Abstract
Introduction
The present study describes three new dactylogyrid species infecting the gill filaments of cichlid fishes (Cichliformes: Cichlidae) from the Amazon basin, Peru: Sciadicleithrum amazoniensis n. sp. on Biotodoma cupido (Heckel, 1840), and Sciadicleithrum feliciajaramae n. sp. and Sciadicleithrum souzatecci n. sp. on Bujurquina peregrinabunda Kullander, 1986.
Materials and methods
Some monogeneans were stained with Gomori’s trichrome and mounted in Canada Balsam to determine internal soft structures. Others were cleared in Hoyer’s medium for the study of sclerotized structures. Drawings were made using a drawing tube and a microprojector.
Results
Sciadicleithrum amazoniensis n. sp. is characterized by the presence of a male copulatory organ (MCO) with a coil of approximately 2 counterclockwise rings, an accessory piece articulated to base of the MCO with an expanded proximal end and a bifurcated distal end, and a sinistral vaginal aperture. Sciadicleithrum feliciajaramae n. sp. can be differentiated from all its congeners by its J-shaped MCO with about half a counterclockwise loop and a rod-shaped accessory piece articulated to the base of the MCO, with the distal end bent. Sciadicleithrum souzatecci n. sp. differs from all other members of Sciadicleithrum by having an elongated MCO with about a clockwise loop and a funnel-shaped base. Additionally, Sciadicleithrum souzatecci n. sp. is characterized by its weakly sclerotized, C-shaped accessory piece with a robust middle process.
Conclusions
Present findings are added to the other 26 species previously known in Sciadicleithrum.This is the first data on the parasites of B. peregrinabunda.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Amazonian aquatic ecosystem hosts a diverse range of organisms, spanning from invertebrates to mammals [1]. Fish, in particular, are of paramount ecological importance owing to their remarkable diversity, abundance, and intricate roles within Amazonian communities [2]. Artisanal fishing is linked to the cultural values and socioeconomic lifestyle specific to artisanal fishermen. Short-term fishing activities and the use of small vessels are characteristic of the group. Fishing represents an activity of economic importance and a protein source for the population. Many families have this practice not only for cultural purposes but also as income for their subsistence [3].
The region boasts an extensive catalog of more than 1000 documented species of fish, with approximately 50% of them awaiting formal taxonomic identification [2]. Among the diverse fish families, the cichlids (Cichlidae) stand out, enthralling researchers and enthusiasts alike with their striking coloration, behaviors, dietary preferences, and size variations. This allure has propelled them into a prominent position within the ornamental fish trade [4, 5].
Sciadicleithrum Kritsky, Thatcher & Boeger, 1989, was proposed to accommodate the type species Sciadicleithrum uncinatum Kritsky, Thatcher & Boeger, 1989, parasites of Cichla ocellaris (Cichlidae) from the Negro River near Manaus, Amazonas, Brazil [6]. Species belonging to Sciadicleithrum are characterized mainly by having overlapping gonads, a coiled cirrus with clockwise rings, unmodified anchors, a ventral bar with 2 umbelliform membranes or cavities on the anterior bar margin, and a similar hook with undilated shanks and erect thumbs. Kritsky et al. [6] transferred three species of Urocleidoides Mizelle & Price, 1964 Urocleidoides variablis Mizelle & Kritsky, 1969; Urocleidus aequidens Price & Schlueter, 1967; and Urocleidus cavanaughi Price, 1966 to Sciadicleithrum, due to those three species possessing all the generic features of Sciadicleithrum. Currently, this genus comprises 26 valid species distributed in the Neotropical region [7].
