Abstract
Studies have shown that white matter (WM) volumetric reductions and overall degradation occur with aging. Nonetheless little is known about the WM alterations that may underlie different cognitive status in older individuals. The main goal of the present work was to identify and characterize possible macro and microstructural WM alterations that could distinguish between older healthy individuals with contrasting cognitive profiles (i.e., “poor” vs “good” cognitive performers). Structural and diffusion magnetic resonance imaging was performed in order to quantify local WM volumes, white matter signal abnormalities (WMSA) volume (a measure of lesion burden) and diffusion tensor imaging scalar maps known to probe WM microstructure. A battery of neurocognitive/psychological tests was administered to assess the cognitive performance. Poor performers showed a higher slope for the positive association between WMSA volume and age compared to good performers. Even when controlling for WMSA volume, poor performers also evidenced lower fractional anisotropy, as well as positive associations with age with higher slopes of regression parameters in radial and axial diffusivity. Altogether results suggest that cognitive performance is related to differences in WM, with poor cognitive performers displaying signs of faster aging in WM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The normal aging process is characterized by a natural and progressive cognitive decline (Brickman et al. 2005; Salthouse 2009). Interestingly, the healthy aging pattern seems to be heterogeneous, as different individuals age differently. In fact, cognitive decline is less pronounced in some individuals, compared to others, with this effect being associated with a multitude of factors (MacDonald et al. 2003; Paulo et al. 2011; Santos et al. 2014).
Several changes take place at the brain level with normal aging, including: volume alterations; chemical changes, characterized by decreased synthesis of neurotransmitters, neurotransmitter receptors, and transporters (Ota et al. 2006); cortical thinning (Salat et al. 2004); and functional reorganization (Tomasi and Volkow 2012). Specifically, gray matter (GM) and white matter (WM) alterations in healthy aging have been the scope of many neuroimaging studies (Fjell et al. 2013; Lemaitre et al. 2012; Salat et al. 2009), with some work reporting patterns of WM alterations that accompany cognitive decline in aging. For example, WM degradation in anterior regions has been associated with decreased processing speed and reduced working memory, while degeneration on the posterior parts has been linked to reduced inhibition and higher task switching costs as well as poor episodic memory with WM degradation in central regions of the brain (Kennedy and Raz 2009). Moreover, WM atrophy was associated with a decline in neuropsychological functioning (Brickman et al. 2006).
Another common finding in aging studies is the positive association between aging, cognitive decline, and the presence of WM hyperintensities in T2-weighted MRI images, which have been described as ischemic events (Fischer et al. 2007; Garde et al. 2005). These lesions are frequently associated with axonal loss and demyelination (Wang et al. 2011). Similarly to the volumetric results, WM signal abnormalities (WMSA) seem to present an anterior-to-posterior age pattern, even in healthy individuals, and are associated with poorer cognition and functional status (Maillard et al. 2012; Prins et al. 2005; Wakefield et al. 2010). In a recent study, researchers demonstrated that WMSA were associated with slower search performance in aging, and this association was independent of the generalized decrease in processing speed (Lockhart et al. 2014).
Diffusion tensor imaging (DTI) studies have brought evidence that WM microstructural alterations also occur with aging and may be associated with cognition (for review see (Madden et al. 2011)). Cross sectional studies have described variations on WM properties associated with cognitive performance such as memory, executive functioning, and information processing speed (Deary et al. 2006; Laukka et al. 2013; Vernooij et al. 2009). These have been supported also by longitudinal alterations of perceptual speed and working memory in older adults (Charlton et al. 2010; Lovden et al. 2014). Moreover, studies involving DTI analysis have consistently reported patterns of WM degradation (O’Sullivan et al. 2001), which typically includes both a decrease in Fractional Anisotropy (FA) of normal appearing white matter with increasing age and a decrease in Mean Diffusivity (MD) (Barrick et al. 2010; Kochunov et al. 2007; Pfefferbaum and Sullivan 2003; Salat et al. 2005). Fewer studies have also reported greater age-related increases in Radial Diffusivity (RD) compared to Axial Diffusivity (AD) (Zhang et al. 2010). A recent longitudinal study showed that AD changes with aging were more consistent than FA and RD changes and that both increases and decreases in AD occur along aging (Bender and Raz 2015). There is also an anterior-to-posterior gradient of degeneration with more pronounced effects on the anterior regions (Grieve et al. 2007; Head et al. 2004; Pfefferbaum and Sullivan 2003; Salat et al. 2005). Cognitive impairments associated with WM degradation using DTI metrics have been found in pathological conditions, including Alzheimer’s Disease and mild cognitive impairment (MCI) (Liu et al. 2011; Mielke et al. 2009), multiple sclerosis (MS) (Roosendaal et al. 2009) and major depressive disorder (MDD) (Alves et al. 2012).
Still, despite the findings, most of the previously mentioned studies focused either on an individual dimension of WM assessment (e.g., volumetry, WMSA or DTI indices) or on specific dimensions of cognitive performance (e.g., working memory, executive functioning, processing speed). Moreover, a previous study by Santos et al. (2013) assessed a large sample of older healthy adults with an extensive battery of neurocognitive tests that were grouped in cognitive dimensions. Afterwards, clusters of cognitive performance were obtained using these dimensions and mood status. These results pointed to the fact that some individuals that are better in one dimension of cognition (e.g., memory) are also better at other cognitive dimensions (e.g., executive functioning) and the opposite also holds true. Taking this into account, in the present study through a multimodal neuroimaging approach in a cross-sectional design, we aimed at investigating i) which WM properties could discriminate between overall “good” and “poor” cognitive performance status in healthy older individuals and ii) if age affects each group differently, through a multimodal neuroimaging approach in a cross-sectional design. Specifically, we assessed WM volumetry and WM hypointensities volume (a measure of WMSA volume) through structural MRI and investigated WM microstructural properties through DTI metrics derived from diffusion MRI.
