Abstract
This study aimed to detect alterations in intra- and inter-network functional connectivity (FC) of multiple networks in acute brainstem ischemic stroke patients, and the relationship between FC and movement assessment scores to assess their ability to predict upper extremity motor impairment. Resting-state functional magnetic resonance imaging (rs-fMRI) data were acquired from acute brainstem ischemic stroke patients (n = 50) and healthy controls (HCs) (n = 45). Resting-state networks (RSNs) were established based on independent component analysis (ICA) and the functional network connectivity (FNC) analysis was performed. Subsequently, correlation analysis was subsequently used to explore the relationship between FNC abnormalities and upper extremity motor impairment. Altered FC within default mode network (DMN), executive control network (ECN), the salience network (SN), auditory network (AN), and cerebellum network (CN) were found in the acute brainstem ischemic stroke group relative to HCs. Moreover, different patterns of altered network interactions were found between the patients and HCs, including the SN-CN, SN-AN, and ECN-DMN connections. Correlations between functional disconnection and upper limb dysfunction measurements in acute brainstem ischemic stroke patients were also found. This study intimated that widespread FNC impairment and altered integration existed in brainstem ischemic stroke at acute stage, suggesting that FNC disruption may be applied for early diagnosis and prediction of upper limb dysfunction in acute brainstem ischemic stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute ischemic stroke is a common and frequently-occurring disease, and the incidence is showing a younger trend (Iyer et al., 2019; Zhao et al., 2019). Because of its high disability rate and mortality rate (Powers et al., 2015), it often brings a heavy burden to the family and society. More than one-third of patients with acute ischemic stroke are often accompanied by cognitive and lateral movement disorders, including attention, memory, executive, language, visual field defects, which adversely affects the outcome (Pohjasvaara et al., 2002). Most of these damages are irreversible. Brainstem ischemic stroke accounting for 21.9% has a rapid onset and more dangerous condition compared with ischemic stroke in other parts (Mortality GBD, 2016). Studies have shown that early functional exercise is beneficial to the recovery of dysfunction. Therefore, the evaluation of early cognitive and movement disorders in patients with acute brainstem ischemic stroke is extremely important.
Over recent years, resting-state functional magnetic resonance imaging (rs-fMRI), by measuring the features of low-frequency blood oxygenation level-dependent (BOLD) fluctuations (Biswal et al., 1997; Hampson et al., 2002; Damoiseaux et al., 2006), has grown to become a reliable method for exploring human brain functional connectivity (FC) (Biswal et al., 2010; Guerra-Carrillo et al., 2014; Buckner et al., 2013), especially for evaluating the FC of distant brain regions (Raichle, 2001; Ogawa et al., 1990). Previous studies mostly focused on acute cerebral stroke in the paraventricular and basal ganglia area (Yao et al., 2020). There were few studies exploring the FC changes in patients with acute brainstem ischemic stroke. Our previous research showed significantly decreased functional connectivity in the right medial prefrontal cortex (mPFC) and right precuneus within the anterior and posterior DMN in patients with acute brainstem ischemic stroke, respectively (Jiang et al., 2018). A study of 53 patients with acute ischemic brainstem stroke found significant differences in topological properties, suggesting that the damaged brain network after a brainstem stroke tended to shift toward regular networks. The findings of disrupted topological properties of functional brain networks may help better understand the disease characterization (Shi et al., 2021). The default-mode network (DMN), an important resting-state functional network of the brain, has been associated with emotional and cognitive processing (Broyd et al., 2009; Mantini & Vanduffel, 2013). The cognitive decline has been related to the FC impairment of the DMN in ischemic stroke with variously located lesions (Tuladhar et al., 2013), such as the internal capsule, corona radiata, thalamus, occipital lobe and parietal lobe. However, little is known whether the regional spontaneous activity of the DMN is also damaged in patients with acute brainstem ischemic stroke. These studies mostly focused on a small number of changes in intra-network interactions, and did not analyze the interactions between each network. Independent component analysis (ICA) has been proverbially used for identifying resting-state networks (RSNs) due to its ability to separate various brain function networks to define different remote interaction patterns (De Luca et al., 2006; Fox & Raichle, 2007). For the present, the function network connectivity (FNC) can be used to represent the temporal correlation between these RSNs (Jafri et al., 2008; Qin et al., 2018). Therefore, observation of the RSNs and FNC may come up with more information to proceed the understanding of the underlying neural mechanisms of cognitive and motor impairment in acute brainstem ischemic stroke.
