Abstract
Monogenoids are ectoparasites that have a simple one-host lifecycle, high species diversity, and a relatively high host specificity. During studies on the helminth fauna of fishes from the Jurua River, in Acre State, Brazil, a new species of the monotypic genus Unibarra Suriano & Incorvaia, 1995 was found parasitizing Oxydoras niger Valenciennes, 1821. Unibarra juruaensis n. sp. is allocated in the genus based on the presence of a single haptoral bar, marginal hooks similar in shape and size, gonads partially overlapping, and a conspicuous filament which connects the base of the male copulatory organ with the accessory piece. The new species differs from the only species of the genus by the smaller size of the body and of the structures, by the morphology of copulatory complex, with an accessory piece thinner than that of U. paranoplatensis Suriano & Incorvaia, 1995 and by the presence of two eyespots. The type species, U. paranoplatensis, is referred in a new host, Pimelodus blochii Valenciennes, 1840, with new morphological data. A table of measurements of the new species and previous and the present reports of U. paranoplatensis is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the class Monogenoidea Bychowsky, 1937 are obligate ectoparasites of marine, brackish and freshwater fishes, amphibians, and chelonians that have a direct lifecycle, high species diversity, and a relatively high host-specificity, making them suitable markers to study biodiversity and speciation in groups of closely related [1, 2]. The direct life cycles of monogenoideans allow them to rapidly multiply in lentic and eutrophized environments such as aquaculture, where intensive levels of infections can cause considerable pathogenicity often having a harmful impact on their hosts’ health, provoking economic losses [2,3,4,5].
Among Platyhelminthes, the Monogenoidea represents a diverse group, comprehending about 560 known species in Brazil being that approximately 400 of them were described from Brazilian hosts [6], mainly in freshwater fishes. According to Reis [7], there are 6,025 freshwater fish species in South America, and this high regional biodiversity leads us to wait for high diversity of parasites in these hosts. In this context are included the neotropical catfishes from the order Siluriformes that are known to harbor a rich and diverse fauna of gill monogenoideans [8,9,10]. The Neotropical ichthyofauna is dominated by fishes from the orders Characiformes, Siluriformes, and Gymnotiformes, which constitute about 77% of the species. Siluriformes is a large and diverse order of fishes, with 1700 of the 3000 valid species found in America, mostly in the Neotropical Region [8, 11, 12]. Catfishes have huge economic value are greatly appreciated by aquarists and many species, especially large pimelodids and doradids, are used for local consumption [12, 13]. Most of them are omnivores, nocturnal, and depend mainly on senses other than sight, being preadapted for life in caves, aquifers, and deep river channels [13].
Oxydoras niger Valenciennes, 1821 (Siluriformes: Doradidae) commonly called “cuiucuiu” in Brazil, is a South American endemic species, distributed in the basins of Amazonas River, Araguaia-Tocantins, and Prata. The species is considered the largest Doradidae in Amazon, is sought after for its large size, reaching up to 1.2 m long and 20 kg in weight and for its good meat that looks like salmon in color. The species is omnivorous, feeding on insects’ larvae and other aquatic invertebrates, including shrimps and molluscs, in the debris on the river bottom and it reproduces at the beginning of the rainy season [14, 15].
Pimelodus blochii Valenciennes, 1840 is a siluriform endemic fish from the Neotropical Region, which occurs in the Amazon, Paraná, Orinoco, and Guiana basins [16, 17]. The species is gregarious, living in groups, commonly found under logs in the benthic environments. They feed on fruits, thus helping to scatter several plant seeds, on small fishes and insects, suggesting a large trophic adaptation [18], but may also act as a detritivore [16]. Probably migrate upstream during reproduction. The female lays an average of 50,000 eggs which are fertilized externally [19]. The food diversity of P. blochii associated with its greater use in filleting, indicates a great potential for commercial exploitation as food for the riverside population [20].