Freshwater fish represent a valuable resource, both for the economy and for the environment, which justifies a greater interest in these organisms from the most diverse areas of science. The number of studies on biology in general and on parasitic diseases has been increasing in recent years, although the latter is still far below expectations, given that the ratio of the diversity of Neotropical ichthyofauna does not match the diversity of parasites described to date [8]. During a parasitological survey of freshwater fish from the Peruvian Amazonia, three new species of Sciadicleithrum were found infecting the gills of two cichlid fishes, Biotodoma cupido (Heckel, 1840), and Bujurquina peregrinabunda Kullander, 1986. The new species are described and illustrated herein.
Materials and Methods
Thirty specimens of B. cupido and 30 of B. peregrinabunda were collected between May and June 2023 from the Tanshi Creek (3°54’S, 73°24’W), a tributary of the Nanay River in Iquitos, Loreto region, Peru. The fish hosts were collected by local fishermen and were transported alive to the Laboratory of Parasitology of the Instituto de Investigaciones de la Amazonía Peruana (IIAP), where they were dissected. Scientific names of hosts are those provided in FishBase [9]. The gills were excised and placed in vials containing heated water (approximately 68 °C). Each vial was vigorously shaken, and 96% ethanol was added. In the laboratory, the content of each vial was examined using a stereomicroscope and monogeneans were removed from the gills or sediment using dissection needles. Some specimens were stained with Gomori’s trichrome [10, 11] and mounted in Canada Balsam to study internal organs, while others were cleared in Hoyer’s medium for the study of sclerotized structures [10]. Sclerotized structures of all monogeneans were examined and photographed using a LEICA DM750 phase-contrast microscopy optics with LEICA- ICC50W HD camera and Software LAS (Leica Application Suite), EZ version 1.80, 2009, Switzerland. Measurements are expressed as the range followed by the mean and number (n) of structures measured in parentheses. Measurements, all in micrometers, were made following the procedures of Mizelle & Klucka [12]. The description of the male copulatory organ (MCO) follows Kritsky et al. [13] and Bellay et al. [14]. Lengths of or curved or bent structures (anchors, bars, and accessory pieces) represent the straight-line distances between extreme ends. Illustrations were prepared with the aid of a drawing tube and microprojector.
The type specimens were deposited in the Helminthological Collection of the Museum of Natural History at the San Marcos University (MUSM) Peru and in the collection of the Laboratorio de Parasitología y Sanidad Acuícola of the Instituto de Investigaciones de la Amazonía Peruana (LAPYSA).
To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new taxon have been submitted to ZooBank (i.e., generic name, specific name). The Life Science Identifier (LSID) is reported in the taxonomic summary.
Result
Order Dactylogyridea Bychowsky, 1933
Dactylogyridae Bychowsky, 1933
SciadicleithrumKritsky, Thatcher & Boeger, 1989
Sciadicleithrum amazoniensisn. sp.
Syn. Sciadicleithrum sp. 2 of Morey et al. [13]
(Fig. 1A-G)
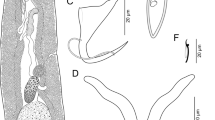
