Abstract
Cognitive impairment (CI), mainly involving attention and processing speed (A-PS), is a common and disabling symptom in multiple sclerosis (MS). Symbol Digit Modalities Test (SDMT) is one of the more sensitive and reliable tests to assess A-PS deficits in MS. Structural MRI correlates of A-PS in MS still need to be clarified. This study aimed to investigate, in a large group of MS patients, the relationship between regional gray matter (GM) atrophy and SDMT performance. 125 relapsing remitting MS patients and 52 healthy controls (HC) underwent a 3 T–MRI protocol including high-resolution 3D–T1 imaging. All subjects underwent a neurological evaluation and SDMT. A Voxel Based Morphometry analysis was performed to assess: 1) correlations between regional GM volume and SDMT performance in MS patients; 2) regional differences in GM volume between MS patients and HC. Thalamic, putamen and cerebellar volumes were also calculated using FIRST tool from the FMRIB Software Library. A linear regression analysis was performed to assess the contribution of each one of these structures to A-PS performance. A significant negative correlation was found between regional GM volume and SDMT score at the level of the thalamus, cerebellum, putamen, and occipital cortex in MS patients. Thalamus, cerebellum and putamen also showed significant GM atrophy in MS patients compared to HC. Thalamic atrophy is also an independent and additional contributor to A-PS deficits in MS patients. These findings support the role of thalamus as the most relevant GM structure subtending A-PS performance in MS, as measured by SDMT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive impairment (CI) is a common and debilitating feature of multiple sclerosis (MS). It occurs in 40%–70% of MS patients and severely impacts on quality of life, employment status and adherence to therapy(Rao et al. 1991b; Julian 2011; DeLuca et al. 2015). Cognitive domains most commonly impaired in MS are attention and processing speed (A-PS), executive functions, immediate and delayed recall or memory and verbal fluency (Chiaravalloti and DeLuca 2008; DeLuca et al. 2015). Heterogeneity in frequency and characteristics of cognitive deficits among patients with MS reflects the influence of many factors, including genetics, sex, intelligence, disease course, comorbid neuropsychiatric illness, and health behaviors (Benedict and Zivadinov 2011). CI starts early in the disease course, generally worsens over time and is not strictly linked to physical disability (Julian 2011). CI has also shown a prognostic value in predicting the conversion from clinically isolated syndrome to MS. For this reason, its detection should influence therapeutic decisions (Zipoli et al. 2010). The Symbol Digit Modalities Test (SDMT), which primarily assesses A-PS and sustained attention (Rao et al. 1991a; Smith 1982), is among the most reliable, sensitive (Parmenter et al. 2007) and clinically meaningful cognitive tests in MS, being also able to predict the vocational status of MS patients (Morrow et al. 2010; Strober et al. 2012).
SDMT performance have shown moderate-to-strong correlations with a series of brain magnetic resonance imaging (MRI) markers of tissue damage (Rao et al. 2014), such as T2 lesion volume (T2-LV), and global brain atrophy (Rao et al. 2014).
Nevertheless, in order to further explore the pathophysiology of A-PS deficits in MS as well as to ameliorate clinical-MRI correlations, regional structural-MRI studies have recently been proposed and implemented. One of the most feasible and promising approaches is represented by voxel based morphometry (VBM) (Good et al. 2001), an unbiased voxel-wise spatially-specific method of MRI analysis, which allows to assess regional gray matter (GM) volume and to compare it between groups of subjects, as well as to correlate it with clinical variables.
Some authors have already used this approach to explore the relationship between regional GM atrophy and SDMT performance (Sastre-Garriga et al. 2009; Nocentini et al. 2014). These studies, however, have showed conflicting results. In particular, Sastre Garriga et al. described no significant correlation between SDMT and regional brain volumes (Sastre-Garriga et al. 2009). On the contrary, Nocetini et al. found that performances in SDMT were associated with a reduction of GM volumes in several regions, such as the prefrontal cotex, the pre- and postcentral gyrus, and in the right temporal cortex (Nocentini et al. 2014). However, it is worth noting that both studies (Nocentini et al. 2014; Sastre-Garriga et al. 2009) were conducted on a small sample size - 15 MS patients in the Sastre-Garriga study; 18 MS patients and 18 healthy controls (HC) in the Nocentini study - and with VBM analysis not controlling for the potential detrimental impact of T1 WM lesions on brain segmentation (i.e. without a lesion-filling of 3D T1 images) (Ceccarelli et al. 2012; Battaglini et al. 2012). Furthermore, the Sastre-Garriga study only included cognitively impaired MS patients and was conducted using a 1.5 T MRI scanner (Sastre-Garriga et al. 2009).