The only monogenoid genus reported from O. niger is Cosmetocleithrum Kritsky, Thatcher & Boeger, 1986, with six species described from this host species, Cosmetocleithrum confusus Kritsky, Thatcher & Boeger, 1986, Cosmetocleithrum gussevi Kritsky, Thatcher & Boeger, 1986, Cosmetocleithrum parvum Kritsky, Thatcher & Boeger, 1986, Cosmetocleithrum rarum Kritsky, Thatcher & Boeger, 1986, Cosmetocleithrum sobrinus Kritsky, Thatcher & Boeger, 1986 all from Brazil and more recently Cosmetocleithrum gigas Morey, Cachique & Babilonia, 2019 was described from this host from Peru [21, 22]. Monogenoid parasites from P. blochii were reported by Negreiros et al. [23, 24] collected from Acre, namely Ameloblastella amazonica Negreiros, Tavares-Dias & Pereira, 2019, Demidospermus leptosynophallus Kritsky & Gutiérrez, 1998, Demidospermus peruvianus Mendoza-Palermo & Scholz, 2011, Demidospermus striatus Mendoza-Palermo & Scholz, 2011, and the gyrodactylid Scleroductus yuncensi Jara & Cone, 1989.
During studies on the helminth fauna of fishes from the Jurua River, in Acre State, Brazil, a new species of the monotypic genus Unibarra was found parasitizing O. niger, in addition of U. paranaplatensis found parasitizing P. blochii. The monogenoid species are described and original drawings are presented herein.
Materials and Methods
Between June and July 2019, seven O. niger (standard length 31–40 cm; total weight 531–736 g) and eight P. blochii (standard length 14–20 cm; total weight 106–136 g) were captured with gill nets and hook and line from Jurua River, Acre, Brazil (7°40′34.1″S, 72°39′39.5″W). The gills were removed and placed in vials containing hot water (~ 65ºC) that were shaken. Absolute ethanol was added to reach a concentration of 70%. Monogenoids were picked from the sediment and gill arches in the laboratory with the aid of a stereoscopic microscope. Some specimens were mounted unstained in Hoyer’s medium for study of the sclerotized parts and others were stained with Gomori’s trichrome and mounted in Canada balsam [25]. Photomicrographs were taken with a digital camera Sony coupled at Axioskop light microscope. All measurements are in micrometers, and the range is followed by the mean in parentheses and the number of specimens measured when more than two. Dimensions of organs and other structures represent the greatest distance; lengths of curved or bent structures (bar and accessory piece) represent the straight line distances between extreme ends. Measurements of haptoral parts follow Freitas et al. [26]. Numbering of hook pairs follows Mizelle [27]. Holotype and paratypes are deposited in the helminthological collection of Instituto Oswaldo Cruz (CHIOC) (Fig. 1).
Results
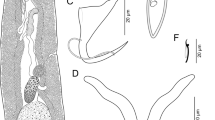
Unibarra juruaensis sp. n. (Figs. 2a–g, 4a,c,e)
Host: Oxydoras niger.
Site: gills.
Type-locality: Jurua River, Acre, Brazil (7°40′34.1″S, 72°39′39.5″W).
Parasitological indexes: Total number of hosts: 5; number of infected hosts: 2; total number of parasites: 7.
Deposited specimens: Holotype CHIOC 39953 a; paratypes CHIOC 39953 b–f, 39954.
Etymology: The specific name refers to the type and the locality of the parasite, Juruá River.