Methods
Ethics statement
The current study was part of the Switchbox (www.switchbox-online.eu/) project. Study goals and tests were explained to all participants. Informed written consent was obtained from all study participants. The study was conducted in accordance with the principles expressed in the Declaration of Helsinki and was approved by the local and national ethics committees.
Neurocognitive assessment
The selection of participants was based on cognitive data obtained from a representative sample of the general Portuguese older population in terms of age, gender, and education [n = 1051, after inclusion/exclusion criteria; subjects randomly selected from the Guimarães and Vizela local area health authority registries (Costa et al. 2013; Santos et al. 2013, 2014)]. A team of trained psychologists performed the neurocognitive/neuropsychological assessments. The test battery included the following instruments: digit-span forward and backward test, Stroop color and word test, controlled oral word association test (COWAT, letters F-A-S), selective reminding test (SRT), digit symbol substitution test (DSST), mini-mental state examination (MMSE), geriatric depression scale (GDS, long-version) and the Graffar socio-demographic scale (Santos et al. 2014). Regarding the MMSE scores, recommendations state that the corresponding threshold should be adjusted depending on factors such as age and/or education (Busch and Chapin 2008; Grigoletto et al. 1999). The following adjusted thresholds for cognitive impairment were calculated and applied: MMSE score < 17 if individual with ≤ 4 years of formal school education and/or ≥ 72 years of age, and MMSE score < 23 otherwise [follows the MMSE validation study for the Portuguese population (Guerreiro et al. 1994)].
Principal component analysis and clusters of cognitive performance
In a previous study, Principal Component Analysis (PCA) was performed in a subsample of 487 participants in order to allocate the multiple test variables into composite components/dimensions, thus reducing data dimensionality with the least possible loss of information (Santos et al. 2013). PCA resulted in the identification of four significant dimensions: memory (MEM) (SRT test variables: consistent long-term retrieval (CLTR), long-term storage (LTS) and delayed recall); executive function (EXEC) (FAS admissible parameter; Stroop parameters: words, colors, and words/colors; digits parameters: forward and backward); global cognitive status (MMSE) and mood (GDS). Further details regarding factor loadings can be consulted in the original publication of the exploratory factor analysis (Santos et al. 2013).
Finally, a cluster analysis on the PCA-derived dimensions (i.e., MEM, EXEC, MMSE and GDS z-scores) was performed in order to identify groups sharing similar characteristics across these dimensions and a four-cluster membership was considered to provide the best solution (Santos et al. 2013). Further analysis of the resulting clusters (C1 to C4) revealed that the subjects included in C1 had the highest MEM, EXEC and MMSE z-scores, and that the clusters followed a consistent rank order of cognitive performance in all cognitive dimensions (i.e., EXEC, MEM and MMSE): C1 > C2 > C3 > C4. Thus, this ranked order of performance in cognitive dimensions corresponded to “very good”, “good”, “poor”, and “very poor” cognitive performers. The clusters differed significantly from each other in all cognitive dimensions (ANOVAs, followed by Games-Howell post hoc tests). GDS scores differed significantly between C1 and C2 clusters and between the C3 and C4 clusters, but not between C1 and C3 and C2 and C4 (Santos et al. 2013).
Participants
In the Switchbox project, from the considered cohort (Santos et al. 2013), 60 subjects from the cluster exhibiting “very good” (C1) cognitive performance and 60 subjects from the “very poor” (C4) cognitive performers cluster were recruited for MRI screening. Participants were pseudo-randomly selected from each cluster in order to obtain two groups matched for age and gender. Selectivity indexes for these background variables ([Msubsample - Mtotal sample] / SDtotal sample) were −0.131 for age and 0.400 for years of formal school education and the ratio of males/females was 0.533 in the original sample and 0.467 in this sample. The primary exclusion criteria were inability to understand the informed consent, participant choice to withdraw from the study, incapacity and/or inability to attend the MRI session, dementia and/or diagnosed neuropsychiatric and/or neurodegenerative disorder (medical records).
In the present manuscript “very good” and “very poor” cognitive performers will be referred simply as good and poor performers, respectively. This setting enabled us to test which WM correlates could distinguish between overall cognitive performance groups. From the 120 subjects initially recruited, nine refused to undergo MRI screening at the time of the evaluation, one subject did not finish the diffusion acquisition from the screening protocol, and four subjects had brain lesions/pathology. In total, 106 subjects (ranging from 51 to 87 years of age) participated in the present study: 58 good and 48 poor cognitive performers.
Data acquisition
All participants underwent the same acquisition protocol implemented on a clinical approved Siemens Magnetom Avanto 1.5 T (Siemens Medical Solutions, Erlangen, Germany) at Hospital de Braga (Braga, Portugal) using a Siemens 12-channel receive-only head coil. The acquisition protocol included a structural 3D T1-weighted magnetization prepared rapid gradient echo (MPRAGE) with the following parameters: repetition time (TR) = 2730 ms, echo time (TE) = 3.48 ms, flip angle = 7°, 176 sagittal slices, in-plane resolution = 1 × 1 mm2 and slice thickness = 1 mm. A Diffusion Weighted Imaging (DWI) scan was also performed using a spin-echo echo-planar imaging (SE-EPI) sequence: TR = 8800 ms, TE = 99 ms, FoV = 240 × 240 mm, acquisition matrix = 120 × 120, 61 2-mm axial slices with no gap, 30 non-collinear gradient directions with b = 1000 s mm−2, one b = 0 s mm−2 acquisition and 1 repetition.
Before any data pre-processing, the raw DWI acquisitions from all subjects were visually inspected by the authors, including a certified neuroradiologist, to confirm that none of the participants had brain lesions and/or critical head motion or artifacts that could compromise data quality.
