elliptical Fourier analysis. Scales: A = 5 mm in scorpion, 0.5 mm in hemispermatophore, B, C, G, H = 1 mm; D–F = 0.5 mm
Morphometric Studies
Classic Morphometric Analysis
For the analysis of chelicerae and the pectines, we employed linear measurements due to methodological constraints in applying geometrical morphometrics. We measured both absolute and relative lengths, with prosome length serving as body size proxy (McLean et al., 2018) (Table 1). These measurements were acquired from photographs obtained for each character using ImageJ software tools (Schneider et al., 2012). To ensure accuracy, measurements were taken three times by the same person, and the measurement error was calculated (Sokal & Rohlf, 1995).
Geometric Morphometric Analysis
We captured digital images of the selected characters in both male and female scorpions, with a scale close to character, and the images were assembled with TPSutil software (Rohlf, 2015). Sets of anatomical landmarks (Bookstein, 1991) and semilandmarks were established using TPSDig2 (Bookstein, 1997; Gunz & Mitteroecker, 2013; Rohlf, 2004). We placed landmarks in the prosome, the hemispermatophore lamella, the pedipalp, and the apophysis of this structure (Tables 1, S1; Fig. 2). In cases where curvatures between adjacent landmarks were of interest, sliding landmarks or semilandmarks were used to provide additional geometric information, specifically in the pedipalp, the pedipalp apophysis, and the hemispermatophore lamella (Fig. 2). For other characters such as the hemispermatophore capsular lobe, telson vesicle and the telson gland we quantified shape using an elliptic Fourier analysis (EFA) (following Santibáñez-López et al., 2017, 2021) that allowed us to explore subtle differences in defined shapes from contour characterization (Ferson et al., 1985; Hammer & Harper, 2006; Kuhl & Giardina, 1982) (Fig. 2).
The shape coordinates of each character were subjected to a Generalized Procrustes Analysis (GPA) (Gower, 1975) with the ‘gpagen’ function of the geomorph package (Adams et al., 2017; Schlager, 2017) in R software (R Core Team, 2021) to remove non-shape variables (translation, rotation, size) from the dataset to compare shape by contrasting with a mean generated from a consensus matrix (Adams et al., 2017; Rohlf & Slice, 1990). The size proxy of each character was retained from the GPA analysis (i.e., centroid size) for subsequent analyses (Bookstein, 1991; Zelditch et al., 2004). To account for semilandmarks in the GPA calculation, we used the ‘slider2d’ function of the Morpho package (Schlager et al., 2021). EFA was performed using the momocs package (Bonhomme et al., 2014; Iwata & Ukai, 2002).
We conducted a Principal Component Analysis (PCA) to visualize and explore the general trends in the distribution of total morphological variation in morphospace from both the data yielded by the GPA as well as the data obtained from the EFA using the ‘plotTangentSpace’ function of the geomorph package. Principal components can be viewed as reorganized and uncorrelated morphological features representing distinct aspects of the total shape variation within the dataset. Additionally, vectors that reflected shape variation along x/y axes were used to visualize magnitudes and overall shape changes with the geomorph package (Bookstein, 1991). We performed a multivariate analysis of variance (MANOVA) with the function ‘procD.lm’ of the geomorph package with resampling permutations procedure to calculate the significance of shape variables. We focused on the variation in shape of the first two principal components (since they captured more than 70% of the morphological variation). First, we checked the allometric component (influence of size on shape) of the characters with the functions ‘procD.lm’ and ‘plotAllometry’ of the geomorph package. If we found allometry in the sample, we calculated residual values of the shape variables for subsequent analyses (Outomuro & Johansson, 2017).
Statistical Analysis to Test RCD
To compare the measurements obtained by classical and geometric morphometrics between species and contexts (sympatry versus allopatry) we utilized linear mixed models (LMMs) in R. Separate models were conducted for each character and sex (because in some characters the number of landmarks was not equal for males and females) where we set as response variables the linear measurements, size variables (centroid size) or shape variables (PCs scores) and the fixed effects were species (levels: U. achalensis/U. brachycentrus) and contexts (levels: sympatry/allopatry). The interaction between these fixed effects was evaluated to corroborate RCD patterns. We added populations of origin as random effects to account for the variability contributed to this factor. Due to the influence of altitude on morphological variability (see section “Influence of Environmental Factors on Morphological Characters”), we added the altitude where individuals were collected as another random effect. Analyses were performed with the package lme4 (Bates et al., 2011) and lsmeans (Lenth, 2016) for a posteriori test (with Bonferroni correction) if necessary. Model validation was assessed graphically and by residual analysis.
Influence of Environmental Factors on Morphological Characters
In addition, a subset of data was subjected to an analysis to investigate whether environmental factors might correlate with any of the phenotypic characters measured. We recognized that factors such as clinal or geographic variation in our study system could potentially influence the observed patterns (Goldberg & Lande, 2006). As altitude may be strongly associated with temperature and humidity, we considered the variation of these environmental variables in our analysis. We obtained the mean annual temperature and mean annual rainfall of the study area from Geoportal IDESA http://geoportal.idesa.gob.ar (data from last year available: 2017). With the QGIS program 3.26 (QGIS Development Team, 2020), we mapped the distribution of the collected individuals (using the geo-referenced latitude and longitude data for each individual). We used the ‘extractRandomClim’ function of the raster package (Hijmans et al., 2015) in R to extract the values of the variables of interest for each collection point. Subsequently, we explored the relationships between these environmental factors with size (centroid size, absolute length) and shape (PCs scores) previously calculated (see section “Geometric Morphometric Analysis”) with linear mixed models (LMMs). We acknowledge that other environmental factors (e.g., soil characteristics, atmospheric pressure, food availability) may affect some of the phenotypic variation among species and populations. Still, the scoring of these factors was beyond the scope of this study, so our estimates of environmental effects on phenotype are prospective.
Results
Morphological Variation Across Contexts
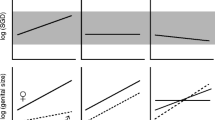
We compared multiple genital and somatic characters in males and female scorpions from sympatric and allopatric contexts. We observed different patterns of phenotypic variation in different directions (convergences and divergences) in each species (Fig. 3), and the shape and size appear to respond independently to different selective pressures. The morphometric results for each character analyzed in both sexes are detailed below, first evaluating the size and then the variation in shape.
Diagrams showing the summary of morphological variation in size and shape of somatic and genitalia characters in scorpions in different contexts of sympatry and allopatry. Each character is scaled at the intrasexual level. Gray area in the middle of the plate indicates sympatric zone. Gray arrows, characters undergoing convergence; black arrows, characters undergoing divergence (RCD). ♂: males, ♀: females, ≠ : Statistical differences between species in sympatry (Color figure online)
Chelicerae and Pecten: Asymmetric Convergence in Size Only in Females
We observed an asymmetric convergence in the absolute length of both chelicerae (χ2 = 34.180, p < 0.001) and pectines (χ2 = 45.894, p < 0.001) in females (being U. brachycentrus more similar to U. achalensis in sympatry) (Fig. 3). Neither contexts nor species showed differences in the relative lengths of chelicerae or pectines. We only found interspecific differences in the relative cheliceral length in males, with U. brachycentrus males having larger chelicerae (χ2 = 64.348, p < 0.001) than U. achalensis males. However, all the other variables did not differ between species or contexts.
Prosome and Telson Vesicle: Size Convergence
The centroid size of the prosome exhibited symmetric convergence in females of both scorpion species, with species becoming more similar in sympatry than in allopatry (χ2 = 26.907, p < 0.001). In males, however, we noted asymmetric convergence, with U. brachycentrus more similar in sympatry than in allopatry) (χ2 = 8.507, p = 0.004) (Fig. 3). In terms of shape, the Procrustes MANOVA showed no significant variation according to species and context. PC1 comprised almost half of the morphological variation (Females: 46.49%, Males: 45.85%), showing interspecific differences (U. brachycentrus more compressed prosome than U. achalensis) (Females: χ2 = 31.992, p < 0.001; Males: χ2 = 19.895, p < 0.001) (Fig. 3). PC2 explained an 18.44% of the variation in females and 13.82% in males and showed no differences between species or contexts in either sex. PC3 accounted for 13.37% of the variability in females without differences between species or contexts. In contrast, PC3 in males, representing 12.52% of morphological variability, was different between species (χ2 = 9.783, p = 0.002) and contexts (χ2 = 6.827, p = 0.006) but we found no significant interaction between these factors.
Regarding the telson vesicle, in females, we found a pattern of symmetric convergence in the centroid size with both species becoming more similar in sympatry than in allopatry (χ2 = 32.176, p < 0.001) (Fig. 3). In males the convergence was asymmetric, as only males of U. brachycentrus presented a shift in the size of this character in sympatry (χ2 = 6.118, p = 0.013). The Procrustes MANOVA showed significant shape variation according to species in both sexes (Females: F = 4.269, p = 0.001; Males: F = 4.404, p = 0.001), but the interaction between species and context was not significant (Fig. 3). In females, we found significant differences between species in telson vesicle shape reflected in PC1 (54%) (χ2 = 22.441, p < 0.001) and PC2 (19.57%) (χ2 = 21.034, p < 0.001). Also, in males, PC1 (67.48%) showed differences between species (χ2 = 36.965, p < 0.001) (Fig. 3), while in PC2 (12.21%) there were no significant differences between species or contexts.
Pedipalp in Females: Asymmetric Convergence in Size and Divergence in Shape
We found asymmetric convergence in pedipalp centroid size, with species more similar in sympatry than in allopatry due to a shift of U. brachycentrus (χ2 = 19.812, p < 0.001) (Figs. 3, 4A). In terms of shape, the Procrustes MANOVA showed significant variation according to species and context (F = 7.788, p = 0.001). PC1 explained 38.10% of morphological variability, and we found asymmetric divergence in PC1, with U. brachycentrus females showing a shift relative to sympatric U. achalensis females and allopatric females (χ2 = 8.294, p = 0.004) (Fig. 4B). PC2 explained 26.95% and PC3 10.60% of morphological variation although these shape variables showed no significant differences between species or contexts.