Description: (based on 7 specimens). Body fusiform, comprising cephalic region, trunk, peduncle and haptor, 650–1000 (811; n = 7) long by 200–400 (278; n = 7) wide. Tegument smooth. Cephalic region presenting cephalic lobes poorly developed and three pairs of head organs. Eyespots 2, accessory granules not observed. Pharynx muscular, glandular, spherical, 52 by 50, esophagus short, bifurcating into intestinal caeca. Intestinal caeca confluent posteriorly, lacking diverticula. Peduncle short, presenting the same width of body. Haptor wider than body, 130–300 (215; n = 6) by 300–800 (429; n = 6). Ventral bar long, delicate, broadly V-shaped, with posteromedial process directed posteriorly, 97–137 (121; n = 6) long by 3–7 (5; n = 6) wide, without the medial projection and 10–22 (15; n = 5) considering the projection. Anchors dissimilar in shape and size, covered by a sclerotized cap in anterior portion. Ventral anchor with rectangular-shaped superficial root, rounded deep root, evenly curved shaft and long point, passing from the level of tip of superficial root, 78–84 (79; n = 7) long, 35–45 (42; n = 7) base; dorsal anchor with well-developed roots, elongated superficial root, rounded deep root, short and curved shaft, long point, extending level of tip of superficial root, 35–40 (38; n = 10) long, 35–45 (39; n = 10) base. Hooks similar in shape, with protruding thumb and delicate point, dilated shank, with a groove in the anterior third. FH loop ¼ shank length. Hooks: pair 1, 30–42 (36; n = 10); pair 2, 30–42 (37; n = 12); pair 3, 31–40 (37; n = 11), pair 4, 30–40 (35; n = 8); pair 5, 30–38 (35; n = 10); pair 6, 30–40 (34; n = 9); pair 7, 30–42 (35; n = 7). Gonads inter-caecal, overlapping. Testes dorsal to germarium, ovate, vas deferens running toward anterior region, looping left intestinal caeca; seminal vesicle a sigmoid dilation of vas deferens. Copulatory complex comprising male copulatory organ (MCO) and accessory piece. MCO a long slender tube, with slightly sclerotized walls, tapering distally, slightly expanded base, 95–137 (125; n = 7), accessory piece not articulated with MCO base, distal rod, distal portion sheath-like, serving as a guide for MCO, 125–162 (143; n = 7) by 10–12 (11; n = 4), with a filament attached on proximal portion, 30–40 (34; n = 4). Ovary long. Oviduct, ootype, and uterus not observed. Vaginal aperture sinistral, in the middle region of the body ventral surface, vaginal vestibule large, cup-shaped, strongly sclerotized, with ridges in posterior wall, with a veil surrounding the anterior portion. Seminal receptacle not observed. Vitellaria scattered throughout the trunk, except in the region of gonads, passing through the intestinal caeca.
Remarks: The new species is allocated in Unibarra based on the following characters: the presence of a single haptoral bar, marginal hooks similar in shape and size, gonads partially overlapping, and a conspicuous filament in the proximal portion of the accessory piece. Unibarra juruaensis sp. n. differs from the only species of the genus by the smaller size of the body, by the well-defined roots and a more sclerotized cap in the anchors of the new species, by the morphology of haptoral bar and the copulatory complex, with an accessory piece thinner than that of U. paranoplatensis and by the presence of two eyespots, feature that was considered as absent in the original diagnosis of the genus.
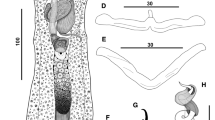
Unibarra paranoplatensis Suriano & Incorvaia, 1995 (Figs. 3a–g, 4b,d,f)
Host: Pimelodus blochii Valenciennes, 1840 (Siluriformes, Pimelodidae), Juruá River, Acre State, Brazil (present study).
Parasitological indexes: Total number of hosts: 8; number of infected hosts: 3; total number of parasites: 11.
Type-host and locality: Zungaro zungaro (Humboldt, 1821) (Siluriformes, Pimelodidae); Parana River, Puerto Italia, Province of Corrientes, Argentina [28]
Others hosts and localities: Pimelodus maculatus Lacepéde, 1803 (Siluriformes, Pimelodidae); de La Plata River, Buenos Aires Harbour, Argentina [28], Aguarunichthys torosus Stewart, 1986 (Siluriformes, Pimelodidae), Santa Clara, Iquitos, Peru [12], Sorubim lima (Bloch & Schneider, 1801) (Siluriformes, Pimelodidae); Acre River, Acre State, Brazil [29].
Deposited specimens: Vouchers CHIOC 39955, 39956 a–d, 39957 a–c, 39958 a–c.