White matter segmentation and WMSA volume estimation
T1-weighted MPRAGE images were processed using the standard semi-automated workflow implemented in Freesurfer toolkit version 5.1 (http://surfer.nmr.mgh.harvard.edu). The pipeline and procedures employed have been validated against manual segmentations (Fischl et al. 2002) and are considered reliable across sessions, scanner platforms, updates, and field strengths (Jovicich et al. 2009). Several improvements were made during the last decade and the technicalities of the procedures are described elsewhere (Desikan et al. 2006; Destrieux et al. 2010; Fischl et al. 2002). Briefly, the entire pipeline involves 31 processing steps which include the spatial normalization to Talairach standard space, skull stripping, intensity normalization, tessellation of GM-WM boundary, and cortical, subcortical, and WM segmentation. For the present study, only the volumes of regions of interest (ROI) resulting from WM segmentation and total WM hypointensities (i.e., WMSA) volume were considered. Freesurfer labels the WMSA using probabilistic procedures that were extended to WM lesion identification (Fischl et al. 2002). This T1-based WMSA volume estimate has been successfully used as a measure of WM lesion volume (Salat et al. 2012), and showed sensitivity in measuring WM lesions in Alzheimer’s disease (Salat et al. 2010), as well as to correlate with estimates based on FLAIR acquisitions and to correlate better with clinical symptoms in MS (Bagnato et al. 2010).
DWI data pre-processing and tensor fitting
All data pre-processing was performed with tools provided with the FMRIB Software Library (FSL v5.0; http://fsl.fmrib.ox.ac.uk/fsl/). The DWI images were initially corrected for motion and eddy current distortions using FMRIB’s Diffusion Toolbox (FDT). The affine transformations used to register each volume were also used to rotate the gradient vectors accordingly using FSL’s fdt_rotate_bvecs script. The first b0 volume of each subject’s dataset was then extracted and skull stripped using the BET tool, thus generating a brain mask that was then applied to the remaining volumes in order to remove non-brain structures.
Tensor fitting and scalar maps computation steps were performed with the tools provided with Camino software package (http://cmic.cs.ucl.ac.uk/camino/) which implements the Robust Estimation of Tensors by Outlier Rejection (RESTORE) algorithm (Chang et al. 2005). The RESTORE algorithm performs nonlinear least squares fitting with constant weights after rejecting potential outliers with an iteratively reweighted least squares regression and inspection of the reweighted fit residuals. After tensor fitting, scalar maps of FA, AD, and RD were generated.
Tract based spatial statistics
Voxel-wise analysis of scalar maps was performed using the TBSS (Smith et al. 2006) procedures, also implemented in FSL. Initially, the FA maps from all participants were slightly eroded and the end slices were zeroed in order to further remove potential outliers. Next, all FA images were nonlinearly registered into a 1 × 1 × 1 mm standard space. In order to perform this, the FA image from each subject was nonlinearly registered to each other in order to find the “most representative one” (i.e., the one that requires the least warping to align all images) that served as the study specific template. This template image was then affine transformed into Montreal Neurological Institute (MNI) 152 standard space and each FA map was transformed into standard space by combining the nonlinear transformation to the FA target with the affine transformation into MNI space. All FA images were then averaged and the resulting image was skeletonized. The skeleton was then thresholded at 0.3 in order to remove from the skeleton regions encompassing multiple tissue types. Finally, all scalar maps (FA, AD, and RD) were projected into this FA skeleton using the transformations applied to the FA images.
Statistical analysis
The main focus of the present work was on the effects of overall cognitive functioning, age and their interactions in relation with WM. Multiple group confirmatory factor analysis (MGCFA) was performed in order to ensure that the cognitive factor structure was valid and invariant across age categories. This can be confirmed by the absence of differences between the fit of unconstrained and constrained models (Δχ2 = 8.22, Δdf = 14, p = 0.877) supporting metric invariance. Footnote 1
Volumetric data analysis was performed ROI-wise and the statistical analysis was performed with SPSS version 22 (IBM, SPSS, Chicago, IL, USA). For each WM ROI, a general linear model analysis of covariance (ANCOVA) was performed with the ROI volume as the dependent variable, group as the fixed factor, and age, sex, years of formal school education, and intracranial volume (ICV) as covariates. For the present study, only the main effects of age, group, and age by group interaction were considered of interest. Only results surviving a significance level of p < 0.05 after correction for multiple comparisons using the false discovery rate (FDR) criterion were reported. Analyses of the WMSA volume, as well as the volumes from the left caudal anterior cingulate and right pars triangularis, rostral anterior cingulate and superior temporal, were performed on natural log transformations of the estimated volume in order to meet the normality assumptions of the residuals of the ANCOVA models. The reason for this transformation was their positively skewed distribution. Systolic and diastolic blood pressure (BP) did not differ between groups (Table 1) and it was not a significant predictor of WMSA volume. For these reasons, and in order to reduce the number of independent variables in the models, we did not control for BP.
Statistical analysis of the skeletonized maps of FA, AD, and RD was performed using the permutation methods employed in “randomise”, distributed with FSL. In total, 4 models were used. In the first model we tested for the group by age interaction; thus, testing if the slopes of the age associations for each group differed significantly. In the second model, regions with significant interactions were masked out, thus enabling the estimation of the main effects of group and age. The third and fourth models were similar to the first two but with an additional regressor for the estimated volume of WMSA. These models enabled the estimation of the same effects as the previous two while controlling for a measure of WM lesion. Sex and years of formal school education were always entered in the models as covariates. Ten thousand random permutations were used in the inference of the contrasts of interest. Threshold-free cluster enhancement (TFCE) was used to detect widespread differences and family-wise error (FWE) correction at p < 0.05 was used to correct for multiple comparisons. The projected regions showing significant results were then labeled according to the John Hopkins University ICBM-DTI-81 WM labels atlas (Hua et al. 2008) distributed with FSL. For visualization purposes, the significant results were dilated with tbss_fill tool (distributed with FSL).
Results
Sample characteristics
Table 1 presents a basic demographic, cognitive, and physiological characterization of the two cognitive performance groups enrolled in the present study.
White matter volumetry
Analysis of WM regional volumes yielded no significant effects for group by age interactions. Similarly, no statistically significant differences were found between good and poor cognitive performers. However, several WM regions showed statistically significant (FDR corrected at p < 0.05) negative associations with age in both hemispheres (Table 2). Lateral Orbitofrontal, Superior Frontal, Inferior Temporal, Fusiform, and the Posterior Cingulate appeared to show the largest age differences.