Interspecific and intraspecific morphological variation in pedipalp and male pedipalp apophysis in Urophonius achalensis and U. brachycentrus from sympatric and allopatric zones. A Pedipalp size of males (first box) and females (second box) indicated by centroid size. B Pedipalp shape (PC1) of males (first box) and females (second box) and differences between species and contexts. C Shape of pedipalp apophysis of males (PC2) and differences between species and contexts. Statistical differences indicated in each graph: continued line showed interspecific differences, dashed line: intraspecific differences (between allopatric and sympatric contexts), ♂: males, ♀: females. D Male pedipalp morphospace indicating the morphological distribution of individuals along two principal components of variation. Numbers in parentheses on each axis showing percentage of variance explained by each principal component. Color reference following A–C. E Summary of morphological changes in PC scores of extremes individuals (minimum in sympatric population and maximum in allopatric population) of U. brachycentrus, Top: shape of male pedipalp (PC1 scores); Below: shape of male pedipalp’ apophysis (PC2 scores); black dots showing landmarks and semilandmarks showing consensus conformation, orientation of arrows (vectors) indicating direction of morphological change and arrow longitude indicating magnitude of change (Color figure online)
Pedipalp and Apophysis in Males: Asymmetric Divergence in Shape
Male pedipalp size showed only interspecific differences, with larger pedipalp and apophysis in U. achalensis than U. brachycentrus (χ2 = 84.839, p < 0.001) (Figs. 3, 4A). The Procrustes MANOVA showed significant variation by species and context (F = 3.321, p = 0.006). Regarding the pedipalp, PC1 explained 45.25% of the morphological variability, and we found a pattern of asymmetric divergence in PC1 (U. brachycentrus males with higher pedipalp and shorter fixed fingers than allopatric males and sympatric U. achalensis males) (χ2 = 10.069, p = 0.002) (Fig. 4B, D, E). PC2 accounted for 20.21% and PC3 a 9.99% of the variability, and this component showed no differences between species or contexts (Fig. 4D).
For the pedipalp apophysis size, we found interspecific differences (χ2 = 38.651, p < 0.001), with apophysis of U. achalensis being larger than those of U. brachycentrus (Figs. 3, 4C). The Procrustes MANOVA showed significant variation in the interaction between species and context (F = 3.419, p = 0.014). PC1 (accounting for 31.11% of the variation) showed no significant differences between species or contexts. In contrast, PC2 explaining 21.07% of the morphological variation, showed significant differences between species in sympatry, and not in allopatry (χ2 = 10.221, p = 0.002) (Fig. 4C, E). Moreover, the shape of the apophysis was different between sympatric and allopatric populations of U. brachycentrus so that this displacement pattern would be an asymmetric divergence. Morphological variability was also distributed between PC3 (9.34%) and PC4 (8.56%), although these variables were not different between contexts and only between species in PC4 (χ2 = 8.685, p = 0.003).
Telson Gland: Asymmetric Convergence in Size
Telson gland size showed a pattern of asymmetric convergence, with U. brachycentrus males more similar to U. achalensis males in sympatry and differing significantly from allopatric population males (with smaller gland) (χ2 = 10.087, p = 0.002) (Fig. 3). The Procrustes MANOVA showed significant variation only according to species (F = 155.064, p < 0.001), but the interaction between species and context was not significant. Regarding shape, PC1 almost completely comprised all morphological variability (92.81%), and we only found significant interspecific differences (U. brachycentrus showing a more compressed and wider telson gland than U. achalensis) (χ2 = 155.774, p < 0.001). PC2, with an explanation of only 2.86% of the morphological variation, did not differ between species or contexts.
Hemispermatophore Lamella: Asymmetrical Divergence in Shape
Hemispermatophore lamella size varied only at the interspecific level (χ2 = 86.714, p < 0.001), with lamella of U. achalensis males always being larger than those of U. brachycentrus (Figs. 3, 5A). In terms of shape, the Procrustes MANOVA showed significant variation according to species and context (F = 3.223, p = 0.006). Almost half of the lamella morphological variation was represented by PC1 (43.41%) (Fig. 5B, C). This shape showed asymmetric divergence, as U. brachycentrus males differed from their allopatric conspecifics with a wider lamella, also differing from sympatric U. achalensis males (χ2 = 6.791, p = 0.009) (Fig. 5C, D). PC2 comprised 15.33% and the PC3 14.02% of the morphological variation but these shape variables showed no differences between species or contexts (Fig. 5C).
Interspecific and intraspecific morphological variation in the hemispermatophore lamella of Urophonius achalensis and U. brachycentrus males from sympatric and allopatric zones. A Size of hemispermatophore lamella indicated by centroid size. B Hemispermatophore lamella shape (PC1) and differences between species and contexts. Statistical differences indicated in each graph: continued line showed interspecific differences, dashed line: intraspecific differences (between allopatric and sympatric contexts). C Morphospace indicating the morphological distribution of individuals along two principal components of variation. Numbers in parentheses on each axis showing percentage of variance explained by each principal component. Color reference following A, B. D Summary of morphological changes in PC1 scores of extremes individuals (maximum in sympatric population and minimum in allopatric population) of U. brachycentrus, black dots showing landmarks and semilandmarks showing consensus conformation, orientation of arrows (vectors) indicating direction of morphological change and arrow longitude indicating magnitude of change (Color figure online)
Hemispermatophore Capsular Lobes: Asymmetrical Divergence in Size
We found a pattern of asymmetric divergence in the hemispermatophore capsular lobe size, with males of U. brachycentrus in sympatry having larger lobes than the rest of the male groups (χ2 = 12.784, p < 0.001) (Fig. 3). We found no significant interaction between species and context in the Procrustes MANOVA, but there was variation in shape according to species (F = 4.847, p = 0.001). PC1 explained 31.96% and PC3 16.19% of the morphological variance, and none of the shape variables resulted in a difference between species or contexts. PC2 accounted for the 25.52% and differed between contexts (χ2 = 3.926, p = 0.048) and marginally between species (χ2 = 3.319, p = 0.068), but the interaction between context and species was not significant.
Influence of Environmental Factors on Morphological Characters
We found that the size (centroid size and absolute length) of nearly all the characters we examined exhibited variations correlated with temperature (Table 2). We found a significant statistical interaction between temperature and species in all cases, so temperature-dependent morphological variations were observed only in U. brachycentrus, with no relationship in U. achalensis. In general, both sexes of this species had larger characters in colder areas (at higher altitudes) and smaller characters in warmer areas (at lower altitudes). This pattern was evident in the prosome, pedipalp, chelicerae, pecten, and telson vesicle for both sexes. In males, we also found this same pattern of variation in U. brachycentrus for the telson gland and genital characters, though it was not apparent in the pedipalp apophysis. The observed variation in the size of many characters aligns with the asymmetric convergence found in U. brachycentrus. The shape of none of the analyzed structures showed variation with temperature (Table 2). Our analysis of humidity (rainfall) also revealed patterns of morphological variation of some characters regarding this environmental factor (Table 2). We observed that females of both species exhibited a larger prosoma in more humid areas. Additionally, we found an interaction between humidity and species for telson gland shape (PC1). That is, in U. brachycentrus, males displayed a gland with negative PC1 values in more humid areas. This morphological change was associated with a more slender and less rounded gland. The shape of no other character was affected by humidity.
Discussion
We found great morphological variability between sympatric and allopatric contexts, as well as along the environmental cline in our model scorpion species. This study provided valuable and novel insights into the evolution of somatic and genital characters within an understudied animal model, but with great potential for further research. We uncovered complex patterns of phenotypic variation in different directions, both convergences and divergences in size and shape, which suggest a mosaic evolution in certain sexual characters in these scorpions. Convergence patterns were primarily attributed to an increase in size under lower temperatures, making species more similar at higher altitudes. Our findings suggest an asymmetric RCD in the shape of certain sexual characters of both sexes key for courtship success (i.e., grasping characters) and sperm transfer (i.e., genital characters of the hemispermatophore). In the following discussion, we analyze in depth these patterns of phenotypic variation, explore the possible selection pressures underlying this variability, and consider the implications of the RCD for the mating system and coexistence for these scorpion species.
Size Convergence of Multiple Characters Along an Environmental Cline
Individuals of U. brachycentrus exhibited an increase in size in various characters at lower temperatures (higher altitude) becoming more similar to heterospecifics in sympatric areas (convergence pattern). Notably, this morphological pattern in size aligns with the Atkinson’s rule (1994, 1995), which predicts larger body sizes at lower temperatures (Horne et al., 2015). Many ectotherms grow more slowly and mature with larger body sizes in colder environments (Angilletta et al., 2004). This increase in size may be adaptive as it may enhance fecundity, survival, or reproductive rates (Stearns, 1992). Scorpions are known to be influenced by the number of molts or the intermolt period, which can impact their final body size (Sarmento et al., 2008; Seiter et al., 2020). In scorpions, geographic variability has been documented (Abdel-Nabi et al., 2004; Harington, 1983; Olivero et al., 2012, 2015; Yamashita & Rhoads, 2013), and the size of individuals can be influenced by environmental gradients (Jochim et al., 2020; Lira et al., 2021). For example, Jochim et al. (2020), studying the morphology of a species complex of the Family Vaejovidae, found a pattern of morphological convergence similar to our results, with larger individuals at higher elevations in mountainous regions of Arizona. These authors argue that these scorpions probably follow Bergmann’s rule, although they do not discuss these aspects further (Jochim et al., 2020). Bergmann’s ecological rule (1847) was initially formulated for homeotherms, predicting larger body sizes at higher latitudes (with colder climates). Its application to poikilotherms has yielded mixed evidence, with both supporting and contradictory findings (Angilletta & Dunham, 2003; Shelomi, 2012; Vinarski, 2014). The evaluation of the relevance of eco-geographical rules in scorpions remains a topic that warrants further investigation in future research, and it would also be interesting to consider the phylogeographic perspective of our results by considering a larger number of populations.
Temperature is predicted to impact the body size of individuals of both sexes similarly (Hirst et al., 2015), and in U. brachycentrus, we found that males and females increase in size with cooler temperatures. However, this increment manifests in different characters for each sex. This discrepancy may be attributed to sexual dimorphism resulting from different life habits or sex-specific phenotypic plasticity (Blanckenhorn et al., 2006; Fairbairn, 2005; Stillwell & Fox, 2007). Females had a general increase in size, which includes their chelicerae, a key character for excavation and dig gestation chambers (Maury, 1968, 1969, 1977). Males exhibited size increases in the body and telson gland size, a character used in sexual interactions (Olivero et al., 2015; Peretti, 1997). The telson gland shape changes linked to humidity are intriguing. This gland produces a waxy secretion that could be directly influenced by this environmental factor. This finding allows future studies aimed at manipulating this parameter to investigate its effects on secretory properties and its role during sexual interactions.