Description (based on 6 specimens mounted in Hoyer’s medium and 4 stained with Gomori’s trichrome): with the characteristics of the genus as described by Kritsky et al. [11] and Bellay et al. [12]. Body fusiform (Fig. 1A), 265–339 (301; n = 6) long; greatest width 111–130 (116; n = 6) usually at level of testis. Tegument smooth. Cephalic region moderately broad; cephalic lobes inconspicuous. Three bilateral pairs of inconspicuous head organs. Eyespots 4, with posterior pair being larger; few accessory granules scattered in cephalic region. Pharynx spherical, muscular, glandular, 15–24 (20; n = 6) in diameter. Esophagus short to absent, intestinal caeca confluent posteriorly to gonads, lacking diverticula. Peduncle short, broad. Haptor globose (Fig. 1A), differentiated from body proper, with two pairs of anchors and two bars. Anchors dissimilar, with fine conspicuous alae (Fig. 1B-C). Ventral anchor 47–52 (50; n = 6) long, with elongate superficial root, poorly developed deep root, slightly curved shaft and recurved point (Fig. 1B). Dorsal anchor 21–26 (23; n = 6) long, elongate superficial root, dome-shaped deep root, curved shaft, and recurved point (Fig. 1C). Ventral bar elongate, with bifurcated enlarged ends posteriorly directed (Fig. 1D), 27–40 (33; n = 6) long, 3–5 (4; n = 6) wide. Dorsal bar elongate, almost dumbbell-shaped, with enlarged ends posteriorly directed (Fig. 1E), 28–37 (32; n = 6) long, 2–4 (3; n = 6) wide. Seven pairs of hooks, similar in shape, 10–14 (112; n = 6) long, with distribution as proposed by Mizelle [14] (1–5 pairs ventral, 6–7 pairs dorsal), each with erected thumb, straight shank, and short and recurved point; filamentous hook (FH) loop 2/3 of shank length (Fig. 1F). Male copulatory organ (MCO), 17–26 (22; n = 6) long, comprising a coil of around 2 counterclockwise rings (Fig. 1G). Accessory piece thin, with expanded proximal end, bifurcated distal end, articulated with MCO base (Fig. 1G), 9–13 (1812; n = 6) long. Gonads overlapping, testis dorsal to germarium (Fig. 1A). Testis ovate; vas deferens looping left intestinal caeca, dilating to form a big sigmoid seminal vesicle; oval prostatic reservoir, posterior to MCO; prostatic glands not observed. Germarium elongate, with irregular margin; oviduct, oötype and uterus not observed. Vaginal aperture sinistral, with funnel-shaped, vaginal vestibule inconspicuous, vaginal duct narrow, seminal receptacle small, ovate (Fig. 1A). Vitelline follicles dense, extending from posterior level of pharynx to posterior end of trunk, absent in regions of reproductive organs (Fig. 1A). Eggs not observed.
Taxonomic Summary
Type host: Biotodoma cupido (Heckel, 1840) (Cichliformes: Cichlidae).
Site in host: Gill filaments
Type Locality: Tanshi creek (3°54’S, 73°24’W), Iquitos City, Loreto region, Peru, South America.
Type Material: Holotype (MUSM 5376); 5 paratypes (MUSM 5377 a–e); 4 paratypes (LAYPSA M-88 a–d).
ZooBank Registration: LSIDurn:lsid:zoobank.org:act:27AE2171-260B-4F4E-8978-96FCCC85C260
Parasitological Indices: Examined fish = 30; parasitized fish = 11; prevalence of infestation = 30%; mean intensity of infestation = 1.66; mean abundance of infestation = 0.55.
Etymology: The specific name refers to the ecological region (Amazonia) where the specimens were collected.
Remarks
Based on the morphology of the copulatory complex (a MCO with 2 rings and an accessory piece with bifurcated distal end), Sciadicleithrum amazoniensis n. sp. most closely resembles S. araguariensis Paschoal, Scholz, Tavares-Dias & Luque, 2016, an ectoparasite of Crenicichla labrina (Spix & Agassiz, 1831) in Brazil. Both species can be distinguished by the following characteristics: in S. amazoniensis n. sp., the accessory piece has an expanded proximal end and is articulated with the MCO base, where in S. araguariensis, the accessory piece is unarticulated with the MCO base, and its proximal end is not expanded. In addition, S. amazoniensis n. sp. has a sinistral vaginal aperture (a dextral vaginal aperture in S. araguariensis).
Sciadicleithrum feliciajaramaen. sp.