Similarly to other studies, WMSA volumes were non-normally distributed and positively skewed (skewness of 1.843 and 2.150 for good and poor cognitive performers respectively) (Jacobs et al. 2013). Good cognitive performers presented median WMSA volume of 2109.5 (IQR = 1469.25) and poor cognitive performers presented median WMSA volume of 3052.5 (IQR = 4375.5). These values are comparable to other studies assessing healthy individuals with approximate age ranges (Jacobs et al. 2013; Leritz et al. 2013). Analysis of natural log transformed WMSA volume revealed a significant group by age interaction (F (1,100) = 10.531; p = 0.003; η2 = 0.095). Parameter estimates revealed that while WMSA volume has a positive correlation with age in both groups, the slope of the regression parameter is higher in poor cognitive performers (B = 0.072; SE = 0.012; CI95% = [0.047–0.097]) when compared to the slope in good cognitive performers (B = 0.033; SE = 006; CI95% = [0.020–0.046]). As expected, age was a significant predictor of WMSA volume (F (1,101) = 73.785; p < 0.001; η2 = 0.422) and group differences were also significant (F (1,101) = 8.253; p = 0.005; η2 = 0.076).
Group by age interactions in DTI metrics
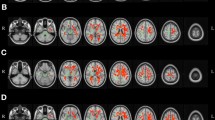
Spatial representations of statistically significant interactions between age and group, with and without inclusion of WMSA volume in the model, are presented in Fig. 1. Significant interactions between age and group in FA maps were found in the posterior thalamic radiation, splenium of corpus callosum and body of corpus callosum, with all clusters localized in the left hemisphere of the brain (Fig. 1a). Post-hoc analysis revealed that the group with poor cognitive performance had a stronger negative association between FA and age when compared to the group with good cognitive performance. The results presented in Fig. 1b indicate that after the inclusion of WMSA in the regression model, no significant interactions between age and group in FA values remained.
Statistically significant group by age interactions in FA, RD, and AD projected maps, before (a, c, e) and after (b, d, f) controlling for WMSA volume. Significance threshold was set to p < 0.05 (FWE corrected for multiple comparisons). Blue/light-blue gradient indicates lower age slopes for good cognitive performers when compared to poor cognitive performers. Red/yellow gradient indicates higher age slopes for the good cognitive performance group
Regarding RD, Fig. 1c shows that several significant interactions could be found between age and group in several widespread clusters and in both hemispheres. Post-hoc analysis revealed an inverse pattern relative to the FA interactions, with the poor cognitive performance group evidencing a higher slope in the positive association of RD and age. After inclusion of WMSA volume in the model, most of the significant interactions remained, being observed the same pattern of higher slope of the RD association with age in poor cognitive performers when compared to good cognitive performers (Fig. 1d).
Analysis of AD maps showed several widespread significant age by group interactions (Fig. 1e). Similarly to the interactions in RD profiles, poor cognitive performers showed higher slopes for the positive association of AD with age when compared to good cognitive performers. As shown in Fig. 1f, after inclusion of WMSA volume in the model, most of the previously described interactions remained.
Age effect on DTI metrics
Maps of the significant effects of age on FA, RD, and AD are presented in Fig. 2. These results were previously masked out with the significant group by age interaction results in order to reflect age effects only. Higher age was associated with lower FA values in almost all the WM tracts (Fig. 2a), and most of those differences remained after controlling for WMSA volume (Fig. 2b). The same pattern, although with a positive correlation with age, was found for RD profiles with and without inclusion of WMSA volume as a covariate (Fig. 2c and d). Age was also positively associated with higher AD values (Fig. 2e). The inclusion of WMSA as covariate seemed to have a low impact in the age effect (Fig. 2f).
Statistically significant associations between age and FA, RD, and AD maps, before (a, c, e) and after (b, d, f) controlling for WMSA volume. Blue/light-blue gradient indicates negative associations with age. Red/yellow gradient indicates positive. All results were considered significant at p < 0.05 (FWE corrected for multiple comparisons)
Group effect on DTI metrics
Analysis of group differences while accounting for age, gender, and years of formal school education, yielded several statistically significant effects (Fig. 3). Similarly to the age effect results, regions where group by age interactions were significant were previously masked out so that the presented results reflect only group differences. Between group analyses of FA maps revealed that good cognitive performers had significantly higher FA values in several wide spread WM tracts when compared with poor cognitive performers (Fig. 3a). Inclusion of WMSA volume in the regression model accounted for some, but not all, of the described between group differences (Fig. 3b).
Statistically significant group differences in FA, RD, and AD maps, before (a, c, e) and after (b, d, f) controlling for WMSA volume. Blue/light-blue gradient indicates lower values for good cognitive performers when compared to poor cognitive performers. Red/yellow gradient indicates higher values for the good cognitive performance group. All results were considered significant at p < 0.05 (FWE corrected for multiple comparisons)
In the same way, several widespread group differences could be found in RD maps, with good cognitive performers showing statistically significant lower values of RD (Fig. 3c). Figure 3d shows that, after regressing out WMSA volume effect, good cognitive performers still showed lower values of RD than poor cognitive performers in several white matter tracts. Regarding AD profiles, no group differences were found in AD before (Fig. 3e) and after (Fig. 3f) including WMSA volume as a covariate in the model.
Discussion
In the present study we investigated which possible WM correlates could underlie the distinction between two opposite cognitive profiles (i.e., ‘good’ and ‘poor’) in older subjects. Considering the effect of age on WM, the results are consistent with the majority of the previously published aging studies. These effects include i) overall volumetric negative associations with age in WM, which are more pronounced in anterior regions (Salat et al. 2009; Westlye et al. 2010), ii) positive associations with volume of WMSAs (Leritz et al. 2013; Smith et al. 2000), and iii) overall lower FA, increased RD and AD in aged individuals, when comparing to younger subjects (Bennett et al. 2010; Leritz et al. 2013). Longitudinal evidence also suggests reduced FA and increased AD and RD with aging (Sexton et al. 2014; Teipel et al. 2010). These results are usually interpreted in the context of axonal injury or loss and demyelination in late stages of life, potentially linked to cognitive decline.