Reproductive Character Displacement in Somatic and Genital Characters
We found evidence of RCD both in the shape and size of multiple somatic characters in U. brachycentrus, while U. achalensis showed no divergence in any character between sympatric and allopatric contexts. In sympatry, individuals of U. brachycentrus, including both males and females, exhibited more globose pedipalps and males had more deeply curved apophyses compared to their allopatric conspecifics and the sympatric U. achalensis. It is noteworthy that RCD patterns were only found in the shape of somatic characters, not in their size. Furthermore, the pressures that drove this divergence were centered in the pedipalps, a particular trait since it serves both a sexual context and other life activities (e.g., feeding, defense). However, within the pedipalp, the apophysis also displayed a clear and specific RCD pattern, and this character is solely used for a sexual function: securing the female during grasping. In the heterospecific courtship, events of female resistance (pulling in the opposite direction to the male) have been shown to be a selective filter imposed by the female as a behavioral reproductive barrier (Oviedo-Diego et al., 2022). RCD may reinforce this mechanism, leading to mechanical incompatibilities (the “lock-and-key” mechanism) that can hinder the completion of heterospecific mating, and might promote reproductive isolation between species (Eberhard, 2004). The evolution of behaviors in one sex (i.e., female resistance) linked to the morphological divergence of associated characters raises questions about the timing of the appearance of these barriers, why they appear together, and the interplay between mechanisms of natural and sexual selection in scorpions.
On the other hand, we found evidence of RCD in characteristics of the hemispermatophores of U. brachycentrus, that exhibit larger capsular lobes and more compressed lamella compared to allopatric males and sympatric U. achalensis males. The larger capsular lobules in this species could be partly attributed to the increased size of females of this species in the sympatric zone, as morphological complementarity is expected for mechanical isolation by the “lock-and-key” mechanism during sperm transfer. While there are instances supporting these mechanism in arthropods (Kubota et al., 2013; Mikkola, 1992, 2008; Nagata et al., 2007; Nishimura et al., 2022; Sota & Kubota, 1998; Sota & Tanabe, 2010; Takami et al., 2007; Tanabe & Sota, 2008; Usami et al., 2006; Wojcieszek, & Simmons, 2012), it has been rejected in several species because, in general, genitalia diverge much more in males than in females, and it is less common to find morphological complementarity (Eberhard, 1985; Masly, 2012; Shapiro & Porter, 1989). To assert that this mechanism occurs in these species, it would be necessary to evaluate the female component and the fit between the genital components of the two species in heterospecific matings. Because the female genital atrium is flexible and has a relatively ‘‘uniform’’ structure (Peretti, 2003, 2010), it would not be expected that female genitalia would mechanically exclude the entry of heterospecific male genitalia, although copulatory mechanics studies would be required to for confirming this.
Moreover, the capsular lobes possess micro-ornamentations that come into contact with the female genital atrium wall (Peretti, 2003), potentially serving a stimulatory role. Larger capsular lobes might be associated with a greater contact surface of ornamentations with the female genital atrium and consequently lead to increased stimulation, which could be linked to cryptic female choice (Peretti, 2003, 2010). Interestingly, certain portions of the lamina have been reported to be under sexual selection pressures (Monod et al., 2017; Peretti, 2003; Peretti et al., 2001). During the copulatory process, the frontal crest of the lamella spermatophore fits into the inter-coxal space of the female, and there could be a ‘passive’ choice by ‘mechanical adjustment’ (Eberhard, 1985; Huber & Eberhard, 1997; Peretti, 2003, 2010). This challenges our understanding of the driving forces behind the morphological evolution of genitalia in these species. Future studies will aim to assess the strength of these selective forces in the different subunits of the same complex structure, by analyzing the evolution of modules and their integration (Genevcius & Schwertner, 2017; Genevcius et al., 2020).
This interaction between sexual and natural selection could provide an explanation for the evolution of genitalia in these species. Males face an intense competition at the both intra- and interspecific levels and exhibit indiscriminateness in their mate decisions (scenario of promiscuity) (Oviedo-Diego et al., 2021). Thus, females must not only exert mate choice at the pre-copulatory level, but copulatory and post-copulatory mechanisms seem to be necessary to avoid hybridization (Oviedo-Diego, 2022; Oviedo-Diego et al., 2022). A similar example seems to occur in hybridizing Drosophila species where the male genitalia differ in size and shape, while the external female genitalia remain uniform across species (Coyne, 1983). During interspecific mating, the intrusion of the male genitalia differentially contacts the female genitalia so that females can store and use sperm based on the specific male’s identity (i.e., cryptic reproductive isolation) (Price et al., 2001). It is increasingly recognized that mate choice and specific recognition are part of a continuum, and the forces of sexual and natural selection may interact in multiple ways to explain patterns of sexual diversification across species (Boake et al., 1997; Liou & Price, 1994; Mendelson & Shaw, 2012; Ryan & Rand, 1993).
Species Asymmetry in Morphological Variability
Asymmetric RI and RCD have been documented multiple times (Bordenstein et al., 2000; Cooley, 2007; Cooley et al., 2006; Costa-Schmidt & Machado, 2012; Hochkirch et al., 2007; Pfennig & Simovich, 2002; Smadja & Ganem, 2005) and it generally occurs when there are interspecific differences in the intensity of selective pressures to avoid heterospecific interactions, primarily because species face different costs associated with the RI (Cooley, 2007; Pfennig & Simovich, 2002). Moreover, asymmetric outcomes in morphological variability between species may indicate interspecific differences in morphological plasticity. Divergent characters can also be plastic or can be expressed facultatively when individuals face competition with heterospecifics, so plasticity has been a proposed mechanism to explain character displacement (Pfennig & Murphy, 2002; Pfennig & Pfennig, 2010; Rice & Pfennig, 2007; Robinson & Wilson, 1994; Stuart et al., 2017). Species with broad distributions, exposed to a wide range of environmental conditions and with ample genetic variation, may display remarkable phenotypic plasticity (DeWitt & Scheiner, 2004; Lavergne et al., 2004; Pigliucci et al., 2006). For example, Crowder et al. (2010) found that the globally distributed whitefly Bemisia tabaci biotype exhibited greater plasticity in reproductive behavior, which could result in greater success in avoiding the costs of RI than other biotypes.
Here, Urophonius species present asymmetries in their RI degree, as males of U. brachycentrus tend to be more indiscriminate in their mating decisions than males of U. achalensis (Oviedo-Diego et al., 2021). Moreover, U. brachycentrus exhibit a higher male-biased operational sex ratios than U. achalensis in the sympatric zone (Oviedo-Diego, 2022), implying that males of this species face more intense competition to find females, therefore, experience greater costs due to RI (Oviedo-Diego et al., 2020, 2021). In turn, U. brachycentrus showed the most remarkable morphological variations, being the most widely distributed species compared to U. achalensis, endemic to the highland area under analysis (Acosta, 1985, 1993; Ojanguren-Affilastro, 2005; Ojanguren-Affilastro et al., 2020). Furthermore, this species exhibited morphological changes associated with an environmental gradient, stressing a great phenotypic plasticity. This increased plasticity may be related to the morphological changes suffered by this species, such as the observed reproductive character displacement (RCD) in the sympatric zone. This complex social and geographic scenario could subject this species to strong selective pressures for interspecific recognition during mating or sperm transfer and the existence of asymmetric RCD patterns.
Mixed Selective Pressures on Multiple Characters in Scorpions
Our results reveal a remarkable morphological variability in the size and shape of somatic and genital characters in two scorpion species that may be reflecting different evolutionary responses in part by natural selection pressures linked to geographic and environmental variations and species recognition mechanisms, and in part by sexual selection pressures at the intra- and interspecific level. We report a pattern of asymmetric morphological divergence where one of the scorpion species (U. brachycentrus) exhibited an increase in size in several characters at lower temperatures becoming more similar to heterospecifics in sympatric areas. These changes may reflect a plastic and adaptive response to these environments, with the size of these traits being shaped by natural selection. However, the increase in size and a scenario of promiscuity probably led to certain characters undergoing intense sexual selection pressures. On the other hand, key mating success-related traits, like grasping or genital characters, exhibited morphological divergence between species in the sympatric area (RCD pattern), reflecting the action of natural selection, possibly to avoid interbreeding due to mechanical incompatibilities between species.
Peretti (2010) emphasized the presence of mixed patterns in scorpion genitalia, where morphological complexity arises from different selective regimes. Similar observations have been made in other arachnids (Huber, 1996, 2004) and insects (Frazee & Masly, 2015; House et al., 2013; Rowe & Arnqvist, 2012; Simmons et al., 2009; Song & Bucheli, 2010; Song & Wenzel, 2008), where characters are subject to multiple, often conflicting pressures. Studies in water striders, for instance, suggest that the non-intromittent genitalia undergo varying degrees of selection (Bertin & Fairbairn, 2005; Danielsson & Askenmo, 1999; Rowe & Arnqvist, 2012). Another example comes from dung beetles, like Onthophagus taurus, where different parts of male genitalia may be under different selective regimes (Simmons et al., 2009; Song & Wenzel, 2008): the shape of the aedeagus is subject to directional sexual selection, but genital sclerites that penetrate the female genitalia are under stabilizing and disruptive nonlinear selection (Simmons et al., 2009). Also, in this species the genitalia shape diverges rapidly due to directional sexual selection, whereas size remains unaffected in the process (Simmons et al., 2009). Similarly, the millipede Antichiropus variabilis has shown that genitalia shape responded to stabilizing pressures, supporting the occurrence of lock-and-key mechanisms, whereas genitalia size did not follow this pattern and responded to environmental gradients (Wojcieszek & Simmons, 2012). In summary, the size and shape of the same structure may respond in this mosaic manner, independently to different selective pressures, possibly due to genetic or developmental decoupling (Macagno et al., 2011; Richmond, 2014; Rowe & Arnqvist, 2012; Wojcieszek & Simmons, 2012). Future studies will aim to assess other environmental variables influencing shape, the consistency of these results with allometric patterns between populations, and the coevolution between female and male characters.
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Abdel-Nabi, I. M., McVean, A., Abdel-Rahman, M. A., & Omran, M. A. (2004). Intraspecific diversity of morphological characters of the burrowing scorpion Scorpio maurus palmatus (Ehrenberg, 1828) in Egypt (Arachnida: Scorpionida: Scorpionidae). Serket, 9(2), 41–67.
Acosta, L. E. (1985). Redescripción de Urophonius achalensis Abalos y Hominal, 1974 (Scorpiones, Bothriuridae). [Redescription of Urophonius achalensis Abalos & Hominal, 1974 (Scorpiones, Bothriuridae)]. Physis, 43(104), 5–12.
Acosta, L. E. (1993). Escorpiones y opiliones de la provincia de Córdoba (Argentina): diversidad y zoogeografía. [Scorpiones and Opiliones of Cordoba Province (Argentina): Diversity and zoogeography]. Bulletin De La Société Neuchâteloise Des Sciences Naturelles, 116(1), 11–17.