We hypothesize that brainstem patients showed different functional brain network disruption and were related to the patients’ upper extremity motor dysfunction. The brainstem is the most common site for subcortical stroke involving the motor pathway and they could cause anterograde and retrograde degeneration in the pyramidal tract in the early stage of stroke. Previous studies indicated that topological properties of functional brain networks were disrupted (Shi et al., 2021). Herein, we aimed to systematically investigate the changes in RSNs of acute brainstem stroke and the interactions between RSNs. The temporal correlation of brain networks’ activity was used to quantify their interactions and to predict the extent to which group differences could forecast the characteristics of movement disorders after stroke. This could guide the early rehabilitation after stroke and reduce the impact of motor dysfunction on the lives of stroke patients.
Materials and methods
Subjects and clinical data
A total of 50 patients with acute brainstem ischemic stroke (28 males and 22 females; age range: 45–87 years) were recruited from the emergency department of our hospital between February 2017 and February 2019. The inclusion criteria for patients with acute brainstem ischemic stroke were as follows: (a) age 45 years or older; (b) an initial Glasgow Coma Score (GCS) of 12–15 in the emergency department; (c) initial emergency room evaluation of acute brainstem ischemic stroke (accompanied by physical weakness, language impairment and other symptoms of acute cerebral stroke and confirmed by magnetic resonance images); and (d) CT scan as clinical evaluation to rule out cerebral hemorrhage. The exclusion criteria were as follows: (a) a history of a preceding acute cerebral stroke; (b) history of drug or alcohol misuse; (c) history of sedative use in hospitals or emergency rooms; and (d) MRI contraindications. In addition, 45 healthy subjects (22 males and 23 females; age range: 48–78 years) were recruited as the healthy control (HC) group and matched for sex, and age. The same exclusion criteria were adopted as the patient group. All HCs underwent the same motor function assessment as acute brainstem ischemic patients.
Motor function assessment
Fugl-Meyer assessment (FMA) was performed to assess upper extremity motor disorder. This test consisted of wrist, elbow, shoulder, forearm, hand movement and coordination. The assessment time was approximately 10 min, with a maximum score of 66, a higher score implies less upper limb dysfunction (Platz et al., 2005). Motor Assessment Scale (MAS) was also performed to assess upper extremity motor disorder (Poole & Whitney, 1988). The assessment time was approximately 10 min, with a maximum score of 48.
Imaging data acquisition
All MRI data were acquired within 8 days after stroke on a 3.0 Tesla MRI scanner (Ingenia, Philips Medical Systems) with an eight-channel phased-array head coil. For this analysis, resting-state functional images were acquired axially using a gradient echo-planar imaging sequence. The scanning parameters were as follows: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; gap = 0 mm; slices = 36; thickness = 4 mm; field of view (FOV) = 240 mm × 240 mm; acquisition matrix = 64 × 64; and flip angle (FA) = 90°. The rs-fMRI sequence scan took 8 min and 6 s. Structural images were obtained using a three-dimensional turbo fast echo (3D-TFE) T1WI sequence and following scan parameters: TR/ TE = 8.1/3.7 ms; slices = 170; thickness = 1 mm; gap = 0 mm; FOV = 256 mm × 256 mm; FA = 8°; and acquisition matrix = 256 × 256. The structural sequence lasted 5 min and 28 s. During the scan, the subjects need to keep quiet and still, close their eyes and not think about any special things. In the meantime, foam padding was used to reduce the involuntary movement of the head, and earplugs were used to reduce the influence of noise on the participants. In addition, 3D gradient echo susceptibility-weighted imaging (SWI) sequences (TR/ TE = 22/34 ms; slice thickness = 1 mm; FA = 20°; matrix = 276 × 319; and FOV = 220 mm × 220 mm) were also implemented to help detect hemorrhagic or other lesions.
Data preprocessing
Graph Theoretical Network Analysis (GRETNA) was applied to preprocess the functional image data for further analysis with the following stages (Wang et al., 2015). First, the first 10 volumes of each time series were removed to allow for participant adaptation to the scanning environment. Then the remaining volumes were slice-timing corrected and calibrated for head motion correction. Participant data were excluded from the analysis, including demonstrating head movement >2.0 mm translation or > 2.0° rotation. The rest of the dataset was spatially normalized to a template from the Montreal Neurological Institute (resampled voxel size = 3 × 3× 3 mm3), followed by a 6-mm spatial sequence with a Gaussian smoothing kernel. The corrected volumes were spatially normalized to the Montreal Neurological Institute space with resampled voxel size = 3 × 3 × 3 mm3, and finally spatially smoothed with a Gaussian smooth kernel (full width at half maximum of 6 mm).