Furthermore, interestingly, when controlling for the effects of age, results indicate that the contrasting cognitive profiles are underlined by differences in WM, which, notably, evolve differently with age. In fact, albeit no volumetric group differences or group by age interactions differences were observed, WMSA volume evidenced a positive association with age. This association had a higher slope in poor cognitive performers in comparison with the slope of association in good cognitive performers. This points to an increased susceptibility for the presence of brain ischemic events in older individuals with poorer cognitive performance, even when these are clinically silent. Moreover, WMSA volume has been shown to be positively associated with vascular risk factors, such as blood pressure (Firbank et al. 2007), as well with executive functioning, processing speed, and memory performance (Jacobs et al. 2013; Maillard et al. 2012; Prins et al. 2005). Thus, poorer cognitive performers not only reveal an augmented presence of WM lesions, which potentially results from loss of axonal integrity (Wang et al. 2011), with repercussion in cognitive functions, but they also exhibit patterns that could reflect an increased vascular risk when compared to those of similar age but overall stronger cognitive performance pattern. However, we did not find differences in BP between groups and BP was not a significant predictor of WMSA. This could be due to the fact that the older Portuguese population is generally hypertensive, independently of cognitive performance, and possibly, other factors may play a role. Some studies suggest that vascular risk factors, including hypertension, affect WM microstructural integrity independently of WMSA (Wang et al. 2015). Additionally, metabolic risk factors could also mediate these effects (Bender and Raz 2015).
When considering the effect in WM microstructure, good cognitive performers evidenced overall higher FA and lower RD and AD when compared to poorer performers. However, since several group by age interactions were found in all metrics, group differences need to be interpreted in the context of these interactions. Regarding FA, only small significant interactions were found in regions close to the left lateral ventricle that were not significant after the inclusion of WMSA volume as regressor. Nevertheless, the volume of WMSA explained only some of the widespread group differences in FA. Of note, lower values of FA are usually associated with age-related cognitive decline. This is thought to reflect axonal damage and decreased efficiency in communication and consequent disconnection of neural networks (Bartzokis et al. 2004; Kochunov et al. 2007; O’Sullivan et al. 2001). Additionally, longitudinal studies showed that alterations in FA in one tract are associated with similar alterations in other tracts (Lovden et al. 2014) thus indicating that FA changes with aging are not mostly tract specific.
Regarding RD, several widespread group by age interactions were found, with most remaining significant after the inclusion of WMSA in the model. This means that the positive association of RD and age had a higher slope in poor cognitive performers compared to good performers. The group comparisons further revealed that poor cognitive performers already showed significantly higher RD values at the mean age of the population under study. RD is the DTI metric mostly associated with myelination levels with increased RD typically reflecting demyelination (Klawiter et al. 2011; Sun et al. 2006). Taking this into account, our results point to an increased rate of demyelination in older individuals with poor cognitive performance, which is a clinically relevant finding.
When analyzing AD maps, no group differences were found with or without inclusion of WMSA volume as a regressor in the model. More interestingly, numerous significant group by age interactions were found with and without inclusion of WMSA volume as a regressor. This suggests that although at the mean age of our sample the groups did not differ significantly in AD after inclusion of WMSA volume, the slopes of the positive associations between AD and age are different, with the poor performance group showing a higher slope in AD compared to the good performance group. Unlike FA and RD, localized or sparse foci of increase (Zhang et al. 2010), decrease (Burzynska et al. 2010) or no effect (Madden et al. 2009) in AD with aging have been reported. Bender and Raz (2015) found, in a longitudinal setting, that aging was associated with both decreases and increases in AD. Whereas, RD is typically associated with myelination levels, AD is usually interpreted in the context of axonal pathology. Small decreases of AD have been reported in the presence of axonal injury (Budde et al. 2007, 2009) suggesting that cellular fragments and gliosis would restrict water diffusion. Sun and colleagues suggest, however, that cellular debris could be later removed by microglia, clearing the lesion area and thus leading to an increase in AD (Sun et al. 2008). As such, the increased slopes of the age regressor in AD could reflect the higher presence of axonal damage and the increased slopes of the regression parameter with RD could reflect a higher axonal myelin loss. Wallerian degeneration theory accounts for both axonal injury (primary degeneration) and loss of myelin following the axonal lesioning (secondary degeneration) (Conforti et al. 2014). Taking all of this into account, we hypothesize that the higher slope of the associations of age with AD and RD could reflect that poor cognitive performers could be suffering a higher rate of Wallerian degeneration compared to good cognitive performers.
In summary, older individuals with poor cognitive performance evidenced lower FA and higher slopes in the association of age with RD and AD, controlling for age, education, and macrostructural lesioning, when compared to good cognitive performers. In the context of aging, a similar pattern has been suggested to reflect chronic WM degeneration (Burzynska et al. 2010). Longitudinal studies reported that changes in perceptual speed are associated with decreased FA and increased MD in the cortical spinal tract (Lovden et al. 2014) and that working memory declines with aging were associated with decreases in MD but not FA (Charlton et al. 2010). In the present study however, we found differences to affect not only these tracts but also almost all WM tracts. This may indicate that overall good performance could be the result of overall preserved WM integrity. This is further supported by the results found for WMSA. However, with the present setting, we cannot exclude the hypothesis that this is the result of the summation of the effects of individual cognitive dimensions that are preserved in good performers. Considering that poor cognitive performers evidence higher slopes for the age regression parameters with diffusivity metrics, we speculate that poor cognitive performers display faster WM degeneration. This view is further reinforced by the similarity between the group comparisons results and the cross sectional effects of age. We aim at further investigating this with longitudinal analysis of the same cohort.