Acosta, L. E. (1995). The scorpions of the Argentinian Western Chaco. I. Community survey in the llanos district. Biogeographica, 7(4), 187–196.
Adams, D. C., Collyer, M. L., Kaliontzopoulou, A., & Sherratt, E. (2017). Geomorph: Software for geometric morphometric analyses. R package version 3.0.6. http://CRAN.R-project.org/package=geomorph
Agnarsson, I., Gotelli, N. J., Agostini, D., & Kuntner, M. (2016). Limited role of character displacement in the coexistence of congeneric Anelosimus spiders in a Madagascan montane forest. Ecography, 39(8), 743–753. https://doi.org/10.1111/ecog.01930
Angilletta, M. J., Jr., & Dunham, A. E. (2003). The temperature-size rule in ectotherms: Simple evolutionary explanations may not be general. The American Naturalist, 162(3), 332–342.
Angilletta, M. J., Jr., Steury, T. D., & Sears, M. W. (2004). Temperature, growth rate, and body size in ectotherms: Fitting pieces of a life-history puzzle. Integrative and Comparative Biology, 44(6), 498–509. https://doi.org/10.1093/icb/44.6.498
Armbruster, W. S., & Herzig, A. L. (1984). Partitioning and sharing of pollinators by four sympatric species of Dalechampia (Euphorbiaceae) in Panama. Annals of the Missouri Botanical Garden. https://doi.org/10.2307/2399053
Atkinson, D. (1994). Temperature and organism size: A biological law for ectotherms? Advances in Ecological Research, 25, 1–58. https://doi.org/10.1016/S0065-2504(08)60212-3
Atkinson, D. (1995). Effects of temperature on the size of aquatic ectotherms: Exceptions to the general rule. Journal of Thermal Biology, 20(1–2), 61–74. https://doi.org/10.1016/0306-4565(94)00028-H
Auber, M. (1963). Reproduction et croissance de Buthus occitanus Amx. Annales des Sciences Naturelles-Zoologie et Biologie Animale, 5(2), 273–285.
Barth, F. G. (1990). Spider courtship: Male vibrations, female responsiveness and reproductive isolation. In F. G. Gribakin, K. Wiese, & A. V. Popov (Eds.), Sensory systems and communication in arthropods (pp. 161–166). Birkhäuser.
Bates, D., Maechler, M., Bolker, B., Walker, S., Christensen, R. H. B., Singmann, H., Dai, B., Scheipl, F., & Grothendieck, G. (2011). Package ‘lme4’. Linear mixed-effects models using S4 classes. R package version, 1(6).
Bennet-Clark, H. C., & Ewing, A. W. (1970). The love song of the fruit fly. Scientific American, 223(1), 84–93.
Bergmann, K. G. L. C. (1847). Über die Verhältnisse der wärmeokönomie der Thiere zu ihrer Grösse. Göttinger Studien, 3, 595–708.
Bertin, A., & Fairbairn, D. J. (2005). One tool, many uses: Precopulatory sexual selection on genital morphology in Aquarius remigis. Journal of Evolutionary Biology, 18, 949–961. https://doi.org/10.1111/j.1420-9101.2005.00913.x
Blanckenhorn, W. U., Stillwell, R. C., Young, K. A., Fox, C. W., & Ashton, K. G. (2006). When Rensch meets Bergmann: Does sexual size dimorphism change systematically with latitude? Evolution, 60(10), 2004–2011. https://doi.org/10.1111/j.0014-3820.2006.tb01838.x
Blows, M. W. (2007). A tale of two matrices: Multivariate approaches in evolutionary biology. Journal of Evolutionary Biology, 20(1), 1–8. https://doi.org/10.1111/j.1420-9101.2006.01164.x
Boake, C. R., DeAngelis, M. P., & Andreadis, D. K. (1997). Is sexual selection and species recognition a continuum? Mating behavior of the stalk-eyed fly Drosophila heteroneura. Proceedings of the National Academy of Sciences of the United States of America, 94(23), 12442–12445. https://doi.org/10.1073/pnas.94.23.12442
Bonhomme, V., Picq, S., Gaucherel, C., & Claude, J. (2014). Momocs: Outline analysis using R. Journal of Statistical Software, 56(13), 24.
Bookstein, F. L. (1991). Morphometric tools for landmark data: Geometry and biology. Cambridge University Press.
Bookstein, F. L. (1997). Landmark methods for forms without landmarks: Morphometrics of group differences in outline shape. Medical Image Analysis, 1(3), 225–243. https://doi.org/10.1016/S1361-8415(97)85012-8
Bordenstein, S. R., Drapeau, M. D., & Werren, J. H. (2000). Intraspecific variation in sexual isolation in the jewel wasp Nasonia. Evolution, 54(2), 567–573. https://doi.org/10.1111/j.0014-3820.2000.tb00059.x
Brennan, P. L., & Prum, R. O. (2015). Mechanisms and evidence of genital coevolution: The roles of natural selection, mate choice, and sexual conflict. Cold Spring Harbor Perspectives in Biology, 7(7), a017749. https://doi.org/10.1101/cshperspect.a017749
Buchanan, K., Burt de Perera, T., Carere, C., Carter, T., Hailey, A., Hubrecht, R., Jennings, D. J., Metcalfe, N. B., Pitcher, T. E., Péron, F., Sneddon, L. U., Sneddon, C., Talling, J., Thomas, R., & Thompson, M. (2012). Guidelines for the treatment of animals in behavioural research and teaching. Animal Behavior, 83, 301–309.
Burdfield-Steel, E. R., & Shuker, D. M. (2011). Reproductive interference. Current Biology, 21(12), R450–R451.
Butlin, R. (1987). Speciation by reinforcement. Trends in Ecology & Evolution, 2(1), 8–13. https://doi.org/10.1016/0169-5347(87)90193-5
Carrera, P. C., Mattoni, C. I., & Peretti, A. V. (2009). Chelicerae as male grasping organs in scorpions: Sexual dimorphism and associated behaviour. Zoology, 112(5), 332–350. https://doi.org/10.1016/j.zool.2009.01.002
Chantall-Rocha, S., & Japyassú, H. F. (2017). Diffuse resistance courtship in the scorpion Rhopalurus rochai (Scorpiones: Buthidae). Behavioural Processes, 135, 45–55.
Cooley, J. R. (2007). Decoding asymmetries in reproductive character displacement. Proceedings of the Academy of Natural Sciences of Philadelphia, 156(1), 89–96.
Cooley, J. R., Marshall, D. C., Hill, K. B., & Simon, C. (2006). Reconstructing asymmetrical reproductive character displacement in a periodical cicada contact zone. Journal of Evolutionary Biology, 19(3), 855–868. https://doi.org/10.1111/j.1420-9101.2005.01056.x
Costa-Schmidt, L. E., & de Araújo, A. M. (2010). Genitalic variation and taxonomic discrimination in the semi-aquatic spider genus Paratrechalea (Araneae: Trechaleidae). The Journal of Arachnology, 38(2), 242–249. https://doi.org/10.1636/JOA_A09-75.1
Costa-Schmidt, L. E., & Machado, G. (2012). Reproductive interference between two sibling species of gift-giving spiders. Animal Behaviour, 84(5), 1201–1211. https://doi.org/10.1016/j.anbehav.2012.08.026
Cothran, R. D. (2015). The importance of reproductive interference in ecology and evolution: From organism to communities. Population Ecology, 57, 339–341. https://doi.org/10.1007/s10144-015-0488-z
Coyne, J. A. (1983). Genetic basis of differences in genital morphology among three sibling species of Drosophila. Evolution. https://doi.org/10.2307/2408834
Coyne, J. A., & Orr, H. A. (2004). Speciation. Sinauer Associates.
Crowder, D. W., Sitvarin, M. I., & Carrière, Y. (2010). Plasticity in mating behaviour drives asymmetric reproductive interference in whiteflies. Animal Behaviour, 79(3), 579–587. https://doi.org/10.1016/j.anbehav.2009.11.025
Dame, E. A., & Petren, K. (2006). Behavioural mechanisms of invasion and displacement in Pacific Island geckos (Hemidactylus). Animal Behaviour, 71(5), 1165–1173. https://doi.org/10.1016/j.anbehav.2005.10.009
Danielsson, I., & Askenmo, C. (1999). Male genital traits and mating interval affect male fertilization success in the water strider Garris lacustris. Behavioral Ecology and Sociobiology, 46(3), 149–156. https://doi.org/10.1007/s002650050604
De La Serna De Esteban, C. J. (1978). Las glándulas tegumentarias del metasoma en algunas especies argentinas de la familia Bothriuridae (Scorpiones). [The tegumentary glands of the metasoma in some Argentine species of the family Bothriuridae (Scorpiones)]. Physis, 94, 121–132.
DeWitt, T. J., & Scheiner, S. M. (2004). Phenotypic plasticity: Functional and conceptual approaches. Oxford University Press.
Dionisio-da-Silva, W., de Araujo Lira, A. F., & de Albuquerque, C. M. R. (2018). Distinct edge effects and reproductive periods of sympatric litter-dwelling scorpions (Arachnida: Scorpiones) in a Brazilian Atlantic Forest. Zoology, 129, 17–24.
Drury, J. P., Okamoto, K. W., Anderson, C. N., & Grether, G. F. (2015). Reproductive interference explains persistence of aggression between species. Proceedings of the Royal Society B: Biological Sciences, 282(1804), 20142256. https://doi.org/10.1098/rspb.2014.2256
Dyer, K. A., White, B. E., Sztepanacz, J. L., Bewick, E. R., & Rundle, H. D. (2014). Reproductive character displacement of epicuticular compounds and their contribution to mate choice in Drosophila subquinaria and Drosophila recens. Evolution, 68(4), 1163–1175. https://doi.org/10.1111/evo.12335
Eberhard, W. G. (1985). Sexual selection and animal genitalia. Harvard University Press.