Syn. Sciadicleithrum sp. 3 of Morey et al. [13]
(Fig. 2A-G)
Description (based on 8 specimens mounted in Hoyer’s medium and 2 stained with Gomori’s trichrome): with the characteristics of the genus as described by Kritsky et al. [11] and Bellay et al. [12]. Body elongate, fusiform (Fig. 2A), 282–434 (352; n = 10) long; greatest width 73–202 (132; n = 10) usually at level of MCO. Tegument smooth. Cephalic region slightly broad; cephalic lobes inconspicuous. Three bilateral pairs of conspicuous head organs. Eyespots 4, with posterior pair being larger; few accessory granules scattered in cephalic region. Pharynx spherical, muscular, glandular, 15–18 (16; n = 10) in diameter. Esophagus short to absent, intestinal caeca confluent posteriorly to gonads, lacking diverticula. Peduncle broad. Haptor subhexagonal (Fig. 2A), differentiated from body proper, with two pairs of anchors and two bars. Anchors dissimilar, with fine conspicuous alae (Fig. 2B-C). Ventral anchor 39–63 (51; n = 10) long, with tapering broad superficial root, dome deep root, slightly curved shaft, and short point (Fig. 2B). Dorsal anchor 35–47 (42; n = 10) long, with slightly appressed roots, subtriangular superficial root, short deep root, curved shaft, and short point (Fig. 2C). Ventral bar almost V-shaped, robust with anteromedial slightly knob and enlarged ends posteriorly directed (Fig. 2D), 52–69 (59; n = 10) long, 7–12 (11; n = 10) wide. Dorsal bar elongate, with inconspicuous anteromedial projection and enlarged ends posteriorly directed (Fig. 2E), 61–85 (73; n = 10) long, 8–13 (11; n = 10) wide. Seven pairs of hooks, similar in shape and size, 14–23 (19; n = 10) long, with distribution as proposed by Mizelle [14] (1–5 pairs ventral, 6–7 pairs dorsal), each with protruding and obtuse thumb, expanded posteriorly shank, and short and recurved point; filamentous hook (FH) loop 2/3 of shank length (Fig. 2F). MCO, 10–28 (20; n = 9) long, short, J-shaped, comprising about half a clockwise loop (Fig. 2G). Accessory piece sinuous, thin, rod-shaped, articulated with MCO base (Fig. 2G), 13–23 (18; n = 8) long. Gonads overlapping, testis dorsal to germarium (Fig. 2A). Testis ovate; vas deferens looping left intestinal caeca, dilating to form a short sack-shaped seminal vesicle; small prostatic reservoir, posterior to MCO; prostatic glands not observed. Germarium elongate; oviduct, oötype and uterus not observed. Vaginal aperture dextral; vaginal vestibule inconspicuous; vaginal duct narrow; seminal receptacle big, drop-shaped (Fig. 2A). Vitelline follicles dense, extending from posterior level of pharynx to posterior end of trunk, absent in regions of reproductive organs (Fig. 2A). Eggs not observed.
Taxonomic Summary
Type host: Bujurquina peregrinabunda Kullander, 1986 (Cichliformes: Cichlidae).
Site in host: Gill filaments.
Type Locality: Tanshi creek (3°54’S, 73°24’W), Iquitos City, Loreto region, Peru, South America.
Type Material: Holotype (MUSM 5378); 5 paratypes (MUSM 5379 a–e); 4 paratypes (LAYPSA M-89 a–d).
ZooBank Registration: LSIDurn:lsid:zoobank.org:act:050E58DA-B664-495C-8F86-DD2635027A60.
Etymology: The new species is named in honor of Felicia Diaz Jarama (National University of Peruvian Amazon) (in memoriam) in recognition of her valuable contribution to the education of young Peruvian professionals.
Remarks
Among of Sciadicleithrum species, the new species most closely resembles Sciadicleithrum umbilicum Kritsky, Thatcher & Boeger, 1989 from Cichla ocellaris Bloch & Schneider, 1801 in Brazil, due to its short MCO and rod-shaped accessory piece. However, the new species is easily differentiated from S. umbilicum by its J-shaped MCO (sinuous MCO in S. umbilicum) and by the morphology of the distal end of the accessory piece (a bent distal end in the new species vs. a ring-shaped distal end in S. umbilicum).
Sciadicleithrum souzateccin. sp.