In recent years, MRI studies have tried to move from group-based analysis, such as VBM, to individual measures, mainly through the implementation of automated methods of brain segmentation, such as the FIRST tool from the FMRIB Software Library (FSL) (Patenaude et al. 2011) or FreeSurfer (Dale et al. 1999). Using these approaches, A-PS performances have been found to relate to deep GM structures volumes, especially the thalamus and putamen (Batista et al. 2012; Schoonheim et al. 2012; Houtchens et al. 2007; Minagar et al. 2013; Bergsland et al. 2016; Benedict et al. 2013).
Based on this knolewdge, the objectives of the present study were: 1) to further explore, by using VBM in a large MS population, which GM regions subtend SDMT performance in MS, after controlling for the effect of focal WM lesions; 2) to assess, by usingVBM, which SDMT-correlated GM regions are atrophic in MS patient compared to HC; 3) to explore which of the SDMT-correlated and atrophic GM regions, identified by VBM and measured by FIRST, contributes to A-PS performance in MS patients.
Our working hypothesis was that A-PS impairment in MS patients might be subtended by atrophy of specific brain GM regions.
Methods
Study population
125 relapsing remitting (RR) MS patients (Polman et al. 2011) were consecutively enrolled at our MS center. 52 HC matched for sex, age and education were recruited from a large HC database available at our Institution (Table 1). Inclusion criteria were: 1) age between 18 and 65 years; 2) no current or past disorders, other than MS (for patients), which could affect cognitive performance at SDMT; 3) no concomitant treatments with psychoactive drugs; 4) absence of motor, sensory and/or visual deficits that might interfere with cognitive test performance; 5) no relapses and/or steroid treatments within 1 month before participating in the study (for patients); 6) normal MRI findings and preservation at SDMT (see the “Clinical and neuropsycological assessment” section) in HC.
The study was approved by the local Ethic Committee and a signed informed consent was obtained from all participants.
Clinical and neuropsycological assessment
On the same day of MRI acquisition all the subjects underwent: 1) a neurological examination including Expanded Disability Status Scale (EDSS) (Kurtzke 1983) (for MS patients); 2) SDMT administration (Rao et al. 1991b): all subjects were required to verbally pair numbers and symbols according to a fixed pattern. The final score was determined by the amount of pairings solved correctly within 90 s (Smith 1982); SDMT scores were converted into z scores using available normative data of the Italian population (Amato et al. 2006). HC with an SDMT Z-scores ≤ 1.5 (Amato et al. 2010) were excluded from the study.
MRI acquisition
Brain MRI scans were acquired on a 3 T GE Medical System (Milwaukee, WI) scanner equipped with an 8-channel parallel head coil. The following images were acquired: 1) DP/T2 weighted (dual-echo (DE) fast spin echo (FSE), repetition time [TR] = 3080 ms, echo time [TE] 1/TE2 = 24/127.5 ms, axial slices =44/44, matrix =256 X 384, field of view [FOV] = 240 mm, slice thickness = 3 mm, interslice gap =0 mm); 2) high-resolution 3D–T1 (magnetization prepared Fast Spoiled Gradient echo , TR = 6988 ms, TI = 650 ms, TE = 2,85 ms, matrix =256 × 256, slice number = 166, sagittal slices, flip angle =8°, voxel size =1 X 1 X 1.2 mm3, FOV = 256, sagittal).
Conventional MRI analysis
The identification of T2 hyperintense lesions in MS patients was conducted on DP/T2 images by a single experienced observer blinded to patients’ clinical characteristics. The MIPAV software (Medical Image Processing, Analysis and Visualization; version 4.2.2; http://mipav.cit.nih.gov) was used to contour lesions and to compute T2-LV of each patient. Normalized brain (NBV), WM (NWMV), GM (NGMV) and peripheral GM volume (NPGMV) volumes were measured on 3D–T1 images using the SIENAx software (Smith et al. 2002), after T1-hypointense lesion refilling (Battaglini et al. 2012).
VBM-analysis (Good et al. 2001)
VBM analysis was performed using SPM12 software (Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) on 3D–T1 lesion-filled images (Battaglini et al. 2012). Images were bias-corrected, tissue-classified, and registered using linear (12-parameter affine) and non-linear transformations (warping) within a unified mode, with default parameters incorporating the DARTEL toolbox (Ashburner 2007). Subsequently, the warped GM segments were affine-transformed into Montreal Neurological Institute (MNI) space and were scaled by the Jacobian determinants of the deformations to account for the local compression and stretching that occurs as a consequence of the warping and affine transformation (modulated GM volumes). The modulated volumes were smoothed with a Gaussian kernel of 8-mm full-width at half maximum (FWHM).