Eberhard, W. G. (2004). Male–female conflict and genitalia: Failure to confirm predictions in insects and spiders. Biological Reviews, 79(1), 121–186. https://doi.org/10.1017/S1464793103006237
Eberhard, W. G. (2010). Evolution of genitalia: Theories, evidence, and new directions. Genetica, 138, 5–18. https://doi.org/10.1007/s10709-009-9358-y
Eberhard, W. G., & Lehmann, G. U. (2019). Demonstrating sexual selection by cryptic female choice on male genitalia: What is enough? Evolution, 73(12), 2415–2435. https://doi.org/10.1111/evo.13863
Fairbairn, D. J. (2005). Allometry for sexual size dimorphism: Testing two hypotheses for Rensch’s rule in the water strider Aquarius remigis. The American Naturalist, 166(S4), S69–S84. https://doi.org/10.1086/444600
Ferson, S., Rohlf, F. J., & Koehn, R. K. (1985). Measuring shape variation of two-dimensional outlines. Systematic Biology, 34(1), 59–68. https://doi.org/10.1093/sysbio/34.1.59
Fox, G. A., Cooper, A. M., & Hayes, W. K. (2015). The dilemma of choosing a reference character for measuring sexual size dimorphism, sexual body component dimorphism, and character scaling: Cryptic dimorphism and allometry in the scorpion Hadrurus arizonensis. PLoS ONE, 10(3), e0120392. https://doi.org/10.1371/journal.pone.0120392
Frazee, S. R., & Masly, J. P. (2015). Multiple sexual selection pressures drive the rapid evolution of complex morphology in a male secondary genital structure. Ecology and Evolution, 5(19), 4437–4450. https://doi.org/10.1002/ece3.1721
Garcia, C. F., Oviedo-Diego, M., Laino, A., Peterson, G., Mattoni, C. I., Peretti, A. V., & Ojanguren-Affilastro, A. A. (2021). Low temperatures induce physiological changes in lipids, fatty acids and hydrocarbons, in two rare winter scorpions of genus Urophonius (Scorpiones, Bothriuridae). Journal of Thermal Biology, 96, 102841. https://doi.org/10.1016/j.jtherbio.2021.102841
Garnier, S., Magniez-Jannin, F., Rasplus, J. Y., & Alibert, P. (2005). When morphometry meets genetics: Inferring the phylogeography of Carabus solieri using Fourier analyses of pronotum and male genitalia. Journal of Evolutionary Biology, 18(2), 269–280. https://doi.org/10.1111/j.1420-9101.2004.00854.x
Genevcius, B. C., & Schwertner, C. F. (2017). Strong functional integration among multiple parts of the complex male and female genitalia of stink bugs. Biological Journal of the Linnean Society, 122(4), 774–786. https://doi.org/10.1093/biolinnean/blx095
Genevcius, B. C., Simon, M. N., Moraes, T., & Schwertner, C. F. (2020). Copulatory function and development shape modular architecture of genitalia differently in males and females. Evolution, 74(6), 1048–1062. https://doi.org/10.1111/evo.13977
Goldberg, E., & Lande, R. (2006). Ecological and reproductive character displacement of an environmental gradient. Evolution, 60(7), 1344–1357. https://doi.org/10.1111/j.0014-3820.2006.tb01214.x
Goodman, A., & Esposito, L. (2020). Niche partitioning in congeneric scorpions. Invertebrate Biology, 139(1), e12280.
Gower, J. C. (1975). Generalized procrustes analysis. Psychometrika, 40(1), 33–51. https://doi.org/10.1007/BF02291478
Graham, M. R., Oláh-Hemmings, V., & Fet, V. (2012). Phylogeography of co-distributed dune scorpions identifies the Amu Darya River as a long-standing component of Central Asian biogeography: (Scorpiones: Buthidae). Zoology in the Middle East, 55(1), 95–110.
Grant, P. R. (1972). Convergent and divergent character displacement. Biological Journal of the Linnean Society, 4(1), 39–68. https://doi.org/10.1111/j.1095-8312.1972.tb00690.x
Grether, G. F., Losin, N., Anderson, C. N., & Okamoto, K. (2009). The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biological Reviews, 84(4), 617–663. https://doi.org/10.1111/j.1469-185X.2009.00089.x
Gröning, J., & Hochkirch, A. (2008). Reproductive interference between animal species. The Quarterly Review of Biology, 83(3), 257–282. https://doi.org/10.1086/590510
Gunz, P., & Mitteroecker, P. (2013). Semilandmarks: A method for quantifying curves and surfaces. Hystrix, the Italian Journal of Mammalogy, 24(1), 103–109. https://doi.org/10.4404/hystrix-24.1-6292
Hammer, Ø., & Harper, D. A. T. (2006). Morphometrics. In Ø. Hammer & D. A. T. Harper (Eds.), Paleontological data analysis (pp. 68–156). Blackwell Publishing.
Harington, A. (1983). Character variation in the scorpion Parabuthus villosus (Peters) (Scorpiones, Buthidae): A case of intermediate zones. Journal of Arachnology, 11, 393–406.
Hettyey, A., & Pearman, P. B. (2003). Social environment and reproductive interference affect reproductive success in the frog Rana latastei. Behavioral Ecology, 14, 294–300. https://doi.org/10.1093/beheco/14.2.294
Hijmans, R. J., Van Etten, J., Cheng, J., Mattiuzzi, M., Sumner, M., Greenberg, J. A., Lamigueiro, O. P., Bevan, A., Racine, E. B., Shortridge, A., & Hijmans, M. R. J. (2015). Package ‘raster’. R package, 734.
Hirst, A. G., Horne, C. R., & Atkinson, D. (2015). Equal temperature-size responses of the sexes are widespread within arthropod species. Proceedings of the Royal Society B: Biological Sciences, 282(1820), 20152475. https://doi.org/10.1098/rspb.2015.2475
Hochkirch, A., Gröning, J., & Bücker, A. (2007). Sympatry with the devil: Reproductive interference could hamper species coexistence. Journal of Animal Ecology, 76(4), 633–642. https://doi.org/10.1111/j.1365-2656.2007.01241.x
Horne, C. R., Hirst, A. G., & Atkinson, D. (2015). Temperature-size responses match latitudinal-size clines in arthropods, revealing critical differences between aquatic and terrestrial species. Ecology Letters, 18(4), 327–335. https://doi.org/10.1111/ele.12413
Hosken, D. J., & Stockley, P. (2004). Sexual selection and genital evolution. Trends in Ecology & Evolution, 19(2), 87–93. https://doi.org/10.1016/j.tree.2003.11.012
Hoskin, C. J., & Higgie, M. (2010). Speciation via species interactions: The divergence of mating traits within species. Ecology Letters, 13(4), 409–420. https://doi.org/10.1111/j.1461-0248.2010.01448.x
House, C. M., Lewis, Z., Hodgson, D. J., Wedell, N., Sharma, M. D., Hunt, J., & Hosken, D. J. (2013). Sexual and natural selection both influence male genital evolution. PLoS ONE, 8(5), e63807. https://doi.org/10.1371/journal.pone.0063807
House, C. M., & Simmons, L. W. (2005). The evolution of male genitalia: Patterns of genetic variation and covariation in the genital sclerites of the dung beetle Onthophagus taurus. Journal of Evolutionary Biology, 18(5), 1281–1292. https://doi.org/10.1111/j.1420-9101.2005.00926.x
Howard, D. J. (1993). Reinforcement: Origin, dynamics, and fate of an evolutionary hypothesis. In R. G. Harrison (Ed.), Hybrid zones and the evolutionary process (pp. 46–69). Oxford University Press.
Huber, B. A. (1996). Genitalia, fluctuating asymmetry, and patterns of sexual selection in Physocyclus globosus (Araneae: Pholcidae). Revue Suisse de Zoologie, 289–294.
Huber, B. A. (2004). Evidence for functional segregation in the directionally asymmetric male genitalia of the spider Metagonia mariguitarensis (González-Sponga) (Pholcidae: Araneae). Journal of Zoology, 262(3), 317–326. https://doi.org/10.1017/S0952836903004709
Huber, B. A., & Eberhard, W. G. (1997). Courtship, copulation, and genital mechanics in Physocyclus globosus (Araneae, Pholcidae). Canadian Journal of Zoology, 75(6), 905–918. https://doi.org/10.1139/z97-109
Iwata, H., & Ukai, Y. (2002). SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. Journal of Heredity, 93(5), 384–385.
Jang, Y., & Gerhardt, H. C. (2006). Divergence in female calling song discrimination between sympatric and allopatric populations of the southern wood cricket Gryllus fultoni (Orthoptera: Gryllidae). Behavioral Ecology and Sociobiology, 60, 150–158. https://doi.org/10.1007/s00265-005-0151-3
Jennions, M. D., & Kelly, C. D. (2002). Geographical variation in male genitalia in Brachyrhaphis episcopi (Poeciliidae): Is it sexually or naturally selected? Oikos, 97(1), 79–86. https://doi.org/10.1034/j.1600-0706.2002.970108.x
Jochim, E. E., Broussard, L. L. M., & Hendrixson, B. E. (2020). Integrative species delimitation and taxonomic status of the scorpion genus Vaejovis Koch, 1836 (Vaejovidae) in the Santa Catalina Mountains, Arizona. Euscorpius, 316, 1–11.
Kawakami, T., & Tatsuta, H. (2010). Variation in the shape of genital appendages along a transect through sympatric and allopatric areas of two brachypterous grasshoppers Parapodisma setouchiensis and Parapodisma subastris (Orthoptera: Podisminae). Annals of the Entomological Society of America, 103, 327–331. https://doi.org/10.1603/AN09074
Kawano, K. (2002). Character displacement in giant rhinoceros beetles. American Naturalist, 159, 255–271. https://doi.org/10.1086/338512
Klingenberg, C. P. (2009). Morphometric integration and modularity in configurations of landmarks: Tools for evaluating a priori hypotheses. Evolution & Development, 11(4), 405–421. https://doi.org/10.1111/j.1525-142X.2009.00347.x
Konuma, J., & Chiba, S. (2012). Ecological character displacement caused by reproductive interference. Journal of Theoretical Biology, 247(2), 354–364. https://doi.org/10.1016/j.jtbi.2007.03.013
Kosuda, S., Sasakawa, K., & Ikeda, H. (2016). Directional mitochondrial introgression and character displacement due to reproductive interference in two closely related Pterostichus ground beetle species. Journal of Evolutionary Biology, 29(6), 1121–1130. https://doi.org/10.1111/jeb.12852
Kraaijeveld, K., Kraaijeveld-Smit, F. J., & Maan, M. E. (2011). Sexual selection and speciation: The comparative evidence revisited. Biological Reviews, 86(2), 367. https://doi.org/10.1111/j.1469-185X.2010.00150.x
Kronforst, M. R., Young, L. G., & Gilbert, L. E. (2007). Reinforcement of mate preference among hybridizing Heliconius butterflies. Journal of Evolutionary Biology, 20(1), 278–285. https://doi.org/10.1111/j.1420-9101.2006.01198.x
Kubota, K., Miyazaki, K., Ebihara, S., & Takami, Y. (2013). Mechanical reproductive isolation via divergent genital morphology between Carabus insulicola and C. esakii with implications in species coexistence. Population Ecology, 55(1), 35–42. https://doi.org/10.1007/s10144-012-0335-4
Kuhl, F. P., & Giardina, C. R. (1982). Elliptic Fourier features of a closed contour. Computer Graphics and Image Processing, 18(3), 236–258. https://doi.org/10.1016/0146-664X(82)90034-X
Kyogoku, D. (2015). Reproductive interference: Ecological and evolutionary consequences of interspecific promiscuity. Population Ecology, 57(2), 253–260. https://doi.org/10.1007/s10144-015-0486-1
Lande, R., & Arnold, S. J. (1983). The measurement of selection on correlated characters. Evolution. https://doi.org/10.2307/2408842
Langerhans, R. B., Layman, C. A., & DeWitt, T. J. (2005). Male genital size reflects a tradeoff between attracting mates and avoiding predators in two live-bearing fish species. Proceedings of the National Academy of Sciences of the United States of America, 102(21), 7618–7623. https://doi.org/10.1073/pnas.0500935102
Lavergne, S., Thompson, J. D., Garnier, E., & Debussche, M. (2004). The biology and ecology of narrow endemic and widespread plants: A comparative study of trait variation in 20 congeneric pairs. Oikos, 107(3), 505–518. https://doi.org/10.1111/j.0030-1299.2004.13423.x
Le Pape, G., & Goyffon, M. (1975). Accouplement interspécifique suivi de parturition dans le genre Androctonus (Scorpionida, Buthidae). Comptes rendus hebdomadaires des seances, Paris, Serie D. Sciences Naturelles, 280, 2005–2008.