Independent component analysis
RSNs were selected with the group ICA software of fMRI toolbox software. ICA analysis is performed in three phases: (1) data reduction, (2) application of the ICA algorithm, and (3) back reconstruction for each individual subject. The number of independent components (ICs) was determined by using the minimum description length (MDL) criteria (Li et al., 2007). In phase one, reduce computational complexity with principal component analysis (PCA), then the remaining reduction step was achieved again using PCA in view of a selected number of ICs. In phase two, run the proper ICA with the infomax algorithm. In the final phase, Single-subject individual time courses and spatial maps were group ICA (GICA) type back reconstructed and results were converted into z-scores to display.
Intra-network connectivity analysis
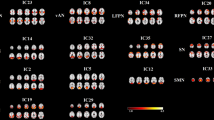
Among the 32 components resulting from ICA, 12 components were selected (7 nonartifactual RSNs) as the focus of the subsequent analyses (Fig. 1) through visual inspection according to previous rs-fMRI studies (Cerliani et al., 2015). The single-sample t test was first used to obtain the z-maps for each group and each RSN. The significance threshold was p < 0.01, corrected for multiple comparisons using FDR. Then, the group comparison of the z-maps of the RSNs was conducted using a two-sample t test restricted to the voxels within a union mask and was determined by the t test results of two single samples. Between-group effects were corrected by FDR correction, the significance threshold was p < 0.01. In the two-sample t test, regions with significant differences were chosen and used in the subsequent analysis from each RSN.
Inter-network connectivity analysis
After ICA, the individual level time courses of the identified RSNs were obtained using the spatiotemporal double regression method. Then, the relationship between different RSNs time courses was studied by FNC analysis. During the analysis, a time-domain band-pass filter (band-pass 0.00–0.25 Hz) was used to reduce the influence of low-frequency drift and high-frequency physiological noise on the time process. Secondly, we calculated the correlations between any two RSNs time courses of each subject. Then the FNC known as temporal correlation is obtained by calculating the Pearson correlation coefficient of the time courses of selected RSNs and generate the matrix of 12 × 12 (RSNs) × 95 (participants). In the general linear model was finally used to analyze which pairs of RSNs were significantly different between controls and patients, the age and sex as covariates. The significance threshold was p < 0.001, corrected for multiple comparisons using FDR.
Correlation analysis
The correlations between FC in the RSNs/FNC and the motor function assessments were calculated in acute brainstem ischemic stroke patients. In the two-sample t test, the brain region with a significant difference for each RSN was selected as the region of interest (ROI) to extract the coordinates of the ROIs. Then the mean z-scores within the ROI was used to perform the relevant calculation. Moreover, the FNC coefficients that showed a highly significant difference between two groups were also used in the correlations with the FMA and MAS scores.
Statistical analysis
An independent t test for continuous variables and a chi-square test for proportions were used to assess clinical characteristic and demographic between patients and HCs by the SPSS 19.0 software package (SPSS, Inc). The significance threshold was set as p < 0.05. Shapiro-Wilk tests were used to evaluate data normality, and p values >0.05 indicate a normal distribution of data. For RSN and FNC analysis, group comparisons between the acute brainstem ischemic stroke and HC group were performed using two-sample t tests. The significance threshold was set at p < 0.01 and p < 0.001 using FDR corrections respectively. Age and sex were used as covariates. Additionally, Pearson’s correlation coefficients between functional connection strength and FMA scores were analyzed with a significance level of p < 0.05. SPM12 was used for voxel-level statistical analysis of the RSNs, and MATLAB (MATLAB 2013a) was used for other statistical analyses, including FNC group comparisons and correlation analyses.
Results
Participants and clinical data
The final cohort in this study consisted of 50 acute brainstem ischemic stroke patients and 45 HC subjects, four patients were excluded because of excessive head motion artifacts after the fMRI data head motion check. The lesions in acute brainstem ischemic stroke group were all laterally distributed, with 25 cases on each side. The mean GCS scores for stroke patients were 14.24 ± 0.91. There was no significant difference in age and gender between the acute brainstem ischemic stroke group and the HC group (Table 1). Moreover, there was no brain hemorrhage or bleeding in the stroke patients.