Thalamic, putamen and cerebellar atrophy measures
Thalamus, Putamen and Cerebellum were segmented from the 3D T1-weighted images in native space using the FIRST tool from the FMRIB Software Library (Patenaude et al. 2011). The results of segmentation were all visually checked. Normalized volumes were calculated from the FIRST output using the SIENAx scaling factor (Smith 2002).
Statistical analysis
A Kolmogorov–Smirnov test was used to verify normal distribution of demographic, clinical and conventional MRI variables. Between-group comparisons were performed using a Student’s t-test for unpaired samples, the Mann-Whitney and Chi-square tests, as appropriate. A p < 0.05 was considered statistically significant. Correction for multiple comparisons was performed by applying the Bonferroni method (SPSS Statistics version 20.0). The GM volume maps were statistically analysed using the general linear model based on Gaussian random field theory. Correlation between GM volume and SDMT Z-scores was assesed both in MS and in HC group using multiple regression analysis with total intracranial volume (TIV), age and sex as covariates of no interest. Regional differences in GM volume between the two experimental groups (MS patients and HC) were also assessed with TIV, age and sex as covariates of no interest. Statistical inference was performed at the voxel level, with a family-wise error (FWE) correction for multiple comparisons. Clusters were considered significant at p < 0.05. Pearson product moment correlations were examined between thalamic, putamen, cerebellar and NPGV volumes and SDMT Z-scores with significance set at p < 0.05, using first age and then age and NPGMV as covariates. As the right and left structures were strongly correlated (data not shown), they were combined by calculating mean values in order to reduce the number of variables (Batista et al. 2012). Only the structures whose volume was significantly correlated with SDMT Z-scores were used in subsequent regression analyses. Stepwise linear regression models (entrance criterion p < 0.05 and exit criterion p = 0.10) were generated in order to determine the strongest predictors of SDMT performance. Age was entered as a covariate and retained in block 1, and the significant GM structures were entered in block 2 using the forward stepwise technique. Finally, each model was repeated with NPGMV added in block 1 (Batista et al. 2012) (SPSS Statistics version 20.0).
Results
Clinical-demographic, neuropsychological and conventional MRI data
Table 1 summarizes the main clinical-demographic, neuropsychological and conventional MRI data in the two studied groups. To better describe the distribution of SDMT scores in the studied populations, MS patients were stratified in three subgroups, based on SDMT z- scores, as follows: i) > −0.99 SD: 46/125, 37%, ii) between −1 and −1.99 SD: 33/125, 26%, iii) ≤ −2 SD: 46/125, 37%.
MS patients had a lower SDMT Z-scores, NBV, NWMV, NGMV and NPGMV than HC (Table 1).
VBM analysis
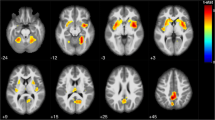
In the MS group, the correlation analysis showed a positive association between SDMT score and regional GM volume in the following brain regions (Fig. 1, Table 2): the posterior lobe of the cerebellum, bilaterally; the thalamus, bilaterally; the left lateral occipital cortex, the occipital pole, bilaterally; the right occipital fusiform gyrus and the left putamen. On the contrary, no correlations were found between SDMT and regional GM volume in the HC group.
Regional pattern of gray matter (GM) volumes positively associated with Symbol Digit Modalities Test (SDMT) Z-scores in multiple sclerosis (MS) patients, superimposed on the customized GM template. Legend: The regions showing significant correlations (p < 0.05) are shown in red–yellow (t-value 0–6). Abbreviations: A = anterior, P = posterior, L = left, R = right, S = superior, I = inferior
Compared to HC, MS patients showed significant regional GM loss in the following areas: the thalamus, bilaterally; the postcentral gyrus, bilaterally; the right precentral gyrus; the right inferior temporal gyrus; the insular cortex, bilaterally; the middle temporal gyrus, bilaterally; the left superior frontal gyrus; the right cerebellum; the left pallidum; the left cingulate gyrus; the right putamen; the right temporal pole and the left inferior frontal gyrus (Fig. 2, Table 3). No areas of regional GM volume reduction were found in HC when compared to MS patients.