The present work presents some limitations that should be considered. One of the main limitation concerns the estimation of WMSA volume that, as in the present study, is estimated from a T1-weighted MPRAGE acquisition. Several different sources of WM lesioning can lead to WMSA and these different sources could not be fully identified with a T1-weighted sequence. Optimally, a combination of T1, T2, proton density (PD), and fluid-attenuated inversion-recovery (FLAIR) sequences should be used (Kim et al. 2008). Another limitation concerns the nature and interpretation of the DTI metrics analyzed. The images acquired in order to estimate those metrics had a limited voxel size of 2 mm. However, these metrics were interpreted as reflecting the properties of WM at the axonal level that has a much smaller scale. This leads to an important issue in inferring WM integrity from DTI scalar metrics. The link between FA, RD and AD and myelination levels or axonal injury is not well established. Other aspects such as crossing fibers, axonal re-arrangement or partial volume effects are known to have some impact on the same metrics (Jones et al. 2013). Nevertheless, the use of multiple DTI and structural metrics, as addressed in this work, may help to overcome part of these limitations. A final limitation of the study is that is has a cross-sectional design. Future work should include a longitudinal perspective of the effects of aging, to clarify how the different groups actually progress with age.
In summary, our results showed that poor cognitive performance in older adults is associated with increased volume of WM lesions and that individuals with a weaker cognitive profile show a higher rate of chronic white matter degeneration. The similarities between the group effects and the age effect suggest that aging has a greater impact in poor cognitive performers compared to stronger performers with similar chronological age.
Notes
Analysis performed on another sample of 435 subjects, similar to the sample used in (Santos et al. 2013) in terms of age and gender (data not published). The sample was divided in age categories (i.e., 50–60, 60–70, 70 or more years of age) for the MGCFA.
References
Alves, G. S., Karakaya, T., Fusser, F., Kordulla, M., O’Dwyer, L., Christl, J., & Pantel, J. (2012). Association of microstructural white matter abnormalities with cognitive dysfunction in geriatric patients with major depression. Psychiatry Research, 203(2–3), 194–200. doi:10.1016/j.pscychresns.2011.12.006.
Bagnato, F., Salman, Z., Kane, R., Auh, S., Cantor, F. K., Ehrmantraut, M., & McFarland, H. F. (2010). T1 cortical hypointensities and their association with cognitive disability in multiple sclerosis. Multiple Sclerosis, 16(10), 1203–1212. doi:10.1177/1352458510377223.
Barrick, T. R., Charlton, R. A., Clark, C. A., & Markus, H. S. (2010). White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. NeuroImage, 51(2), 565–577. doi:10.1016/j.neuroimage.2010.02.033.
Bartzokis, G., Sultzer, D., Lu, P. H., Nuechterlein, K. H., Mintz, J., & Cummings, J. L. (2004). Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiology of Aging, 25(7), 843–851. doi:10.1016/j.neurobiolaging.2003.09.005.
Bender, A. R., & Raz, N. (2015). Normal-appearing cerebral white matter in healthy adults: mean change over two years and individual differences in change. Neurobiol Aging(0). doi: 10.1016/j.neurobiolaging.2015.02.001.
Bennett, I. J., Madden, D. J., Vaidya, C. J., Howard, D. V., & Howard, J. H., Jr. (2010). Age-related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Human Brain Mapping, 31(3), 378–390. doi:10.1002/hbm.20872.
Brickman, A. M., Paul, R. H., Cohen, R. A., Williams, L. M., MacGregor, K. L., Jefferson, A. L., & Gordon, E. (2005). Category and letter verbal fluency across the adult lifespan: relationship to EEG theta power. Archives of Clinical Neuropsychology, 20(5), 561–573. doi:10.1016/j.acn.2004.12.006.
Brickman, A. M., Zimmerman, M. E., Paul, R. H., Grieve, S. M., Tate, D. F., Cohen, R. A., & Gordon, E. (2006). Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry, 60(5), 444–453.
Budde, M. D., Kim, J. H., Liang, H. F., Schmidt, R. E., Russell, J. H., Cross, A. H., & Song, S. K. (2007). Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magnetic Resonance in Medicine, 57(4), 688–695. doi:10.1002/mrm.21200.
Budde, M. D., Xie, M., Cross, A. H., & Song, S. K. (2009). Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. The Journal of Neuroscience, 29(9), 2805–2813. doi:10.1523/JNEUROSCI. 4605-08.2009.
Burzynska, A. Z., Preuschhof, C., Backman, L., Nyberg, L., Li, S. C., Lindenberger, U., & Heekeren, H. R. (2010). Age-related differences in white matter microstructure: region-specific patterns of diffusivity. NeuroImage, 49(3), 2104–2112. doi:10.1016/j.neuroimage.2009.09.041.
Busch, R. M., & Chapin, J. S. (2008). Review of normative data for common screening measures used to evaluate cognitive functioning in elderly individuals. The Clinical Neuropsychologist, 22(4), 620–650. doi:10.1080/13854040701448793.
Chang, L. C., Jones, D. K., & Pierpaoli, C. (2005). RESTORE: robust estimation of tensors by outlier rejection. Magnetic Resonance in Medicine, 53(5), 1088–1095. doi:10.1002/mrm.20426.
Charlton, R. A., Schiavone, F., Barrick, T. R., Morris, R. G., & Markus, H. S. (2010). Diffusion tensor imaging detects age related white matter change over a 2 year follow-up which is associated with working memory decline. Journal of Neurology, Neurosurgery, and Psychiatry, 81(1), 13–19. doi:10.1136/jnnp.2008.167288.
Conforti, L., Gilley, J., & Coleman, M. P. (2014). Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nature Reviews Neuroscience, 15(6), 394–409. doi:10.1038/Nrn3680.
Costa, P. S., Santos, N. C., Cunha, P., Palha, J. A., & Sousa, N. (2013). The use of bayesian latent class cluster models to classify patterns of cognitive performance in healthy ageing. PLoS One, 8(8), e71940. doi:10.1371/journal.pone.0071940.