ICA and component selection
A total of 32 ICs was extracted by ICA in this study, among which 12 components were selected as the RSNs for further analysis in the light of previously published results (Fig. 1). Subsequently, seven networks with these components were labeled as follows: the default mode network (DMN) (IC11 + 14 + 19) typically included the posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), inferior parietal, precuneus, and bilateral angular gyrus nodes. The executive control network (ECN) (IC16 + 20) included the left lateral frontoparietal network (LFPN) and the right lateral frontoparietal network (RFPN). The LFPN was mainly focused at the left middle frontal gyrus, superior parietal lobule, inferior parietal lobule, and angular gyrus; The spatial distribution of RFPN is similar to that of LFPN. The salience network (SN) (IC7) showed spatial patterns mainly consisting of the dorsal anterior cingulate (dACC), anterior insular cortices, part of the prefrontal areas. The visual network (VN) (IC1+ 10 + 12) included the primary visual cortex [the bilateral calcarine sulcus and medial extra-striate regions (eg. the lingual gyrus and cuneus)] and the occipital part of the fusiform gyrus and extravisual (the occipital pole, the lateral occipital cortex). The auditory network (AN) (IC24) mainly included the bilateral intraparietal sulcus, middle temporal lobe and frontal eye field. The sensorimotor network (SMN) (IC23) comprised SMN1 that included the paracentral lobule, the supplementary motor area (SMA), and the pre- and postcentral gyri; SMN2 was mainly focused at the bilateral primary somatosensory cortex, including postcentral and precentral gyri areas. The cerebellum network (CN) (IC15) had spatial patterns that primarily encompassed the cerebellum anterior lobe, cerebellum posterior lobe, and declive.
Altered FC within RSNs
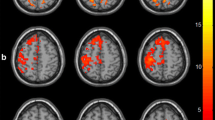
We found significant differences between the patient and HC groups within two RSNs, including the DMN and ECN (Table 2 and Fig. 2A). Compared with the HC group, the patients group exhibited decreased FC within the DMN [right frontal inferior orbital gyrus (R_FIO) and left temporal middle gyrus (L_TM)] and ECN [right frontal middle gyrus (R_FM)]. Based on voxel-wise analysis, acute brainstem ischemic stroke group compared with the HC group did not demonstrate altered resting-state FC in the other selected RSNs.
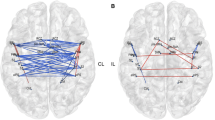
Altered FC within and between each RSNs. (A) Group FC differences within RSNs. Significant differences between the HC group and acute brainstem ischemic stroke group were found within three RSNs. (B) Group differences in static FNC. Three connections were found to be significantly altered (P < .001). DMN, default mode network; ECN, executive control network; L, left; TM, temporal middle gyrus; R, right; FIO, frontal inferior orbital gyrus; FM, frontal middle gyrus
Altered inter-network FC
Three connections were found to be significantly altered for the FNC analysis. Relative to the HC group, the acute brainstem ischemic stroke group exhibited significantly decreased negative interactions in two RSN connections, including the SN-CN connection and SN-AN connection. Moreover, compared with the HC group, the patient group showed increased FNC in the interaction between the DMN cortices (IC14) and ECN cortices (IC20). Additionally, DMN (IC14)-DMN (IC19), was also found to be significantly decreased in the patients group compared with the HC group (Fig. 2B).
Correlation analysis
Correlations were performed between the mean z-scores of four ROIs in the five RSNs and FMA scores, we found no significant statistical differences. After performing the correlations between the FNC coefficients and the FMA scores in the acute brainstem ischemic stroke group, only the positive correlation was found between FC value of the SN-CN and FMA scores (r = 0.299, p = 0.035) (Fig. 3A). FC value of the SN-CN and MAS scores were also found to be positively correlated (r = 0.336, p = 0.017, Fig. 3B).
Discussion
Extensive changes in network function connections after acute stroke will occur immediately and are important for recovery (Carter et al., 2012; Grefkes & Ward, 2014). Rs-fMRI research demonstrates that the FC between the ipsilesional and the contralesional primary sensorimotor cortex is significantly diminished in acute ischemic stroke (Wang et al., 2010; Park et al., 2011; Golestani et al., 2013). Decreased FC with the ipsilesional primary motor cortex (M1) was also found in other brain regions such as the bilateral secondary somatosensory cortex, bilateral SMA, bilateral thalamus, bilateral cerebellum, contralesional premotor cortex, and contralesional posterior parietal cortex (Carter et al., 2010).