Regional pattern of gray matter (GM) atrophy in patients with multiple sclerosis (MS), compared to healthy controls (HC), superimposed on the customized GM template. Legend: The regions of significant reduced GM volume in MS patients (p < 0.05) are shown in red-yellow (t-value 0–8). Abbreviations: A = anterior, P = posterior, L = left, R = right, S = superior, I = inferior
Thalamic, putamen and cerebellar atrophy measures
In MS patients the mean thalamic volume was 10.1 mL (1.1 SD), the mean putamen volume was 4.5 mL (0.9 SD) and the mean cerebellar volume was 79.6 mL (4.6 SD).
Correlation analysis and linear regression analysis
Correlations analysis between NPGMV, thalamic, putamen and cerebellar volumes and SDMT Z-scores are presented in Table 4. All significant correlations were positive. SDMT Z-scores showed a significant correlation with: 1) thalamic volumes, putamen volumes and NPGMV; 2) thalamic and putamen volumes, controlling for age; 3) only thalamic volumes, controlling for age and NPGMV.
In the linear regression model (including thalamus and putamen), the thalamus was the only independent predictor of SDMT Z-score, after controlling for age and for age + NPGMV (Table 5).
Discussion
Despite the high frequency and the negative impact of CI on patients’ daily life, neuropsychological (NP) assessment is not currently included in routine clinical evaluation of MS patients. The principal factors that limit its use are center-dependent (dedicated rooms, scheduling, etc.), rater-dependent (training, time of administration, etc.), test-dependent (screening vs comprehensive batteries) and patient-dependent (i.e. confounders such as depression, fatigue, physical impairment, etc.) (Chiaravalloti and DeLuca 2008).
For the abovementioned reasons, SDMT, a simple and short test which explores those cognitive domains frequently impaired in MS patients (Smith 1982), has been proposed as a screening test for routine clinical assessment of cognitive dysfunction in MS (Lopez-Gongora et al. 2015). Indeed, it has been shown that SDMT is able to correctly classify MS patients as cognitively impaired (Parmenter et al. 2007; Lopez-Gongora et al. 2015) and to discriminate between MS patients and HC (Benedict et al. 2008). Moreover, among various MS-related factors (e.g. disability, disease duration, MS course, other cognitive tests, and personality characteristics) SDMT is the most reliable predictor of employment status (Strober et al. 2012) and its decline over time predicts a transition from fulfilling employment to vocational disability (Morrow et al. 2010). Finally, SDMT is simple and fast to administer, lasting a total of five minutes (Drake et al. 2010). Based on these premises, in the present study VBM analysis was applied to a large population of MS patients to investigate the contribution of regional GM damage to A-PS deficits, as measured by SDMT. Subsequently, we tested if volumes of SDMT-related GM regions independently contribute to A-PS performances in MS patients.
Our results showed that lower performance in SDMT was associated with a reduction of GM volumes in the cerebellum, bilaterally (posterior lobe), the thalamus, bilaterally, the left putamen and the occipital cortex.
These results are in contrast with those of previous studies using SDMT and a VBM approach. In particular, Sastre-Garriga et al. reported no significant correlation between regional GM volumes and SDMT scores (Sastre-Garriga et al. 2009), while Nocetini et al. found a correlation between SDMT performance and GM atrophy of specific regions located in the frontal, parietal and temporal cortex (Nocentini et al. 2014). However, these discrepancies might reflect the small sample size (Nocentini et al. 2014; Sastre-Garriga et al. 2009), the characteristics of studied populations (e.g. Cognitive status of enrolled MS patients) (Sastre-Garriga et al. 2009) and/or methodological issues (Nocentini et al. 2014; Sastre-Garriga et al. 2009) (e.g. using VBM without lesion filling of 3D–T1 images) (Battaglini et al. 2012).
On the other hand, in agreement with other studies, using an individual based method (Batista et al. 2012; Schoonheim et al. 2012; Houtchens et al. 2007; Minagar et al. 2013; Bergsland et al. 2016; Benedict et al. 2013), we were also able to observe that global thalamic and putamen volumes are related to SDMT Z-scores. The absence of correlation of SDMT Z-scores with cerebellar and neocortical (as measured by NPGMV) volumes could depend on the fact that VBM results included only sub-regions of these relatively large structures. Furthermore, in the linear regression analysis, we were able to confirm that thalamic atrophy is an independent and strong contributor to MS-related A-PS deficit, also controlling for age and neocortical atrophy (Batista et al. 2012; Benedict et al. 2013).