Syn. Sciadicleithrum sp. 4 of Morey et al. [13]
(Fig. 3A-G)
Description (based on 8 specimens mounted in Hoyer’s medium and 2 stained with Gomori’s trichrome): with the characteristics of the genus as described by Kritsky et al. [11] and Bellay et al. [12]. Body elongate, fusiform (Fig. 3A), 205–451 (306; n = 10) long; greatest width 64–151 (104; n = 10) usually at level of MCO. Tegument smooth. Cephalic region slightly broad; cephalic lobes poorly developed. Three bilateral pairs of conspicuous head organs. Eyespots 4, with posterior pair being larger; few accessory granules scattered in cephalic region. Pharynx spherical, muscular, glandular, 23–32 (27; n = 10) in diameter. Esophagus short to absent, intestinal caeca confluent posteriorly to gonads, lacking diverticula. Peduncle broad. Haptor subhexagonal (Fig. 3A), differentiated from body proper, with two pairs of anchors and two bars. Anchors dissimilar, with fine conspicuous alae (Fig. 3B-C). Ventral anchor 29–53 (36; n = 10) long, with tapering broad superficial root, inconspicuous deep root, slightly curved shaft, and short point (Fig. 3B). Dorsal anchor 33–47 (38; n = 10) long, with slightly appressed roots, tapered superficial root, short deep root, curved shaft, and short point (Fig. 3C). Ventral bar almost W-shaped, robust with posteromedial notch and enlarged ends anteriorly directed (Fig. 3D), 23–57 (43; n = 10) long, 7–11 (8; n = 10) wide. Dorsal bar elongate, rod-shaped, with posterolateral notches (Fig. 3E), 29–66 (47; n = 10) long, 6–12 (9; n = 10) wide. Seven pairs of hooks, similar in shape, 12–17 (14; n = 10) long, with distribution as proposed by Mizelle [14] (1–5 pairs ventral, 6–7 pairs dorsal), each with erected thumb, slightly curved shank, and short and recurved point; filamentous hook (FH) loop 2/3 of shank length (Fig. 3F). MCO 19–32 (23; n = 9) long, elongated, tubular, slender, comprising about a clockwise loop, funnel-shaped base (Fig. 3G). Accessory piece articulated with MCO base, weakly sclerotized, almost C-shaped, with robust middle process (Fig. 3G), 13–32 (21; n = 8) long. Gonads overlapping, testis dorsal to ovary (Fig. 3A). Testis ovate; vas deferens looping left intestinal caeca, dilating to form a short fusiform seminal vesicle; elongate prostatic reservoir, posterior to MCO; prostatic glands not observed. Ovary elongate; oviduct, oötype and uterus not observed. Vaginal aperture sinistral; vaginal vestibule inconspicuous; vaginal duct narrow; seminal receptacle big, ovate (Fig. 3A). Vitelline follicles dense, extending from posterior level of pharynx to posterior end of trunk, absent in regions of reproductive organs (Fig. 3A). Eggs not observed.
Taxonomic Summary
Type host: Bujurquina peregrinabunda Kullander, 1986 (Cichliformes: Cichlidae).
Site in host: Gill filaments.
Type Locality: Tanshi creek (3°54’S, 73°24’W), Iquitos City, Loreto region, Peru, South America.
Type Material: Holotype (MUSM 5380); 5 paratypes (MUSM 5381 a–e); 4 paratypes (LAYPSA M-90 a–d).
ZooBank Registration: LSIDurn:lsid:zoobank.org:act:50CC3105-B800-445E-B755-C4E250DF65E8.
Etymology: The new species is named in honor of Javier Souza Tecco (National University of Peruvian Amazon) (in memoriam) in recognition of his valuable contribution to the education of young Peruvian professionals.
Remarks
Sciadicleithrum souzatecci n. sp. can be distinguished from all its congeners by the presence of a slender MCO with about a clockwise loop and a funnel-shaped base. Additionally, Sciadicleithrum souzatecci n. sp. is characterized by its weakly sclerotized, C-shaped accessory piece with a robust middle process.