Deary, I. J., Bastin, M. E., Pattie, A., Clayden, J. D., Whalley, L. J., Starr, J. M., & Wardlaw, J. M. (2006). White matter integrity and cognition in childhood and old age. Neurology, 66(4), 505–512. doi:10.1212/01.wnl.0000199954.81900.e2.
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., & Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31, 968–980.
Destrieux, C., Fischl, B., Dale, A., & Halgren, E. (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage, 53(1), 1–15. doi:10.1016/j.neuroimage.2010.06.010.
Firbank, M. J., Wiseman, R. M., Burton, E. J., Saxby, B. K., O’Brien, J. T., & Ford, G. A. (2007). Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. Journal of Neurology, 254(6), 713–721. doi:10.1007/s00415-006-0238-4.
Fischer, P., Krampla, W., Mostafaie, N., Zehetmayer, S., Rainer, M., Jungwirth, S., & Tragl, K. H. (2007). VITA study: white matter hyperintensities of vascular and degenerative origin in the elderly. Journal of Neural Transmission, Supplement, 72, 181–188.
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., & Dale, A. M. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355.
Fjell, A. M., Westlye, L. T., Grydeland, H., Amlien, I., Espeseth, T., Reinvang, I., & Alzheimer Disease Neuroimaging, I. (2013). Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiology of Aging, 34(10), 2239–2247. doi:10.1016/j.neurobiolaging.2013.04.006.
Garde, E., Mortensen, E. L., Rostrup, E., & Paulson, O. B. (2005). Decline in intelligence is associated with progression in white matter hyperintensity volume. Journal of Neurology, Neurosurgery & Psychiatry, 76(9), 1289–1291.
Grieve, S. M., Williams, L. M., Paul, R. H., Clark, C. R., & Gordon, E. (2007). Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR - American Journal of Neuroradiology, 28(2), 226–235.
Grigoletto, F., Zappala, G., Anderson, D. W., & Lebowitz, B. D. (1999). Norms for the mini-mental state examination in a healthy population. Neurology, 53(2), 315–320.
Guerreiro, M., Silva, A. P., Botelho, M. A., Leitão, O., Castro-Caldas, A., & Garcia, C. (1994). Adaptação à população portuguesa da tradução do mini mental state examination (MMSE). Revista Portuguesa de Neurologia, 1(9), 9–10.
Head, D., Buckner, R. L., Shimony, J. S., Williams, L. E., Akbudak, E., Conturo, T. E., & Snyder, A. Z. (2004). Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cerebral Cortex, 14(4), 410–423.
Hua, K., Zhang, J., Wakana, S., Jiang, H., Li, X., Reich, D. S., & Mori, S. (2008). Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage, 39(1), 336–347. doi:10.1016/j.neuroimage.2007.07.053.
Jacobs, H. I., Leritz, E. C., Williams, V. J., Van Boxtel, M. P., van der Elst, W., Jolles, J., & Salat, D. H. (2013). Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Human Brain Mapping, 34(1), 77–95. doi:10.1002/hbm.21412.
Jones, D. K., Knosche, T. R., & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage, 73, 239–254. doi:10.1016/j.neuroimage.2012.06.081.
Jovicich, J., Czanner, S., Han, X., Salat, D., van der Kouwe, A., Quinn, B., & Fischl, B. (2009). MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. NeuroImage, 46(1), 177–192. doi:10.1016/j.neuroimage.2009.02.010.
Kennedy, K. M., & Raz, N. (2009). Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia, 47(3), 916–927. doi:10.1016/j.neuropsychologia.2009.01.001.
Kim, K. W., MacFall, J. R., & Payne, M. E. (2008). Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biological Psychiatry, 64(4), 273–280. doi:10.1016/j.biopsych.2008.03.024.
Klawiter, E. C., Schmidt, R. E., Trinkaus, K., Liang, H. F., Budde, M. D., Naismith, R. T., & Benzinger, T. L. (2011). Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. NeuroImage, 55(4), 1454–1460. doi:10.1016/j.neuroimage.2011.01.007.
Kochunov, P., Thompson, P. M., Lancaster, J. L., Bartzokis, G., Smith, S., Coyle, T., & Fox, P. T. (2007). Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. NeuroImage, 35(2), 478–487. doi:10.1016/j.neuroimage.2006.12.021.
Laukka, E. J., Lovden, M., Kalpouzos, G., Li, T. Q., Jonsson, T., Wahlund, L. O., & Backman, L. (2013). Associations between white matter microstructure and cognitive performance in old and very old age. PLoS One, 8(11), e81419. doi:10.1371/journal.pone.0081419.
Lemaitre, H., Goldman, A. L., Sambataro, F., Verchinski, B. A., Meyer-Lindenberg, A., Weinberger, D. R., & Mattay, V. S. (2012). Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging, 33(3), 617 e611-619. doi: 10.1016/j.neurobiolaging.2010.07.013.
Leritz, E. C., Shepel, J., Williams, V. J., Lipsitz, L. A., McGlinchey, R. E., Milberg, W. P., & Salat, D. H. (2013). Associations between T(1) white matter lesion volume and regional white matter microstructure in aging. Human Brain Mapping. doi:10.1002/hbm.22236.
Liu, Y., Spulber, G., Lehtimaki, K. K., Kononen, M., Hallikainen, I., Grohn, H., & Soininen, H. (2011). Diffusion tensor imaging and tract-based spatial statistics in Alzheimer’s disease and mild cognitive impairment. Neurobiology of Aging, 32(9), 1558–1571. doi:10.1016/j.neurobiolaging.2009.10.006.
Lockhart, S. N., Roach, A. E., Luck, S. J., Geng, J., Beckett, L., Carmichael, O., & DeCarli, C. (2014). White matter hyperintensities are associated with visual search behavior independent of generalized slowing in aging. Neuropsychologia, 52, 93–101. doi:10.1016/j.neuropsychologia.2013.10.011.
Lovden, M., Kohncke, Y., Laukka, E. J., Kalpouzos, G., Salami, A., Li, T. Q., & Backman, L. (2014). Changes in perceptual speed and white matter microstructure in the corticospinal tract are associated in very old age. NeuroImage, 102(Pt 2), 520–530. doi:10.1016/j.neuroimage.2014.08.020.