The contribution of the thalamus to cognition is well documented and several previous MRI studies have shown that different thalamic MRI measures (e.g. volume, microstructural damage, etc.) strongly correlate with global and selective CI in MS (Minagar et al. 2013; Bisecco et al. 2015; Batista et al. 2012; Schoonheim et al. 2012; Houtchens et al. 2007; Bergsland et al. 2016; Benedict et al. 2013). Our findings, therefore, strongly confirm and reinforce the role of the thalamus as an independent and additional contributor in determining A-PS functions in MS patients.
With regards to the cerebellum, several studies have demonstrated its key role in cognition, particularly regarding language, executive function and visuo-spatial abilities (Tedesco et al. 2011). It has been hypothesized that the “cognitive” cerebellum stems from its strong anatomical connections with many associative cortical areas, including the prefrontal cortex (Middleton and Strick 2000). Moreover, it has been recently demonstrated that the cerebellum also plays a critical and specific role in MS-related cognitive dysfunction, mostly affecting those cognitive domains related to executive functions, A-PS and verbal fluency (Sarica et al. 2015). Poor performance in A-PS in MS has been related to a disconnection between posterior cerebellum and cortical areas involved in cognition (Rocca et al. 2016). Structural studies have also explored the relationship between A-PS and volume, LV and microstructural damage of the cerebellum, showing conflicting results (Sarica et al. 2015; Weier et al. 2014; Damasceno et al. 2014). Moreover, a lower performance at SDMT has been linked to GM atrophy in cortical areas strictly connected with the cerebellum, such as the dorsolateral prefrontal cortex (Cerasa et al. 2013). Finally, cognitive deficits observed in MS patients seem to be related to a fronto-cerebellar connectivity impairment (Bonnet et al. 2010) that might be partially overcome by cognitive rehabilitation (Sastre-Garriga et al. 2011).
To our knowledge, the present study is the first one that reported a significant correlation between cognitive performance and regional cerebellar atrophy in MS in a whole brain voxel-vise study, thus suggesting that cerebellar damage might be relevant to MS-related CI, and particularly to A-PS deficits. The absence of correlation with global cerebellar volume and the localization of areas SDMT-correlated in the posterior lobe of cerebellum support the hypothesis of a cerebellar sub-regional specialization for cognitive functions (Rocca et al. 2016; Sarica et al. 2015).
As for the abovementioned structures, putamen damage has also been previously associated with A-PS in MS (Batista et al. 2012). This nucleus is involved in voluntary fixational control and saccades, and contributes to planning tasks with a visual searching component (Batista et al. 2012). Here we confirm the role of the putamen in A-PS deficits, emphasizing the role of visual movements in cognitive tasks influenced by visual searching such as SDMT.
A similar interpretation might also be applied to the relationship between occipital cortex atrophy and SDMT performance, since it was demonstrated that occipital regions experience a significant functional MRI activation during SDMT execution in MS patients (Genova et al. 2009).
To assess whether GM regions showing correlations with SDMT performance are also atrophic in MS patients, a whole-brain VBM analysis comparing MS patients and HC was also performed. Our findings are in line with those of previous studies (Prinster et al. 2006; Lansley et al. 2013) reporting regional GM loss at the level of the thalamus, basal ganglia, pre/postcentral and cingulate gyri in MS patients (Lansley et al. 2013). Interestingly, in our study, all SDMT-correlated GM regions, except for occipital cortex, were atrophic in MS patients.
We recognize that generalization of our results might be limited by the lack of a global neuropsychological evaluation. Consequently, we cannot be confident that our MS patients are representative of a “common” MS population. Nevertheless, SDMT has been shown to be able to correctly classify MS patients as cognitively impaired (Parmenter et al. 2007; Lopez-Gongora et al. 2015). Accordingly, the percentage of our MS patients impaired (Amato et al. 2006) at SDMT (37%), was in line with the expected percentage of CI in MS general population (Chiaravalloti and DeLuca 2008). In line with the abovementioned statements, the strong relationship between SDMT performance and CI does not allow us to exclude that our findings reflect global CI (identified by lower scores on the SDMT) rather than just A-PS deficits.
In conclusion, this study suggests that A-PS deficits in MS are associated with GM atrophy of strategic brain structures, directly (such as the thalamus and the cerebellum) or indirectly (through the control of visual functions, such as the putamen and occipital cortex) involved in cognitive functions. Furthermore, thalamic atrophy plays a specific, independent and additional role in determining A-PS deficits in MS.
References
Amato, M. P., Portaccio, E., Goretti, B., Zipoli, V., Ricchiuti, L., De Caro, M. F., et al. (2006). The Rao's brief repeatable battery and Stroop test: normative values with age, education and gender corrections in an Italian population. Multiple Sclerosis, 12(6), 787–793.