Discussion
The present study provides new data on dactylogyrid monogeneans parasitizing cichlid fishes in Peru. Three new species of Sciadicleithrum were found on fish of two genera, i.e. Biotodoma and Bujurquina. In Peru, dactylogyrids of genera Biotodomella, Dactylogyrus, Gussevia, Sciadicleithrum, Urocleidus, Trinidactylus, and Tucunarella, have been reported infecting cichlid fish [15,16,17]. This represents the first data on monogenean species infecting B. peregrinabunda. As for B. cupido, the dactylogyrid Biotodomella mirospinata Morey, Arimuya & Boeger, 2019 is the only known species [17]. The new species infecting the gills of B. cupido described here increases the number of known monogeneans for this fish species to two.
The majority of the species of Sciadicleithrum, including the three new species described here, exhibited a strict (oioxenous) host specificity. This high degree of specificity can be attributed to the evolutionary adaptations that these monogeneans have developed to exploit their particular hosts’ unique physiological and ecological niches. Such specialization often leads to a co-evolutionary relationship where both the parasite and the host exert selective pressures on each other, driving their mutual evolutionary trajectories. However, there are exceptions to this host specificity. Three species have been documented to occur on more than one host species, demonstrating a lower degree of host specificity. For instance, S. joanae Yamada, Takemoto, Bellay & Pavanelli, 2009 has been found on both Crenicichla britskii Kullander, 1982 and C. niederleinii (Holmberg, 1891); S. satonopercae Yamada, Takemoto, Bellay & Pavanelli, 2009 on Satanoperca pappaterra (Heckel, 1840) and S. jurupari (Heckel, 1840); and S. variabile (Mizelle & Kritsky, 1969) Kritsky, Thatcher & Boeger, 1989 on Symphysodon discus Heckel, 1840 and Cichlasoma amazonarum Kullander, 1983 [18]. The ability to infest multiple host species could be advantageous, allowing these parasites to exploit a broader range of ecological niches. This flexibility can reduce their dependency on a single host species and potentially buffer them against host population fluctuations. However, it also requires these parasites to possess or develop adaptations that enable them to overcome the immune defenses and physiological differences of multiple host species.
The data obtained in the present study expands the known number of Sciadicleithrum species to 29, indicating the necessity for further comprehensive investigations to fully elucidate the real diversity within this genus.
References
Milliman J, Farnsworth K (2011) River discharge to the coastal ocean: a global synthesis. Cambridge University Press, Cambridge
Dagosta FCP, de Pinna M (2019) The fishes of the Amazon: distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Museum Nat History 431:1–163
Barbosa LA, Viana DC, Queiroz C (2020) Characterization of artisanal fishing and commercialization of fish in open air markets. Revista eletrônica científica ensino interdisciplinar, 6(19)
Ortega H, Guerra H, Ramírez R (2007) The introduction of nonnative fishes into freshwater systems of Peru. In: Bert TM (ed) Ecological and genetic implications of aquaculture ac-tivities, vol 6. Springer, New York, pp 247–278
Perez-Sanchez E, Páramo-Delgadillo S (2008) The culture of cichlids of southeastern Mexico. Aquac Res 39:777–783
Kritsky DC, Thatcher VE, Boeger WA (1989) Neotropical Monogenea. 15. Dactylogyridae from the gills of Brazilian Cichlidae, with the proposal of Sciadicleithrum gen. n. Proceedings of the Helminthological Society of Washington, 56, 128–140