The frontoparietal lobe is an important part of spatial attention, and the ECN is involved in numerous advanced cognitive tasks and plays an important role in adaptive cognitive control (McHugh et al., 2017). As we know, the DMN controls both primary perception and advanced cognition and is responsible for the integration of these two (Zhao et al., 2018). According to this notion, acute brainstem ischemic stroke patients showed decreased FC in the R_FIO and L_TM within the DMN as well as the R_FM within the ECN, indicating that dysfunction within the DMN and ECN might contribute to the cognitive impairments observed in acute brainstem ischemic stroke patients. And it is a potential biomarker of cognitive impairment caused by brainstem stroke. Longitudinal studies should be carried out to study whether low connectivity in DMN can be used as an early biomarker, so that clinicians can predict the risk of cognitive impairment after acute brainstem stroke patient. Additionally, in this study, we have observed enhanced network connection between DMN and ECN, this result could be interpreted as a recruitment or compensatory reallocation of cognitive resources.
The SN is a large-scale edge network that co-activates signals needed for behavioral changes (Chen et al., 2016). The insular cortex is an important part of SN, which is mainly responsible for emotional processing, control of cognitive functions and behaviors, and also plays a pivotal role in integrating internal and external processes (Uddin, 2015). Cerebellum plays a key role in motor learning and cognitive processes, and previous studies have demonstrated that acute ischemic stroke patients had abnormal reduction between the bilateral cerebellum and the ipsilateral motor cortex27, (Adamaszek et al., 2017; Keren-Happuch et al., 2014; Fan et al., 2019; Stoodley, 2016). In this study, the functional connection between SN and CN networks was decreased, and the FC value was related to the upper limb motor score, which may explain the upper limb motor dysfunction in patients with acute ischemic brainstem stroke. It is worth noting that the functional connection between SN and AN is also reduced. This finding supports the hypothesis of auditory-motor decline (Skipper et al., 2017). The reduction of auditory input from the cochlea to the auditory system relates to a reduction in the recruitment of the articulatory cortex. Auditory impairment during stroke recovery and changes in auditory -related network connections deserve further study. This study further explores the changes in the connections between brain networks after brainstem infarction, indicating that the brainstem is an important part of the connection between the central and peripheral nerves. Later, it affects the connections between a variety of brain networks, including SN-CN, SN-AN, and ECN-DMN. These findings support our hypothesis and are particularly important for understanding the pathophysiology of stroke, because motor reorganization is a mechanism of cortical injury after subcortical stroke, which disrupts the relevant network that supports motor behavior (Zhao et al., 2018). We used the time correlation of brain network activity to quantify brain network interactions and predicted the extent to which group differences can predict the characteristics of movement disorders after stroke. This will guide early recovery after stroke and reduce the impact of motor dysfunction on the lives of stroke patients.
This study has several limitations. First, the sample in this study is limited, and only the functional connections of brain networks in the acute phase of brainstem stroke have been observed. The sample size needs to be expanded to conduct more longitudinal studies on the functional connections within and between networks during the recovery period of brainstem stroke. Second, our research focused more on the relationship between brain network connections and upper limb dysfunction, and lacked an assessment of the connection with cognitive impairment. Our subsequent research will improve cognitive assessment and explore the relationship between the two. Moreover, our research was based on a limited network interaction model. There are other networks that have not been considered that may play an equally important role, such as attention networks (ATN) and self-referential networks (SRN). Alerting, orienting, and executive control are independent components of attention (Rosenberg et al., 2017; Fan et al., 2005). ATN may be related to the following activities of the brain, (a) alertness, or preparing and maintaining alertness and vigilance; (b) orienting, or directing overt or covert attention to a stimulus. SRN is crucial for representing knowledge pertaining to the self and that this is an important function of the resting state. Moreover, areas within this network allow for top-down modulation between sensory, self-referential, and higher-order processing (Bai et al., 2012; Mantini et al., 2007; Northoff et al., 2006). Finally, although using ICA to reveal the advantages of unconstrained brain connections, ICA cannot reveal the directionality of interactions between networks. Further research is needed to evaluate the direct influence and direction of each network in coupling with other networks in patients with acute ischemic brainstem stroke.
In conclusion, this study showed extensive early changes in static FNC during the acute phase of brainstem stroke. These network interactions can provide a powerful way to assess and predict upper limb movement disorders, and help us understand the neural mechanisms of upper limb movement disorders after brainstem stroke.