New data from 11 specimens newly collected: Body 1,030–1,730 (1,310; n = 8) long by 240–380 (320; n = 8) wide. Cephalic margin broad; cephalic lobes moderately developed; three bilateral pairs of head organs; cephalic glands indistinct. Two eyespots. Pharynx spherical; esophagus short, bifurcating into two intestinal caeca; caeca confluent posterior to gonads. Peduncle short, presenting the same body width. Haptor subhexagonal, 165–245 (186; n = 4) long by 162–480 (355; n = 14) wide. Ventral bar V-shaped, 115–140 (125; n = 10) long with posteromedial projection, 10–21 (15; n = 10) by 12–17 (14; n = 10) wide (without posteromedian process) and 20–30 (26; n = 10) (including the posteromedian process). Anchors dissimilar in shape and size and covered by a thin sclerotized cap. Ventral anchor 82–95 (88; n = 20) long, 40–48 (44; n = 20) base, with inconspicuous roots, curved shaft and recurved point; dorsal anchor 32–42 (38; n = 20) long, 32–42 (37; n = 20) base with short superficial and deep roots, short shaft and long point. Hooks: pair 1, 62–70 (64; n = 13); pair 2, 50–80 (71; n = 15); pair 3, 62–83 (69; n = 13), pair 4, 50–77 (64; n = 15); pair 5, 40–65 (59; n = 13); pair 6, 50–67 (61; n = 15); pair 7, 59–80 (70; n = 15), each with erected thumb, shank dilated, FH loop well developed, 0.6 shank length. Copulatory complex comprising male copulatory organ (MCO) and accessory piece. MCO 125–137 (131; n = 10), a thin and slender tube, with expanded base, presenting slightly sclerotized walls; accessory piece 142–157 (153; n = 10) long by 12–22 (18; n = 10), non-articulated to cirrus base, rod-shaped. Vaginal pore medioventral, vaginal vestibule large, cup-shaped, strongly sclerotized. Eggs 60–72 (68; n = 4) by 55–62 (60; n = 4). Gonads slightly overlapping, inter-caecal; germarium ovate with irregular edges. Testis post germarial, elongate to ovate. Vitellarium composed of small follicles, dense, extending from pharyngeal level to posterior to caeca (Fig. 3).
Remarks: The morphology and measurements (Table 1) of the specimens collected from P. blochii agree with the original description, with few differences. In the original description of U. paranoplatensis, Suriano & Incorvaia [28] stated that measurements of anchors were presented following Beverley-Burton & Suriano [30], which have adopted the scheme utilized by Soviet parasitologists (see [31]) but modified as follows: (a) distance from tip of superficial root to curve of blade; (b) distance from superficial root—deep notch to curve of the blade; (c) length of deep root; (d) length of superficial root; and (e) distance from tip of blade to curve of blade and the scheme was followed by Negreiros et al. [29]. Suriano & Incorvaia [28] and Negreiros et al. [29] did not consider the item “c” of Beverley-Burton & Suriano [30]. In Table 1, measurements of the new species and of Unibarra paranoplatensis are presented, including those following the scheme proposed by Beverley-Burton & Suriano [30]. The vagina of Unibarra paranoplatensis was described as non-sclerotized but Suriano & Incorvaia [28] referred vaginal canal as strongly sclerotized.
Discussion
The genus Unibarra was proposed for Unibarra paranoplatensis Suriano & Incorvaia, 1995, which is characterized mainly by presenting a single haptoral bar. This species was originally described in Zungaro zungaro (Humboldt) (= Paulicea luetkeni Steindachner) and recorded in Pimelodus maculatus Lacépede (= Pimelodus clarias maculatus) in Argentina [28], in Pimelodus albicans Valenciennes [32] also from Argentina and in Aguarunichthys torosus Stewart in Peru [12]. The species was first recognized in Brazil by Negreiros et al. [29] in Acre River basin parasitizing Sorubim lima Bloch & Schneider. Species of Unibarra are characterized mainly by presenting a single haptoral bar (Fig. 4).
Light photomicrographs of Unibarra spp. from Juruá River, Acre State, Brazil (a, c, e) Unibarra juruanesis sp. n. parasite from Oxydoras niger: a Copulatory complex, c Vagina, e. Haptor; (b, d, f) Unibarra paranoplatensis Suriano & Incorvaia, 1995 parasite from Pimelodus blochii: b Copulatory complex; d Vagina; f haptor. Scale bars: a, b 50 µm, c, d 30 µm, e, f 100 µm
In the present paper, specimens collected from Juruá River presenting features of the previoulsy monotypic genus Unibarra were found and a new species is described from O. niger, expanding to Doradidae the siluriform families parasitized by this monogenoid genus. Also, the presente report of U. paranoplatensis in P. blochii confirms the host–parasite specificity of Unibarra spp. to fishes belonging to Pimelodidae and Doradidae.
Availability of data and material
Not applicable.