Amato, M. P., Portaccio, E., Goretti, B., Zipoli, V., Iudice, A., Della Pina, D., et al. (2010). Relevance of cognitive deterioration in early relapsing-remitting MS: a 3-year follow-up study. Multiple Sclerosis, 16(12), 1474–1482. doi:10.1177/1352458510380089.
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. doi:10.1016/j.neuroimage.2007.07.007.
Batista, S., Zivadinov, R., Hoogs, M., Bergsland, N., Heininen-Brown, M., Dwyer, M. G., et al. (2012). Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. Journal of Neurology, 259(1), 139–146. doi:10.1007/s00415-011-6147-1.
Battaglini, M., Jenkinson, M., & De Stefano, N. (2012). Evaluating and reducing the impact of white matter lesions on brain volume measurements. Human Brain Mapping, 33(9), 2062–2071. doi:10.1002/hbm.21344.
Benedict, R. H., & Zivadinov, R. (2011). Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nature Reviews. Neurology, 7(6), 332–342. doi:10.1038/nrneurol.2011.61.
Benedict, R. H., Duquin, J. A., Jurgensen, S., Rudick, R. A., Feitcher, J., Munschauer, F. E., et al. (2008). Repeated assessment of neuropsychological deficits in multiple sclerosis using the symbol digit modalities test and the MS neuropsychological screening questionnaire. Multiple Sclerosis, 14(7), 940–946. doi:10.1177/1352458508090923.
Benedict, R. H., Hulst, H. E., Bergsland, N., Schoonheim, M. M., Dwyer, M. G., Weinstock-Guttman, B., et al. (2013). Clinical significance of atrophy and white matter mean diffusivity within the thalamus of multiple sclerosis patients. Multiple Sclerosis, 19(11), 1478–1484. doi:10.1177/1352458513478675.
Bergsland, N., Zivadinov, R., Dwyer, M. G., Weinstock-Guttman, B., & Benedict, R. H. (2016). Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Multiple Sclerosis, 22(10), 1327–1336. doi:10.1177/1352458515616204.
Bisecco, A., Rocca, M. A., Pagani, E., Mancini, L., Enzinger, C., Gallo, A., et al. (2015). Connectivity-based parcellation of the thalamus in multiple sclerosis and its implications for cognitive impairment: a multicenter study. Human Brain Mapping. doi:10.1002/hbm.22809.
Bonnet, M. C., Allard, M., Dilharreguy, B., Deloire, M., Petry, K. G., & Brochet, B. (2010). Cognitive compensation failure in multiple sclerosis. Neurology, 75(14), 1241–1248. doi:10.1212/WNL.0b013e3181f612e3.
Ceccarelli, A., Jackson, J. S., Tauhid, S., Arora, A., Gorky, J., Dell'Oglio, E., et al. (2012). The impact of lesion in-painting and registration methods on voxel-based morphometry in detecting regional cerebral gray matter atrophy in multiple sclerosis. AJNR. American Journal of Neuroradiology, 33(8), 1579–1585. doi:10.3174/ajnr.A3083.
Cerasa, A., Valentino, P., Chiriaco, C., Pirritano, D., Nistico, R., Gioia, C. M., et al. (2013). MR imaging and cognitive correlates of relapsing-remitting multiple sclerosis patients with cerebellar symptoms. Journal of Neurology, 260(5), 1358–1366. doi:10.1007/s00415-012-6805-y.
Chiaravalloti, N. D., & DeLuca, J. (2008). Cognitive impairment in multiple sclerosis. Lancet Neurology, 7(12), 1139–1151. doi:10.1016/S1474-4422(08)70259-X.
Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. doi:10.1006/nimg.1998.0395.
Damasceno, A., Damasceno, B. P., & Cendes, F. (2014). The clinical impact of cerebellar grey matter pathology in multiple sclerosis. PloS One, 9(5), e96193. doi:10.1371/journal.pone.0096193.
DeLuca, G. C., Yates, R. L., Beale, H., & Morrow, S. A. (2015). Cognitive impairment in multiple sclerosis: clinical, radiologic and pathologic insights. Brain Pathology, 25(1), 79–98. doi:10.1111/bpa.12220.
Drake, A. S., Weinstock-Guttman, B., Morrow, S. A., Hojnacki, D., Munschauer, F. E., & Benedict, R. H. (2010). Psychometrics and normative data for the multiple sclerosis functional composite: replacing the PASAT with the symbol digit modalities test. Multiple Sclerosis, 16(2), 228–237. doi:10.1177/1352458509354552.