Data availability
Imaging data could be provided upon request.
References
Adamaszek, M., D'Agata, F., Ferrucci, R., Habas, C., Keulen, S., Kirkby, K. C., et al. (2017). Consensus paper: Cerebellum and emotion. Cerebellum, 16(2), 552–576. https://doi.org/10.1007/s12311-016-0815-8
Bai, F., Shi, Y., Yuan, Y., Wang, Y., Yue, C., Teng, Y., et al. (2012). Altered self-referential network in resting-state amnestic type mild cognitive impairment. Cortex, 48(5), 604–613. https://doi.org/10.1016/j.cortex.2011.02.011
Biswal, B. B., Van Kylen, J., & Hyde, J. S. (1997). Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR in Biomedicine, 10(4–5), 165–170. https://doi.org/10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7
Biswal, B. B., Mennes, M., Zuo, X. N., Gohel, S., Kelly, C., Smith, S. M., et al. (2010). Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America, 107(10), 4734–4739. https://doi.org/10.1073/pnas.0911855107
Broyd, S. J., Demanuele, C., Debener, S., Helps, S. K., James, C. J., & Sonuga-Barke, E. J. (2009). Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience and Biobehavioral Reviews, 33(3), 279–296. https://doi.org/10.1016/j.neubiorev.2008.09.002
Buckner, R. L., Krienen, F. M., & Yeo, B. T. (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nature Neuroscience, 16(7), 832–837. https://doi.org/10.1038/nn.3423
Carter, A. R., Astafiev, S. V., Lang, C. E., Connor, L. T., Rengachary, J., Strube, M. J., et al. (2010). Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Annals of Neurology, 67(3), 365–375. https://doi.org/10.1002/ana.21905
Carter, A. R., Shulman, G. L., & Corbetta, M. (2012). Why use a connectivity-based approach to study stroke and recovery of function? NeuroImage, 62(4), 2271–2280. https://doi.org/10.1016/j.neuroimage.2012.02.070
Cerliani, L., Mennes, M., Thomas, R. M., Di Martino, A., Thioux, M., & Keysers, C. (2015). Increased functional connectivity between subcortical and cortical resting-state networks in autism Spectrum disorder. JAMA Psychiatry, 72(8), 767–777. https://doi.org/10.1001/jamapsychiatry.2015.0101
Chen, T., Cai, W., Ryali, S., Supekar, K., & Menon, V. (2016). Distinct global brain dynamics and spatiotemporal Organization of the Salience Network. PLoS Biology, 14(6), e1002469. https://doi.org/10.1371/journal.pbio.1002469
Damoiseaux, J. S., Rombouts, S. A., Barkhof, F., Scheltens, P., Stam, C. J., Smith, S. M., et al. (2006). Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America, 103(37), 13848–13853. https://doi.org/10.1073/pnas.0601417103
De Luca, M., Beckmann, C. F., De Stefano, N., Matthews, P. M., & Smith, S. M. (2006). fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage, 29(4), 1359–1367. https://doi.org/10.1016/j.neuroimage.2005.08.035
Fan, J., McCandliss, B. D., Fossella, J., Flombaum, J. I., & Posner, M. I. (2005). The activation of attentional networks. Neuroimage, 26(2), 471–479. https://doi.org/10.1016/j.neuroimage.2005.02.004
Fan, L., Hu, J., Ma, W., Wang, D., Yao, Q., & Shi, J. (2019). Altered baseline activity and connectivity associated with cognitive impairment following acute cerebellar infarction: A resting-state fMRI study. Neuroscience Letters, 692, 199–203. https://doi.org/10.1016/j.neulet.2018.11.007
Fox, M. D., & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700–711. https://doi.org/10.1038/nrn2201
Golestani, A. M., Tymchuk, S., Demchuk, A., Goodyear, B. G., & Group V-S. (2013). Longitudinal evaluation of resting-state FMRI after acute stroke with hemiparesis. Neurorehabilitation and Neural Repair, 27(2), 153–163. https://doi.org/10.1177/1545968312457827
Grefkes, C., & Ward, N. S. (2014). Cortical reorganization after stroke: How much and how functional? The Neuroscientist : a Review Journal Bringing Neurobiology, Neurology and Psychiatry, 20(1), 56–70. https://doi.org/10.1177/1073858413491147
Guerra-Carrillo, B., Mackey, A. P., & Bunge, S. A. (2014). Resting-state fMRI: A window into human brain plasticity. The Neuroscientist : a Review Journal Bringing Neurobiology, Neurology and Psychiatry, 20(5), 522–533. https://doi.org/10.1177/1073858414524442
Hampson, M., Peterson, B. S., Skudlarski, P., Gatenby, J. C., & Gore, J. C. (2002). Detection of functional connectivity using temporal correlations in MR images. Human Brain Mapping, 15(4), 247–262. https://doi.org/10.1002/hbm.10022
Iyer, D. G., Shah, N. S., Hastings, K. G., Hu, J., Rodriguez, F., Boothroyd, D. B. et al. (2019). Years of Potential Life Lost Because of Cardiovascular Disease in Asian-American Subgroups, 2003–2012. Journal of the American Heart Association, e010744.