References
Van Steenberge M, Pariselle A, Huyse T, Volckaert FAM, Snoeks J, Vanhove MPM (2015) Morphology, molecules, and monogenoidean parasites: an example of an integrative approach to cichlid biodiversity. PLoS ONE 10(4):e0124474. https://doi.org/10.1371/journal.pone.0124474
Tavares-Dias M, Silva LMA, Oliveira MSB (2022) Geographic range, distribution patterns and interactions of Monogenea Van Beneden 1858, with species of native host freshwater fishes from Brazil. Braz J Vet Parasitol 31(3):e005722. https://doi.org/10.1590/S1984-29612022048
Ogawa K (2015) Diseases of cultured marine fishes caused by Platyhelminthes (Monogenea, Digenea, Cestoda). Parasitology 142(1):178–195
Reed P, Francis-Floyd R, Klinger R, Petty D (2019) Monogenean parasites of fish. Fisheries and aquatic sciences, IFAS Extension, University of Florida. FA28. https://edis.ifas.ufl.edu
Norbury LJ, Shirakashi S, Power C, Nowak BF, Bott NJ (2022) Praziquantel use in aquaculture—current status and emerging issues. Int J Parasitol Drugs Drug Resist 18:87–102
Boeger WA, Cohen SC, Domingues MV, Justo MCN, Pariselle A (2022) Monogenoidea in Catálogo Taxonômico da Fauna do Brasil. PNUD. Available at http://fauna.jbrj.gov.br/fauna/faunadobrasil/65
Reis RE (2013) Conserving the freshwater fishes of South America. Int Zoo Yearbook 47:65–70
Acosta AA, Scholz T, Blasco-Costa I, Alves PV, Silva RJ (2018) A new genus and two new species of dactylogyrid monogeneans from gills of Neotropical catfishes (Siluriformes: Doradidae and Loricariidae). Parasitol Int 67:4–12
Cohen, SC, Cárdenas MQ, Justo MCN (2021) Checklist de Monogenoidea parasitos de peixes Siluriformes do Brasil. In: Atualidades em Medicina Tropical na America do Sul: Veterinaria/Melchior LAK et al (org.). Stricto Sensu Editora, Rio Branco. doi: https://doi.org/10.35170/ss.ed.9786586283594.02
Yamada POF, Yamada FH, Silva RJ (2021) Three new species of Cosmetocleithrum (Monogenea, Dactylogyridae) gill parasites of Trachelyopterus galeatus (Siluriformes, Auchenipteridae) in Southern Brazil. Acta Parasitol 66:436–445. https://doi.org/10.1007/s11686-020-00282-3
Albert JS, Reis RE (2011) Introduction of neotropical freshwater. In: Albert JS, Reis RE (eds) Historical biogeography of neotropical freshwater fishes, 1st edn. University of California Press, California, pp 3–19
Mendoza-Palmero CA, Blasco-Costa I, Scholz T (2015) Molecular phylogeny of neotropical monogeneans (Platyhelminthes: Monogenea) from catfishes (Siluriformes). Parasit Vectors 8:164
Lundberg JG, Friel JP (2003) Siluriformes. Catfishes, version 20 January 2003 (under construction). http://tolweb.org/Siluriformes/15065/2003.01.20 in The Tree of Life Web Project (http://tolweb.org).
Santos RBS, Tavares-Dias M (2010) Células sanguíneas e resposta hematológica de Oxydoras niger (Pisces, Doradidae) oriundos da bacia do médio rio Solimões, estado do Amazonas, Brasil, naturalmente parasitados. Bol Inst Pesca SP 36:283–292
Silva AMO, Tavares-Dias M, Jerônimo GT, Martins ML (2011) Parasite diversity in Oxydoras niger (Osteichthyes: Doradidae) from the basin of Solimões River, Amazonas state, Brazil, and the relationship between monogenoidean and condition factor Braz. J Biol 71:791–796
Lundberg JG, Littmann MW (2003) Pimelodidae (long-whiskered catfishes). In: Reis RE, Kullander SO, Ferraris CJ (eds) Checklist of the freshwater fishes of South and Central America. EDIPUCRS, Porto Alegre, pp 432–446
Fricke R, Eschmeyer WN, Van der Laan R (eds) (2022) Eschmeyer’s catalog of fishes: genera, species, references. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp. Electronic version accessed 10 oct 2022.