Lenth, R. V. (2016). Least-squares means: The R package lsmeans. Journal of Statistical Software, 69, 1–33. https://doi.org/10.18637/jss.v069.i01
Leonard, J., & Córdoba-Aguilar, A. (2010). The evolution of primary sexual characters in animals. Oxford University Press.
Levin, D. A. (1970). Reinforcement of reproductive isolation: Plants versus animals. The American Naturalist, 104(940), 571–581. https://doi.org/10.1086/282691
Liou, L. W., & Price, T. D. (1994). Speciation by reinforcement of premating isolation. Evolution, 48, 1451–1459. https://doi.org/10.1111/j.1558-5646.1994.tb02187.x
Lira, A. F., Foerster, S. I., Albuquerque, C. M., & Moura, G. J. (2021). Contrasting patterns at interspecific and intraspecific levels in scorpion body size across a climatic gradient from rainforest to dryland vegetation. Zoology, 146, 125908. https://doi.org/10.1016/j.zool.2021.125908
Lira, A. F., Pordeus, L. M., Rego, F. N., Iannuzzi, K., & Albuquerque, C. M. (2018). Sexual dimorphism and reproductive behavior in the Brazilian scorpion Tityus pusillus (Scorpiones, Buthidae). Invertebrate Biology, 137(3), 221–230.
Maan, M. E., & Seehausen, O. (2011). Ecology, sexual selection and speciation. Ecology Letters, 14(6), 591–602. https://doi.org/10.1111/j.1461-0248.2011.01606.x
Macagno, A. L., Pizzo, A., Parzer, H. F., Palestrini, C., Rolando, A., & Moczek, A. P. (2011). Shape-but not size-codivergence between male and female copulatory structures in Onthophagus beetles. PLoS ONE, 6(12), e28893. https://doi.org/10.1371/journal.pone.0028893
Marshall, D. C., & Cooley, J. R. (2000). Reproductive character displacement and speciation in periodical cicadas, with description of a new species, 13-year Magicicada neotredecim. Evolution, 54(4), 1313–1325. https://doi.org/10.1111/j.0014-3820.2000.tb00564.x
Masly, J. P. (2012). 170 years of “lock-and-key”: Genital morphology and reproductive isolation. International Journal of Evolutionary Biology. https://doi.org/10.1155/2012/247352
Matsumoto, T., Takakura, K.-I., & Nishida, T. (2010). Alien pollen grains interfere with the reproductive success of native congener. Biological Invasions, 12, 1617–1626. https://doi.org/10.1007/s10530-009-9574-5
Matthiesen, F. A. (1968). On the sexual behaviour of some Brazilian scorpions. Revista Brasileira de Pesquisas Médicas e Biológicas, 1(2), 93–96.
Mattoni, C. I., Ochoa, J. A., Ojanguren Affilastro, A. A., & Prendini, L. (2012). Orobothriurus (Scorpiones: Bothriuridae) phylogeny, Andean biogeography, and the relative importance of genitalic and somatic characters. Zoologica Scripta, 41(2), 160–176. https://doi.org/10.1111/j.1463-6409.2011.00508.x
Maury, E. A. (1968). Aportes al conocimiento de los escorpiones de la Republica Argentina. I. Observaciones biologicas sobre Urophonius brachycentrus (Thorell, 1877) (Bothriuridae). [Contributions to the knowledge of the scorpions of the Argentine Republic. I. Biological observations on Urophonius brachycentrus (Thorell, 1877) (Bothriuridae)]. Physis, 27(75), 407–418.
Maury, E. A. (1969). Observaciones sobre el ciclo reproductivo de Urophonius brachycentrus (Thorell, 1877) (Scorpiones, Bothriuridae). [Observations on the reproductive cycle of Urophonius brachycentrus (Thorell, 1877) (Scorpiones, Bothriuridae)]. Physis, 32(85), 131–139.
Maury, E. A. (1977). Comentarios sobre dos especies de escorpiones del género Urophonius (Bothriuridae). [Comments on two species of scorpions of the genus Urophonius (Bothriuridae)]. Revista del Museo Argentino de Ciencias Naturales. Entomología, 5(7), 143–160.
Mayr, E. (1963). Animal species and evolution. Harvard University Press.
McLean, C. J., Garwood, R. J., & Brassey, C. A. (2018). Sexual dimorphism in the Arachnid orders. PeerJ, 6, e5751. https://doi.org/10.7717/peerj.5751
McPeek, M. A., Shen, L., & Farid, H. (2009). The correlated evolution of three-dimensional reproductive structures between male and female damselflies. Evolution: International Journal of Organic Evolution, 63(1), 73–83. https://doi.org/10.1111/j.1558-5646.2008.00527.x
Mendelson, T. C., & Shaw, K. L. (2012). The (mis)concept of species recognition. Trends in Ecology & Evolution, 27(8), 421–427. https://doi.org/10.1016/j.tree.2012.04.001
Mikkola, K. (1992). Evidence for lock-and-key mechanisms in the internal genitalia of the Apamea moths (Lepidoptera, Noctuidae). Systematic Entomology, 17(2), 145–153. https://doi.org/10.1111/j.1365-3113.1992.tb00327.x
Mikkola, K. (2008). The lock-and-key mechanisms of the internal genitalia of the Noctuidae (Lepidoptera): How are they selected for? European Journal of Entomology, 105(1), 13–25.
Monod, L., Cauwet, L., González-Santillán, E., & Huber, S. (2017). The male sexual apparatus in the order Scorpiones (Arachnida): A comparative study of functional morphology as a tool to define hypotheses of homology. Frontiers in Zoology, 14(1), 1–48. https://doi.org/10.1186/s12983-017-0231-z
Muster, C., & Michalik, P. (2020). Cryptic diversity in ant-mimic Micaria spiders (Araneae, Gnaphosidae) and a tribute to early naturalists. Zoologica Scripta, 49(2), 197–209. https://doi.org/10.1111/zsc.12404
Nagata, N., Kubota, K., Yahiro, K., & Sota, T. (2007). Mechanical barriers to introgressive hybridization revealed by mitochondrial introgression patterns in Ohomopterus ground beetle assemblages. Molecular Ecology, 16(22), 4822–4836. https://doi.org/10.1111/j.1365-294X.2007.03569.x
Nime, M. F., Casanoves, F., & Mattoni, C. I. (2014). Scorpion diversity in two different habitats in the Arid Chaco, Argentina. Journal of Insect Conservation, 18, 373–384.
Nishimura, T., Nagata, N., Terada, K., Xia, T., Kubota, K., Sota, T., & Takami, Y. (2022). Reproductive character displacement in genital morphology in Ohomopterus ground beetles. The American Naturalist, 199(3), E76–E90. https://doi.org/10.1086/717864
Ojanguren-Affilastro, A. (2005). Estudio monográfico de los escorpiones de la República Argentina. [Monographic study of the scorpions of the Argentine Republic]. Revista Ibérica de Aracnología, 11, 75–241.
Ojanguren-Affilastro, A., Pizarro-Araya, J., & Santibáñez-López, C. E. (2023). Old and cold: Diverse phylogenomic datasets support an ancient transantarctic dispersive route on the scorpion family Bothriuridae in temperate Gondwana. Molecular Phylogenetics and Evolution, 187, 107886.
Ojanguren-Affilastro, A., Ramírez, M. J., & Pizarro-Araya, J. (2020). Phylogenetic analysis of the winter and southernmost scorpion genus Urophonius (Bothriuridae), with the description of two new Patagonian species. Zoologischer Anzeiger, 289, 50–66. https://doi.org/10.1016/j.jcz.2020.09.003
Olivero, P. A., González, A., Mattoni, C. I., & Peretti, A. V. (2015). Chemical caressess: Geographical variation of male sexual signals in a Neotropical scorpion. Behaviour, 152(12–13), 1745–1763. https://doi.org/10.1163/1568539X-00003302
Olivero, P. A., Mattoni, C. I., & Peretti, A. (2012). Morphometry and geographical variation of Bothriurus bonariensis (Scorpiones: Bothriuridae). Journal of Arachnology, 40(1), 113–122. https://doi.org/10.1636/B11-27.1
Olivero, P. A., Vrech, D. E., Oviedo-Diego, M. A., Mattoni, C. I., & Peretti, A. V. (2019). Courtship performance as function of body condition in an ‘ancient’ form of sperm transfer. Animal Biology, 69(1), 33–46. https://doi.org/10.1163/15707563-00001041
Olivero, P. A., Vrech, D. E., Peretti, A. V., & Mattoni, C. I. (2014). Patterns of asymmetry in body traits and genitalia in two distant populations of a Neotropical scorpion. Journal of Natural History, 49(15–16), 853–872. https://doi.org/10.1080/00222933.2014.951086
Outomuro, D., & Johansson, F. (2017). A potential pitfall in studies of biological shape: Does size matter? Journal of Animal Ecology, 86(6), 1447–1457. https://doi.org/10.1111/1365-2656.12732
Oviedo-Diego, M. (2022). Biología Reproductiva y Evolución en arácnidos: estudio multidisciplinario en dos especies modelo de escorpiones (Scorpiones, Bothriuridae, Urophonius) [Reproductive biology and evolution in arachnids: A multidisciplinary study in two model species of scorpions (Scorpiones, Bothriuridae, Urophonius)] (Doctoral Dissertation). Córdoba, Argentina: Universidad Nacional de Córdoba.