Jafri, M. J., Pearlson, G. D., Stevens, M., & Calhoun, V. D. (2008). A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage, 39(4), 1666–1681. https://doi.org/10.1016/j.neuroimage.2007.11.001
Jiang, L., Geng, W., Chen, H., Zhang, H., Bo, F., Mao, C. N., et al. (2018). Decreased functional connectivity within the default-mode network in acute brainstem ischemic stroke. European Journal of Radiology, 105, 221–226. https://doi.org/10.1016/j.ejrad.2018.06.018
Keren-Happuch, E., Chen, S. H., Ho, M. H., & Desmond, J. E. (2014). A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Human Brain Mapping, 35(2), 593–615. https://doi.org/10.1002/hbm.22194
Li, Y. O., Adali, T., & Calhoun, V. D. (2007). Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping, 28(11), 1251–1266. https://doi.org/10.1002/hbm.20359
Mantini, D., & Vanduffel, W. (2013). Emerging roles of the brain’s default network. The Neuroscientist : a Review Journal Bringing Neurobiology, Neurology and Psychiatry, 19(1), 76–87. https://doi.org/10.1177/1073858412446202
Mantini, D., Perrucci, M. G., Del Gratta, C., Romani, G. L., & Corbetta, M. (2007). Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 104(32), 13170–13175. https://doi.org/10.1073/pnas.0700668104
McHugh, M. J., Gu, H., Yang, Y., Adinoff, B., & Stein, E. A. (2017). Executive control network connectivity strength protects against relapse to cocaine use. Addiction Biology, 22(6), 1790–1801. https://doi.org/10.1111/adb.12448
Mortality GBD. (2016). Causes of death C. global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the global burden of disease study 2015. Lancet, 388(10053), 1459–1544. https://doi.org/10.1016/S0140-6736(16)31012-1
Northoff, G., Heinzel, A., de Greck, M., Bermpohl, F., Dobrowolny, H., & Panksepp, J. (2006). Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage, 31(1), 440–457. https://doi.org/10.1016/j.neuroimage.2005.12.002
Ogawa, S., Lee, T. M., Kay, A. R., & Tank, D. W. (1990). Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America, 87(24), 9868–9872. https://doi.org/10.1073/pnas.87.24.9868
Park, C. H., Chang, W. H., Ohn, S. H., Kim, S. T., Bang, O. Y., Pascual-Leone, A., et al. (2011). Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke, 42(5), 1357–1362. https://doi.org/10.1161/STROKEAHA.110.596155
Platz, T., Eickhof, C., van Kaick, S., Engel, U., Pinkowski, C., Kalok, S., et al. (2005). Impairment-oriented training or Bobath therapy for severe arm paresis after stroke: A single-blind, multicentre randomized controlled trial. Clinical Rehabilitation, 19(7), 714–724. https://doi.org/10.1191/0269215505cr904oa
Pohjasvaara, T., Vataja, R., Leppavuori, A., Kaste, M., & Erkinjuntti, T. (2002). Cognitive functions and depression as predictors of poor outcome 15 months after stroke. Cerebrovascular Diseases, 14(3–4), 228–233. https://doi.org/10.1159/000065667
Poole, J. L., & Whitney, S. L. (1988). Motor assessment scale for stroke patients: Concurrent validity and interrater reliability. Archives of Physical Medicine and Rehabilitation, 69(3 Pt 1), 195–197.