Cavalcante PH, Silva MT, Pereira A, Gentile R, Santos CP (2020) helminth diversity in Pimelodus blochii Valenciennes, 1840 (Osteichthyes: Pimelodidae) in two Amazon rivers. Parasitol Res 119:4005–4015
Froese R, Pauly D (Eds) (2022) FishBase. World Wide Web electronic publication. www.fishbase.org, version Jun 2022
Maciel LG, Santos JS, de Araújo JA (2014) Relação das características morfométricas externas do mandi (Pimelodus blochii) em relação ao seu potencial de produção de filé. Rev Agrotec 35:113–120
Kritsky DC, Thatcher VE, Boeger WA (1986) Neotropical Monogenea. 8. Revision of Urocleidoides (Dactylogyridae, Ancyrocephalinae). Proc Helminthol Soc Wash 53:1–37
Morey GAM, Cachique JCZ, Babilonia JJS (2019) Cosmetocleithrum gigas sp. N. (Monogenoidea: Dactylogyridae) from the gills of Oxidoras niger (Siluriformes: Doradidae) from the Peruvian Amazon. Biología. https://doi.org/10.2478/s11756-019-00331-x
Negreiros LP, Tavares-Dias M, Pereira FB (2018) Community structure of metazoan parasites from Pimelodus blochii in two rivers of the Western Brazilian Amazon: same seasonal traits, but different anthropogenic impacts. Parasitol Res 117:3791–3798. https://doi.org/10.1007/s00436-018-6082-5
Negreiros LP, Tavares-Dias M, Periera FB (2019) Monogeneans of the catfish Pimelodus blochii Valenciennes (Siluriformes: Pimelodidae) from the Brazilian Amazon, with a description of a new species of Ameloblastella Kritsky, Mendoza-Franco & Scholz, 2000 (Monogenea: Dactylogyridae) Syst Parasitol 96:399–406. https://doi.org/10.1007/s11230-019-09862-y
Humason GL (1979) Animal tissue techniques. W.H. Freeman Co., USA, p 661
Freitas AJB, Bezerra CAM, Meneses YC, Justo MCN, Viana DC, Cohen SC (2021) Three new species of Urocleidoides (Monogenoidea: Dactylogyridae) parasitizing characiforms (Actinopterygii: Characiformes) in Tocantins River, states of Tocantins and Maranhão, and new record for U. triangulus in Guandu River, state of Rio de Janeiro, Brazil. Zoologia 38:e65001
Mizelle JD (1936) New species of trematodes from the gills of Illinois fishes. Am Mid Natur 17:785–806
Suriano DM, Incorvaia IS (1995) Ancyrocephalid (Monogenea) parasites from siluriform fishes from the ParaneanPlatean icthyogeographical province in Argentine. Acta Parasitol 40:113–124
Negreiros LP, Oliveira MSB, Tavares-Dias M (2019) First record of Unibarra paranoplatensis Suriano & Incorvaia, 1995 (Dactylogyridae: Monogenea) on Sorubim lima (Siluriformes: Pimelodidae) from Brazil. Braz J Vet Parasitol. https://doi.org/10.1590/S1984-29612019012
Beverley-Burton M, Suriano DM (1980) Cleidodiscus robustus Mueller, 1934 (Monogenea: Ancyrocephalidae) from Lepomis gibbosus L. (Pisces: Centrarchidae) in Ontario, Canada: anatomy and systematic position. Can J Zool 58:654–660
Gussev AV (1973–1974) Freshwater Indian Monogenoidea. Principles of systematics, analysis of the world faunas and their evolution. Indian J Helminthol 25–26:1–241
Gutiérrez PA (2001) Monogenean community structure on the gills of Pimelodus albicans from Rio de La Plata (Argentina): a comparative approach. Parasitology 122:465–470
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable institutional, national, and international guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Justo, M.C.N., de Oliveira Martins, W.M. & Cohen, S.C. A New Species of Unibarra (Monogenoidea, Dactylogyridae) Parasite of Oxydoras niger From Juruá River, State of Acre, Brazil and New Data for U. paranoplatensis. Acta Parasit. 68, 439–446 (2023). https://doi.org/10.1007/s11686-023-00681-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00681-2