Oviedo-Diego, M., Costa-Schmidt, L. E., Mattoni, C. I., & Peretti, A. V. (2021). Interaction between sexual communication functions leads to reproductive interference in two syntopic scorpion species. Animal Behaviour, 181, 83–93. https://doi.org/10.1016/j.anbehav.2021.08.029
Oviedo-Diego, M. A., Mattoni, C. I., & Peretti, A. V. (2019). Specificity of the female’s local cellular immune response in genital plug producing scorpion species. PLoS ONE, 14(2), e0208682. https://doi.org/10.1371/journal.pone.0208682
Oviedo-Diego, M., Mattoni, C. I., & Peretti, A. V. (2022). Males make mistakes but females call the shots: Female resistance as a partial reproductive isolating mechanism in scorpions, AAS Annual Meeting, California, United States.
Oviedo-Diego, M. A., Mattoni, C. I., Vrech, D. E., Michalik, P., & Peretti, A. V. (2020). The morphology of mating plugs and its formation in scorpions: Implications for intersexual participation. Journal of Morphology, 281(6), 620–635. https://doi.org/10.1002/jmor.21125
Peretti, A. V. (1993). Estudio de la biología reproductiva en escorpiones argentinos (Arachnida, Scorpiones): un enfoque etológico. [Study of reproductive biology in Argentine scorpions (Arachnida, Scorpiones): An ethological approach] (Doctoral Dissertation). Córdoba, Argentina: Universidad Nacional de Córdoba.
Peretti, A. V. (1997). Relación de las glándulas caudales de Males de escorpiones Bothriuridae con el comportamiento sexual (Scorpiones). [Relationship of the caudal glands of Males of Bothriuridae scorpions to sexual behavior (Scorpiones)]. Revue Arachnologique, 12(3), 31–41.
Peretti, A. V. (2003). Functional morphology of spermatophores and female genitalia in bothriurid scorpions: Genital courtship, coercion and other possible mechanisms. Journal of Zoology, 261(2), 135–153. https://doi.org/10.1017/S095283690300400X
Peretti, A. V. (2010). An ancient indirect sex model: Single and mixed patterns in the evolution of scorpion genitalia. In J. Leonard & A. Cordoba-Aguilar (Eds.), The evolution of primary sexual characters in animal (pp. 218–248). Oxford University Press.
Peretti, A. V. (2013). Sexual selection in Neotropical species: Rules and exceptions. In R. Macedo & G. Machado (Eds.), Sexual selection (pp. 33–52). Amsterdam: Academic Press.
Peretti, A. V., Acosta, L. E., & Martínez, M. A. (2000). Comportamiento de apareamiento en tres especies de Bothriurus del grupo prospicuus: estudio comparado y su relación con Bothriurus flavidus (Scorpiones, Bothriuridae). [Mating behavior in three Bothriurus species of the prospicuus group: A comparative study and its relationship with Bothriurus flavidus (Scorpiones, Bothriuridae)]. Revue Arachnolgique, 13(5), 73–91.
Peretti, A. V., Depiante, M. L., & Battán-Horenstein, M. (2001). Patterns of allometry and asymmetry of body characters and spermatophores in Bothriurus bonariensis (C.L. Koch, 1842) (Scorpiones: Bothriuridae). Scorpions (pp. 331–341).
Pfennig, D. W., & Murphy, P. J. (2002). How fluctuating competition and phenotypic plasticity mediate species divergence. Evolution, 56(6), 1217–1228. https://doi.org/10.1111/j.0014-3820.2002.tb01433.x
Pfennig, D. W., & Pfennig, K. S. (2010). Character displacement and the origins of diversity. The American Naturalist, 176(S1), S26–S44. https://doi.org/10.1086/657056
Pfennig, K. S., & Simovich, M. A. (2002). Differential selection to avoid hybridization in two toad species. Evolution, 56(9), 1840–1848. https://doi.org/10.1111/j.0014-3820.2002.tb00198.x
Pigliucci, M., Murren, C. J., & Schlichting, C. D. (2006). Phenotypic plasticity and evolution by genetic assimilation. Journal of Experimental Biology, 209(12), 2362–2367. https://doi.org/10.1242/jeb.02070
Polis, G. A., & McCormick, S. J. (1986). Patterns of resource use and age structure among species of desert scorpion. The Journal of Animal Ecology, 55, 59–73.
Polis, G. A., & Sissom, W. D. (1990). Life history. In G. A. Polis (Ed.), The biology of scorpions (pp. 161–223). Stanford University Press.
Price, C. S., Kim, C. H., Gronlund, C. J., & Coyne, J. A. (2001). Cryptic reproductive isolation in the Drosophila simulans species complex. Evolution, 55(1), 81–92. https://doi.org/10.1111/j.0014-3820.2001.tb01274.x
Probst, P. J. (1972). Zur Fortpflanzungsbiologie und zur Entwicklung der Giftdrusen beim Skorpion Isometrus maculatus (De Geer, 1778) (Scorpiones: Buthidae). Acta Tropica, 29, 1–87.
Proctor, H. C. (1998). Indirect sperm transfer in arthropods: Behavioral and evolutionary trends. Annual Review of Entomology, 43, 153.
Quantum GIS Development Team. (2020). Quantum GIS geographic information system (2.18). [Open source geospatial foundation project]. http://qgis.osgeo.org
R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rice, A. M., & Pfennig, D. W. (2007). Character displacement: In situ evolution of novel phenotypes or sorting of pre-existing variation? Journal of Evolutionary Biology, 20(2), 448–459. https://doi.org/10.1111/j.1420-9101.2006.01187.x
Richmond, M. P. (2014). The role of aedeagus size and shape in failed mating interactions among recently diverged taxa in the Drosophila mojavensis species cluster. BMC Evolutionary Biology, 14(1), 1–9. https://doi.org/10.1186/s12862-014-0255-3
Robinson, B. W., & Wilson, D. S. (1994). Character release and displacement in fishes: A neglected literature. The American Naturalist, 144(4), 596–627. https://doi.org/10.1086/285696
Robson, G. C., & Richards, O. W. (1936). The variation of animals in nature. Longmans Green.
Rohlf, F. J. (2004). tpsDig-Thin plate spline digitizer, version 1.40. Department of Ecology and Evolution, State University of New York at Stony Brook.
Rohlf, F. J. (2015). The tps series of software. Hystrix, 26, 1–4. https://doi.org/10.4404/hystrix-26.1-11264
Rohlf, F. J., & Slice, D. (1990). Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Biology, 39(1), 40–59. https://doi.org/10.2307/2992207
Romero-Lebrón, E., Oviedo-Diego, M. A., Elias, D., Vrech, D. E., & Peretti, A. V. (2019). Effect of the mating plug on female chemical attractiveness and mating acceptance in a scorpion. Ethology, 125(4), 184–194. https://doi.org/10.1111/eth.12842
Rowe, L., & Arnqvist, G. (2012). Sexual selection and the evolution of genital shape and complexity in water striders. Evolution: International Journal of Organic Evolution, 66(1), 40–54. https://doi.org/10.1111/j.1558-5646.2011.01411.x
Rundle, H. D., & Dyer, K. A. (2015). Reproductive character displacement of female mate preferences for male cuticular hydrocarbons in Drosophila subquinaria. Evolution, 69(10), 2625–2637. https://doi.org/10.1111/evo.12761
Ryan, M. J., & Rand, A. S. (1993). Species recognition and sexual selection as a unitary problem in animal communication. Evolution, 47(2), 647–657. https://doi.org/10.1111/j.1558-5646.1993.tb02118.x
Safran, R. J., Scordato, E. S., Symes, L. B., Rodríguez, R. L., & Mendelson, T. C. (2013). Contributions of natural and sexual selection to the evolution of premating reproductive isolation: A research agenda. Trends in Ecology & Evolution, 28(11), 643–650. https://doi.org/10.1016/j.tree.2013.08.004
Sánchez-Quirós, C., Arévalo, E., & Barrantes, G. (2012). Static allometry and sexual size dimorphism in Centruroides margaritatus (Scorpiones: Buthidae). The Journal of Arachnology, 40(3), 338–344.
Santibáñez-López, C. E., Cushing, P. E., Powell, A. M., & Graham, M. R. (2021). Diversification and post-glacial range expansion of giant North American camel spiders in genus Eremocosta (Solifugae: Eremobatidae). Scientific Reports, 11(1), 1–11. https://doi.org/10.1038/s41598-021-01555-1
Santibáñez-López, C. E., Kriebel, R., & Sharma, P. P. (2017). eadem figura manet: Measuring morphological convergence in diplocentrid scorpions (Arachnida: Scorpiones: Diplocentridae) under a multilocus phylogenetic framework. Invertebrate Systematics, 31(3), 233–248. https://doi.org/10.1071/IS16078
Sarmento, S. M., de Souza, A. M., Meiado, M. V., & de Albuquerque, C. M. (2008). Notes on the life history traits of Rhopalurus rochai (Scorpiones, Buthidae) under different feeding regimes. The Journal of Arachnology, 36(2), 476–479. https://doi.org/10.1636/Csh07-122.1
Schlager, S. (2017). Morpho and Rvcg—Shape analysis in R. In G. Zheng, S. Li, & G. J. Szekely (Eds.), Statistical shape and deformation analysis (pp. 217–256). Academic Press.
Schlager, S., Jefferis, G., Ian, D., & Schlager, M. S. (2021). Package ‘Morpho’. Cran R. http://cran.r-project.org/web/packages/Morpho/Morpho.pdf
Schluter, D., & Nychka, D. (1994). Exploring fitness surfaces. The American Naturalist, 143(4), 597–616. https://doi.org/10.1086/285622
Schneider, C. A., Rasband, W. S., & Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9(7), 671–675.
Seiter, M., Mosetig, L., & Milasowszky, N. (2020). The trade-off between adult size and development time due to different feeding regimes in the scorpion Tityus neibae. Invertebrate Reproduction & Development, 64(4), 274–280. https://doi.org/10.1080/07924259.2020.1806119
Sentenská, L., Graber, F., Richard, M., & Kropf, C. (2017). Sexual dimorphism in venom gland morphology in a sexually stinging scorpion. Biological Journal of the Linnean Society, 122(2), 429–443. https://doi.org/10.1093/biolinnean/blx067
Servedio, M. R., & Noor, M. A. (2003). The role of reinforcement in speciation: Theory and data. Annual Review of Ecology, Evolution, and Systematics, 34(1), 339–364.