Paschoal F, Scholz T, Tavares-Dias M, Luque J (2016) Dactylogyrids (Monogenea) parasitic on cichlids from northern Brazil, with description of two new species of Sciadicleithrum and new host and geographical records. Acta Parasitol 61:158–164
Cohen SC, Justo MCN, Cárdenas MQ, Meneses YC, Bezerra CA, Viana DC (2020) Conceitos básicos e estado da arte dos helmintos parasitos de peixes da bacia Tocantins-Araguaia. In J. A. Prandel (Org.), Conhecimentos Teóricos, Metodológicos e Empíricos para o Avanço da Sustentabilidade no Brasil (1st ed, pp. 54–74). Editora Atena, Paraná. https://doi.org/10.22533/at.ed.943203001
Froese R, Pauly D (2023) FishBase. World Wide Web electronic publication. http://www.fishbase.org. Accessed on 19 Janeyro, 2023
Boeger W, Vianna N (2006) Monogenoidea. Joachim Adis; Jorge Arias; Guillermo Rueda-Delgado; Karl Matthias Wantzen Organization. Biodiversidad Acuática en Latinoamerica. Amazon fish parasites, pp 42–116
Humason GL (1979) Animal tissue techniques, 4th edn. W. H. Freeman and Co., San Francisco, p 661
Mizelle JD, Klucka AR (1953) Studies on monogenetic trematodes. XVI. Dactylogyridae from Wisconsin fishes. Am Midl Nat 49:720–733
Kritsky DC, Boeger WA, Thatcher VE (1985) Neotropical Monogenea. 7. Parasites of the pirarucu Arapaima gigas (Cuvier), with descriptions of two new species and redescription of Dawestrema cycloancistrium Price and Nowlin, 1967 (Dacty-logyridae: Ancyrocephalinae). Proceedings of the Biological Society of Washington, 98, 321–331
Bellay S, Takemoto RM, Yamada FH, Pavanelli GC (2009) Two new species of Sciadicleithrum (Monogenea: Dactylogyridae), gill parasites of Geophagus proximus (Castelnau) (Teleostei: Cichlidae), from the upper Paraná River floodplain, Brazil. Zootaxa 2081:57–66
Mizelle JD, Price CE (1963) Additional haptoral hooks in the genus Dactylogyrus. J Parasitol 19:785–806
Morey GAM, Tafur KMR, de Souza AL, Guimaraes JLC, Rojas CAT, Vela LJH, Garcia SMC, Pizango HAD, Alvarado CJS, Chota HDR, Navas ME, Reátegui RN, Guerra RC, Isern ER (2023) Species of Monogenoidea from cichlids with commercial importance in the Peruvian Amazon. Neotropical Helminthology 17:191–195
Morey GAM, Arimuya MV, Boeger WA (2019) Neotropical Monogenoidea 62. Biotodomella mirospinata gen. nov., sp. nov.(Polyonchoinea: Dactylogyridae): a parasite of the gills of Biotodoma cupido (cichliformes: Cichlidae), from the Peruvian Amazon. Zoologia (Curitiba), 36, e38455
Yamada FH, Takemoto RM, Bellay S, Pavanelli GC (2009) Twonew species of Sciadicleithrum (Monogenea: Dactylogyridae) parasites of neotropical cichlid fishes from the Paraná River,Brazil. Acta Parasitol 54:6–11
Acknowledgements
We would like to express our gratitude to the ACUIPRO project of the IIAP for the support provided in conducting this study.
Author information
Authors and Affiliations
Contributions
The study was designed by GAM, HAM, and ALS. Collection of parasites was performed by GAM, HAM, MVA and ALS. Laboratory were performed by GAM, CLC and JDC. The manuscript was written by GAM, CLC and JDC and subsequently revised by all other authors.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable institutional, national, and international guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morey, G.A.M., Dávila, H.A., Arimuya, M.V. et al. Three New Species of Sciadicleithrum (Monogenoidea, Dactylogyridae) Parasitizing Cichlid Fishes (Cichliformes: Cichlidae) in the Northeastern Peru. Acta Parasit. 69, 1674–1681 (2024). https://doi.org/10.1007/s11686-024-00895-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-024-00895-y