Genova, H. M., Hillary, F. G., Wylie, G., Rypma, B., & Deluca, J. (2009). Examination of processing speed deficits in multiple sclerosis using functional magnetic resonance imaging. Journal of the International Neuropsychological Society, 15(3), 383–393. doi:10.1017/S1355617709090535.
Good, C. D., Johnsrude, I. S., Ashburner, J., Henson, R. N., Friston, K. J., & Frackowiak, R. S. (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14(1 Pt 1), 21–36. doi:10.1006/nimg.2001.0786.
Houtchens, M. K., Benedict, R. H., Killiany, R., Sharma, J., Jaisani, Z., Singh, B., et al. (2007). Thalamic atrophy and cognition in multiple sclerosis. Neurology, 69(12), 1213–1223. doi:10.1212/01.wnl.0000276992.17011.b5.
Julian, L. J. (2011). Cognitive functioning in multiple sclerosis. Neurologic Clinics, 29(2), 507–525. doi:10.1016/j.ncl.2010.12.003.
Kurtzke, J. F. (1983). Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology, 33(11), 1444–1452.
Lansley, J., Mataix-Cols, D., Grau, M., Radua, J., & Sastre-Garriga, J. (2013). Localized grey matter atrophy in multiple sclerosis: a meta-analysis of voxel-based morphometry studies and associations with functional disability. Neuroscience and Biobehavioral Reviews, 37(5), 819–830. doi:10.1016/j.neubiorev.2013.03.006.
Lopez-Gongora, M., Querol, L., & Escartin, A. (2015). A one-year follow-up study of the symbol digit modalities test (SDMT) and the paced auditory serial addition test (PASAT) in relapsing-remitting multiple sclerosis: an appraisal of comparative longitudinal sensitivity. BMC Neurology, 15, 40. doi:10.1186/s12883-015-0296-2.
Middleton, F. A., & Strick, P. L. (2000). Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research. Brain Research Reviews, 31(2–3), 236–250.
Minagar, A., Barnett, M. H., Benedict, R. H., Pelletier, D., Pirko, I., Sahraian, M. A., et al. (2013). The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology, 80(2), 210–219. doi:10.1212/WNL.0b013e31827b910b.
Morrow, S. A., Drake, A., Zivadinov, R., Munschauer, F., Weinstock-Guttman, B., & Benedict, R. H. (2010). Predicting loss of employment over three years in multiple sclerosis: clinically meaningful cognitive decline. The Clinical Neuropsychologist, 24(7), 1131–1145. doi:10.1080/13854046.2010.511272.
Nocentini, U., Bozzali, M., Spano, B., Cercignani, M., Serra, L., Basile, B., et al. (2014). Exploration of the relationships between regional grey matter atrophy and cognition in multiple sclerosis. Brain Imaging and Behavior, 8(3), 378–386. doi:10.1007/s11682-012-9170-7.
Parmenter, B. A., Weinstock-Guttman, B., Garg, N., Munschauer, F., & Benedict, R. H. (2007). Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Multiple Sclerosis, 13(1), 52–57.
Patenaude, B., Smith, S. M., Kennedy, D. N., & Jenkinson, M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage, 56(3), 907–922. doi:10.1016/j.neuroimage.2011.02.046.
Polman, C. H., Reingold, S. C., Banwell, B., Clanet, M., Cohen, J. A., Filippi, M., et al. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69(2), 292–302. doi:10.1002/ana.22366.
Prinster, A., Quarantelli, M., Orefice, G., Lanzillo, R., Brunetti, A., Mollica, C., et al. (2006). Grey matter loss in relapsing-remitting multiple sclerosis: a voxel-based morphometry study. NeuroImage, 29(3), 859–867. doi:10.1016/j.neuroimage.2005.08.034.
Rao, S. M., Leo, G. J., Bernardin, L., & Unverzagt, F. (1991a). Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology, 41(5), 685–691.
Rao, S. M., Leo, G. J., Ellington, L., Nauertz, T., Bernardin, L., & Unverzagt, F. (1991b). Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology, 41(5), 692–696.
Rao, S. M., Martin, A. L., Huelin, R., Wissinger, E., Khankhel, Z., Kim, E., et al. (2014). Correlations between MRI and information processing speed in MS: a meta-analysis. Multiple Sclerosis International, 2014, 975803. doi:10.1155/2014/975803.