Shapiro, A. M., & Porter, A. H. (1989). The lock-and-key hypothesis: Evolutionary and biosystematic interpretation of insect genitalia. Annual Review of Entomology, 34(1), 231–245. https://doi.org/10.1146/annurev.en.34.010189.001311
Shelomi, M. (2012). Where are we now? Bergmann’s rule sensu lato in insects. The American Naturalist, 180(4), 511–519.
Shuker, D. M., & Burdfield-Steel, E. R. (2017). Reproductive interference in insects. Ecological Entomology, 42, 65–75. https://doi.org/10.1111/een.12450
Simmons, L. W. (2014). Sexual selection and genital evolution. Austral Entomology, 53(1), 1–17. https://doi.org/10.1111/aen.12053
Simmons, L. W., House, C. M., Hunt, J., & García-González, F. (2009). Evolutionary response to sexual selection in male genital morphology. Current Biology, 19(17), 1442–1446. https://doi.org/10.1016/j.cub.2009.06.056
Sloan, N. S., & Simmons, L. W. (2019). The evolution of female genitalia. Journal of Evolutionary Biology, 32(9), 882–899. https://doi.org/10.1111/jeb.13503
Smadja, C., & Ganem, G. (2005). Asymmetrical reproductive character displacement in the house mouse. Journal of Evolutionary Biology, 18(6), 1485–1493. https://doi.org/10.1111/j.1420-9101.2005.00944.x
Sobroza, T. V., Gordo, M., Pequeno, P. A., Dunn, J. C., Spironello, W. R., Rabelo, R. M., & Barnett, A. (2021). Convergent character displacement in sympatric tamarin calls (Saguinus spp.). Behavioral Ecology and Sociobiology, 75(5), 1–13. https://doi.org/10.1007/s00265-021-03028-x
Sokal, R. R., & Rohlf, F. J. (1995). Biometry: The principles and practice of statistics in biological research. Freeman and Co.
Song, H., & Bucheli, S. R. (2010). Comparison of phylogenetic signal between male genitalia and non-genital characters in insect systematics. Cladistics, 26(1), 23–35. https://doi.org/10.1111/j.1096-0031.2009.00273.x
Song, H., & Wenzel, J. W. (2008). Mosaic pattern of genital divergence in three populations of Schistocerca lineata Scudder, 1899 (Orthoptera: Acrididae: Cyrtacanthacridinae). Biological Journal of the Linnean Society, 94(2), 289–301. https://doi.org/10.1111/j.1095-8312.2008.00983.x
Sota, T., & Kubota, K. (1998). Genital lock-and-key as a selective agent against hybridization. Evolution, 52(5), 1507–1513. https://doi.org/10.1111/j.1558-5646.1998.tb02033.x
Sota, T., Takami, Y., Kubota, K., Ujiie, M., & Ishikawa, R. (2000). Interspecific body size differentiation in species assemblages of the carabid subgenus Ohomopterus in Japan. Population Ecology, 42(3), 279–291. https://doi.org/10.1007/PL00012006
Sota, T., & Tanabe, T. (2010). Multiple speciation events in an arthropod with divergent evolution in sexual morphology. Proceedings of the Royal Society B: Biological Sciences, 277(1682), 689–696. https://doi.org/10.1098/rspb.2009.1822
Stearns, S. C. (1989). The evolutionary significance of phenotypic plasticity. BioScience, 39(7), 436–445. https://doi.org/10.2307/1311135
Stearns, S. C. (1992). The evolution of life histories. Oxford University Press.
Stillwell, R. C., & Fox, C. W. (2007). Environmental effects on sexual size dimorphism of a seed-feeding beetle. Oecologia, 153(2), 273–280. https://doi.org/10.1007/s00442-007-0724-0
Stratton, G. E. (1997). Investigation of species divergence and reproductive isolation of Schizocosa stridulans (Araneae: Lycosidae) from Illinois. Bulletin-British Arachnological Society, 10, 313–321.
Stuart, Y. E., Inkpen, S. A., Hopkins, R., & Bolnick, D. I. (2017). Character displacement is a pattern: So, what causes it? Biological Journal of the Linnean Society, 121(3), 711–715. https://doi.org/10.1093/biolinnean/blx013
Svensson, E. I., & Gosden, T. P. (2007). Contemporary evolution of secondary sexual traits in the wild. Functional Ecology, 21(3), 422–433. https://doi.org/10.1111/j.1365-2435.2007.01265.x
Takakura, K. I., Nishida, T., & Iwao, K. (2015). Conflicting intersexual mate choices maintain interspecific sexual interactions. Population Ecology, 57(2), 261–271. https://doi.org/10.1007/s10144-015-0492-3
Takami, Y., Nagata, N., Sasabe, M., & Sota, T. (2007). Asymmetry in reproductive isolation and its effect on directional mitochondrial introgression in the parapatric ground beetles Carabus yamato and C. albrechti. Population Ecology, 49(4), 337–346. https://doi.org/10.1007/s10144-007-0052-6
Tanabe, T., & Sota, T. (2008). Complex copulatory behavior and the proximate effect of genital and body size differences on mechanical reproductive isolation in the millipede genus Parafontaria. The American Naturalist, 171(5), 692–699. https://doi.org/10.1086/587075
Tobias, J. A., Cornwallis, C. K., Derryberry, E. P., Claramunt, S., Brumfield, R. T., & Seddon, N. (2014). Species coexistence and the dynamics of phenotypic evolution in adaptive radiation. Nature, 506(7488), 359–363. https://doi.org/10.1038/nature12874
Tuxen, S. L. (1970). Taxonomist’s glossary of genitalia in insects. Monksgaard.
Usami, T., Yokoyama, J., Kubota, K., & Kawata, M. (2006). Genital lock-and-key system and premating isolation by mate preference in carabid beetles (Carabus subgenus Ohomopterus). Biological Journal of the Linnean Society, 87(1), 145–154. https://doi.org/10.1111/j.1095-8312.2006.00562.x
Vignoli, V., Salomone, N., Caruso, T., & Bernini, F. (2005). The Euscorpius tergestinus (CL Koch, 1837) complex in Italy: Biometrics of sympatric hidden species (Scorpiones: Euscorpiidae). Zoologischer Anzeiger - A Journal of Comparative Zoology, 244(2), 97–113.
Vinarski, M. V. (2014). On the applicability of Bergmann’s rule to ectotherms: The state of the art. Biology Bulletin Reviews, 4, 232–242.
Visser, J. H., & Geerts, S. (2021). Static allometry and sexual dimorphism in the Striped Lesser-thicktail Scorpion Uroplectes lineatus. Arachnology, 18(7), 700–707. https://doi.org/10.13156/arac.2020.18.7.700
Waage, J. K. (1979). Reproductive character displacement in Calopteryx (Odonata: Calopterygidae). Evolution. https://doi.org/10.2307/2407369
Werner, M., & Simmons, L. W. (2008). The evolution of male genitalia: Functional integration of genital sclerites in the dung beetle Onthophagus taurus. Biological Journal of the Linnean Society, 93(2), 257–266. https://doi.org/10.1111/j.1095-8312.2007.00924.x
West-Eberhard, M. J. (2003). Developmental plasticity and evolution. Oxford University Press.
Weygoldt, P. (1990). Arthropoda-Chelicerata: Sperm transfer. In K. G. Adiyodi & R. G. Adiyodi (Eds.), Reproductive biology of the invertebrates (pp. 77–119). Wiley.
Wheatcroft, D. (2015). Reproductive interference via display signals: The challenge of multiple receivers. Population Ecology, 57(2), 333–337. https://doi.org/10.1007/s10144-015-0487-0
Whitman, D. W., & Agrawal, A. A. (2009). What is phenotypic plasticity and why is it important. In D. W. Whitman & T. N. Ananthakrishnan (Eds.), Phenotypic plasticity of insects: Mechanisms and consequences (pp. 1–63). Science Publishers.
Wilson, J. D., Zapata, L. V., Barone, M. L., Cotoras, D. D., Poy, D., & Ramírez, M. J. (2021). Geometric morphometrics reveal sister species in sympatry and a cline in genital morphology in a ghost spider genus. Zoologica Scripta, 50(4), 485–499. https://doi.org/10.1111/zsc.12478
Wojcieszek, J. M., & Simmons, L. W. (2012). Evidence for stabilizing selection and slow divergent evolution of male genitalia in a millipede (Antichiropus variabilis). Evolution: International Journal of Organic Evolution, 66(4), 1138–1153. https://doi.org/10.1111/j.1558-5646.2011.01509.x
Yamaguchi, R., & Iwasa, Y. (2015). Reproductive interference can promote recurrent speciation. Population Ecology, 57(2), 343–346. https://doi.org/10.1007/s10144-015-0485-2
Yamashita, T., & Rhoads, D. D. (2013). Species delimitation and morphological divergence in the scorpion Centruroides vittatus (Say, 1821): Insights from phylogeography. PLoS ONE, 8(7), e68282. https://doi.org/10.1371/journal.pone.0068282
Yukilevich, R. (2021). Reproductive character displacement drives diversification of male courtship songs in Drosophila. The American Naturalist, 197(6), 690–707. https://doi.org/10.5061/dryad.m63xsj41k
Zelditch, M. L., Swiderski, D. L., & Sheets, H. D. (2004). Geometric morphometrics for biologists: A primer. Elsevier.
Funding
This study was funded by Consejo Nacional de Investigaciones Científicas y Técnicas (Grant No. BID-PICT 2014-2069).
Author information
Authors and Affiliations
Contributions
AP, CM, MO conceived the project and designed morphological analysis. AP, CM, MO, FB collected and maintained the individuals in the laboratory. MO compiled the raw data, performed the morphometric studies and statistical analyses, ES collaborated with the morphometric and refined statistical analyses, FB help with the graphs and the manuscript writing. MO wrote the manuscript with input from AP, CM, ES and FB. All authors contributed to revisions of project design and gave final approval for publication. AP and CM supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests with regard to this manuscript and the material implicated.
Ethical Approval
We declare that the experiments comply with the current laws of Argentina. This investigation adheres to the ASAB/ABS Guidelines for the Use of Animals in Research (Buchanan et al., 2012), and the use of animals was reviewed and approved by the animal care review committee at the Instituto de Diversidad y Ecología Animal (IDEA), CONICET-UNC, Argentina, where we performed the experiment.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oviedo-Diego, M.A., Mattoni, C.I., Bollatti, F.A. et al. Mosaic Evolution of Grasping and Genital Traits in Two Sympatric Scorpion Species with Reproductive Interference. Evol Biol 51, 124–148 (2024). https://doi.org/10.1007/s11692-023-09623-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-023-09623-2