Powers, W. J., Derdeyn, C. P., Biller, J., Coffey, C. S., Hoh, B. L., Jauch, E. C., et al. (2015). 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early Management of Patients with Acute Ischemic Stroke Regarding Endovascular Treatment: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 46(10), 3020–3035. https://doi.org/10.1161/STR.0000000000000074
Qin, Y., Li, Y., Sun, B., He, H., Peng, R., Zhang, T., et al. (2018). Functional connectivity alterations in children with spastic and Dyskinetic cerebral palsy. Neural Plasticity, 2018, 7058953. https://doi.org/10.1155/2018/7058953
Raichle, M. E. (2001). Cognitive neuroscience. Bold insights. Nature, 412(6843), 128–130. https://doi.org/10.1038/35084300
Rosenberg, M. D., Finn, E. S., Scheinost, D., Constable, R. T., & Chun, M. M. (2017). Characterizing attention with predictive network models. Trends in Cognitive Sciences, 21(4), 290–302. https://doi.org/10.1016/j.tics.2017.01.011
Shi, M., Liu, S., Chen, H., Geng, W., Yin, X., Chen, Y. C., et al. (2021). Disrupted brain functional network topology in unilateral acute brainstem ischemic stroke. Brain Imaging and Behavior, 15(1), 444–452.
Skipper, J. I., Devlin, J. T., & Lametti, D. R. (2017). The hearing ear is always found close to the speaking tongue: Review of the role of the motor system in speech perception. Brain and Language, 164, 77–105. https://doi.org/10.1016/j.bandl.2016.10.004
Stoodley, C. J. (2016). The cerebellum and neurodevelopmental disorders. Cerebellum, 15(1), 34–37. https://doi.org/10.1007/s12311-015-0715-3
Tuladhar, A. M., Snaphaan, L., Shumskaya, E., Rijpkema, M., Fernandez, G., Norris, D. G., et al. (2013). Default mode network connectivity in stroke patients. PLoS One, 8(6), e66556. https://doi.org/10.1371/journal.pone.0066556
Uddin, L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16(1), 55–61. https://doi.org/10.1038/nrn3857
Wang, L., Yu, C., Chen, H., Qin, W., He, Y., Fan, F., et al. (2010). Dynamic functional reorganization of the motor execution network after stroke. Brain: a Journal of Neurology, 133(Pt 4), 1224–1238. https://doi.org/10.1093/brain/awq043
Wang, J., Wang, X., Xia, M., Liao, X., Evans, A., & He, Y. (2015). GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Frontiers in Human Neuroscience, 9, 386. https://doi.org/10.3389/fnhum.2015.00386
Yao, G., Li, J., Liu, S., Wang, J., Cao, X., Li, X., et al. (2020). Alterations of functional connectivity in stroke patients with basal ganglia damage and cognitive impairment. Frontiers in Neurology, 11, 980. https://doi.org/10.3389/fneur.2020.00980
Zhao, Z., Wu, J., Fan, M., Yin, D., Tang, C., Gong, J., et al. (2018). Altered intra- and inter-network functional coupling of resting-state networks associated with motor dysfunction in stroke. Human Brain Mapping, 39(8), 3388–3397. https://doi.org/10.1002/hbm.24183
Zhao, D., Liu, J., Wang, M., Zhang, X., & Zhou, M. (2019). Epidemiology of cardiovascular disease in China: Current features and implications. Nature Reviews Cardiology, 16(4), 203–212. https://doi.org/10.1038/s41569-018-0119-4
Funding
This work was supported by the Jiangsu Provincial Special Program of Medical Science (No. BE2021604), Natural Science Foundation of Jiangsu Province (No. BK20201118) and 333 High-level Talents Training Project of Jiangsu Province (No. BRA2019122).
Author information
Authors and Affiliations
Contributions
WG. and JZ. are co-first authors of this paper, they design the experiment, analyze the data and draft the paper for the work. SS., HC. and MS. help to acquire the clinical and fMRI data. LJ. helps to revise the paper critically for important intellectual content. XY. and YCC. are co-corresponding authors of this paper, they did the financial support, review and final approval of the paper to be published. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
The current study was approved by the Research Ethics Committee of the Nanjing Medical University.
Consent to participate
All participants provided written informed consent before undergoing MR imaging.
Consent to publish
Not applicable.
Competing interests
The authors declare that there is no potential conflict of interests regarding the publication of this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Geng, W., Zhang, J., Shang, S. et al. Reduced functional network connectivity is associated with upper limb dysfunction in acute ischemic brainstem stroke. Brain Imaging and Behavior 16, 802–810 (2022). https://doi.org/10.1007/s11682-021-00554-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-021-00554-0