Rocca, M. A., Valsasina, P., Meani, A., Falini, A., Comi, G., & Filippi, M. (2016). Impaired functional integration in multiple sclerosis: a graph theory study. Brain Structure & Function, 221(1), 115–131. doi:10.1007/s00429-014-0896-4.
Sarica, A., Cerasa, A., & Quattrone, A. (2015). The neurocognitive profile of the cerebellum in multiple sclerosis. International Journal of Molecular Sciences, 16(6), 12185–12198. doi:10.3390/ijms160612185.
Sastre-Garriga, J., Arevalo, M. J., Renom, M., Alonso, J., Gonzalez, I., Galan, I., et al. (2009). Brain volumetry counterparts of cognitive impairment in patients with multiple sclerosis. Journal of the Neurological Sciences, 282(1–2), 120–124. doi:10.1016/j.jns.2008.12.019.
Sastre-Garriga, J., Alonso, J., Renom, M., Arevalo, M. J., Gonzalez, I., Galan, I., et al. (2011). A functional magnetic resonance proof of concept pilot trial of cognitive rehabilitation in multiple sclerosis. Multiple Sclerosis, 17(4), 457–467. doi:10.1177/1352458510389219.
Schoonheim, M. M., Popescu, V., Rueda Lopes, F. C., Wiebenga, O. T., Vrenken, H., Douw, L., et al. (2012). Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology, 79(17), 1754–1761. doi:10.1212/WNL.0b013e3182703f46.
Smith, A. (1982). Symbol digits modalities test: Manual. Los Angeles: Western Psychological Services.
Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. doi:10.1002/hbm.10062.
Smith, S. M., Zhang, Y., Jenkinson, M., Chen, J., Matthews, P. M., Federico, A., et al. (2002). Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage, 17(1), 479–489.
Strober, L. B., Christodoulou, C., Benedict, R. H., Westervelt, H. J., Melville, P., Scherl, W. F., et al. (2012). Unemployment in multiple sclerosis: the contribution of personality and disease. Multiple Sclerosis, 18(5), 647–653. doi:10.1177/1352458511426735.
Tedesco, A. M., Chiricozzi, F. R., Clausi, S., Lupo, M., Molinari, M., & Leggio, M. G. (2011). The cerebellar cognitive profile. Brain, 134(Pt 12), 3672–3686. doi:10.1093/brain/awr266.
Weier, K., Penner, I. K., Magon, S., Amann, M., Naegelin, Y., Andelova, M., et al. (2014). Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PloS One, 9(1), e86916. doi:10.1371/journal.pone.0086916.
Zipoli, V., Goretti, B., Hakiki, B., Siracusa, G., Sorbi, S., Portaccio, E., et al. (2010). Cognitive impairment predicts conversion to multiple sclerosis in clinically isolated syndromes. Multiple Sclerosis, 16(1), 62–67. doi:10.1177/1352458509350311.
Acknowledgements
The authors thank all subjects, especially the patients with MS for the time and effort devoted to this study; Antonella Paccone, MRI Center “SUN-FISM,” University of Campania “Luigi Vanvitelli” and Institute of Diagnosis and Care “Hermitage-Capodimonte,” Naples, Italy for technical support; Mattia Siciliano, Department of Medical, Surgical, Neurological, Metabolic and Aging Sciences, University of Campania “Luigi Vanvitelli”, Italy for contributing to statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
Alvino Bisecco, Svetlana Stamenova, Giuseppina Caiazzo, Alessandro d’Ambrosio, Rosaria Sacco, Renato Docimo, Sabrina Esposito, Mario Cirillo and Fabrizio Esposito have no disclosures. Simona Bonavita received speakers’ honoraria from Biogen Idec, Novartis, and Merck-Serono. Gioacchino Tedeschi has received compensation for consulting services and/or speaking activities from Bayer Schering Pharma, Biogen Idec, Merck Serono, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck Serono, and Fondazione Italiana Sclerosi Multipla. Antonio Gallo received honoraria for speaking and travel grants from Biogen, Sanofi-Aventis, Merck Serono, Genzyme, Teva, Bayer-Schering and Novartis.
Ethical approval
This study was approved by the by the local Ethic Committee. All procedures performed in this study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Alvino Bisecco and Svetlana Stamenova contributed equally to the development of the study.
Rights and permissions
About this article
Cite this article
Bisecco, A., Stamenova, S., Caiazzo, G. et al. Attention and processing speed performance in multiple sclerosis is mostly related to thalamic volume. Brain Imaging and Behavior 12, 20–28 (2018). https://doi.org/10.1007/s11682-016-9667-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9667-6