MacDonald, S. W., Hultsch, D. F., & Dixon, R. A. (2003). Performance variability is related to change in cognition: evidence from the Victoria Longitudinal Study. Psychology and Aging, 18(3), 510.
Madden, D. J., Bennett, I. J., Burzynska, A., Potter, G. G., Chen, N. K., & Song, A. W. (2011). Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica et Biophysica Acta. doi:10.1016/j.bbadis.2011.08.003.
Madden, D. J., Spaniol, J., Costello, M. C., Bucur, B., White, L. E., Cabeza, R., & Huettel, S. A. (2009). Cerebral white matter integrity mediates adult age differences in cognitive performance. Journal Cognitive Neuroscience, 21(2), 289–302. doi:10.1162/jocn.2009.21047.
Maillard, P., Carmichael, O., Fletcher, E., Reed, B., Mungas, D., & DeCarli, C. (2012). Coevolution of white matter hyperintensities and cognition in the elderly. Neurology, 79(5), 442–448. doi:10.1212/WNL.0b013e3182617136.
Mielke, M. M., Kozauer, N. A., Chan, K. C., George, M., Toroney, J., Zerrate, M., & Albert, M. (2009). Regionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. NeuroImage, 46(1), 47–55. doi:10.1016/j.neuroimage.2009.01.054.
O’Sullivan, M., Jones, D. K., Summers, P. E., Morris, R. G., Williams, S. C., & Markus, H. S. (2001). Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology, 57(4), 632–638.
Ota, M., Yasuno, F., Ito, H., Seki, C., Nozaki, S., Asada, T., & Suhara, T. (2006). Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L-[beta-11C]DOPA. Life Sciences, 79(8), 730–736. doi:10.1016/j.lfs.2006.02.017.
Paulo, A. C., Sampaio, A., Santos, N. C., Costa, P. S., Cunha, P., Zihl, J., & Sousa, N. (2011). Patterns of cognitive performance in healthy ageing in northern portugal: a cross-sectional analysis. PLoS One, 6(9), e24553. doi:10.1371/journal.pone.0024553.
Pfefferbaum, A., & Sullivan, E. V. (2003). Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magnetic Resonance in Medicine, 49(5), 953–961. doi:10.1002/mrm.10452.
Prins, N. D., van Dijk, E. J., den Heijer, T., Vermeer, S. E., Jolles, J., Koudstaal, P. J., & Breteler, M. M. (2005). Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain, 128(Pt 9), 2034–2041. doi:10.1093/brain/awh553.
Roosendaal, S. D., Geurts, J. J., Vrenken, H., Hulst, H. E., Cover, K. S., Castelijns, J. A., & Barkhof, F. (2009). Regional DTI differences in multiple sclerosis patients. NeuroImage, 44(4), 1397–1403. doi:10.1016/j.neuroimage.2008.10.026.
Salat, D. H., Buckner, R. L., Snyder, A. Z., Greve, D. N., Desikan, R. S., Busa, E., & Fischl, B. (2004). Thinning of the cerebral cortex in aging. Cerebral Cortex, 14(7), 721–730. doi:10.1093/cercor/bhh032.
Salat, D. H., Greve, D. N., Pacheco, J. L., Quinn, B. T., Helmer, K. G., Buckner, R. L., & Fischl, B. (2009). Regional white matter volume differences in nondemented aging and Alzheimer’s disease. NeuroImage, 44(4), 1247–1258. doi:10.1016/j.neuroimage.2008.10.030.
Salat, D. H., Tuch, D. S., Greve, D. N., van der Kouwe, A. J., Hevelone, N. D., Zaleta, A. K., & Dale, A. M. (2005). Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging, 26(8), 1215–1227. doi:10.1016/j.neurobiolaging.2004.09.017.
Salat, D. H., Tuch, D. S., van der Kouwe, A. J., Greve, D. N., Pappu, V., Lee, S. Y., & Rosas, H. D. (2010). White matter pathology isolates the hippocampal formation in Alzheimer’s disease. Neurobiology of Aging, 31(2), 244–256. doi:10.1016/j.neurobiolaging.2008.03.013.
Salat, D. H., Williams, V. J., Leritz, E. C., Schnyer, D. M., Rudolph, J. L., Lipsitz, L. A., & Milberg, W. P. (2012). Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. NeuroImage, 59(1), 181–192. doi:10.1016/j.neuroimage.2011.07.033.
Salthouse, T. A. (2009). Decomposing age correlations on neuropsychological and cognitive variables. Journal of the International Neuropsychological Society, 15(5), 650–661. doi:10.1017/s1355617709990385.
Santos, N. C., Costa, P. S., Cunha, P., Cotter, J., Sampaio, A., Zihl, J., & Sousa, N. (2013). Mood is a key determinant of cognitive performance in community-dwelling older adults: a cross-sectional analysis. Age (Dordrecht, Netherlands), 35(5), 1983–1993. doi:10.1007/s11357-012-9482-y.
Santos, N. C., Costa, P. S., Cunha, P., Portugal-Nunes, C., Amorim, L., Cotter, J., Sousa, N. (2014). Clinical, physical and lifestyle indicators and relationship with cognition and mood in aging: a cross-sectional analysis of distinct educational groups. Frontiers in Aging Neuroscience, 6. doi: 10.3389/fnagi.2014.00021.
Sexton, C. E., Walhovd, K. B., Storsve, A. B., Tamnes, C. K., Westlye, L. T., Johansen-Berg, H., & Fjell, A. M. (2014). Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. The Journal of Neuroscience, 34(46), 15425–15436. doi:10.1523/JNEUROSCI. 0203-14.2014.
Smith, C. D., Snowdon, D. A., Wang, H., & Markesbery, W. R. (2000). White matter volumes and periventricular white matter hyperintensities in aging and dementia. Neurology, 54(4), 838–842.
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., & Behrens, T. E. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505. doi:10.1016/j.neuroimage.2006.02.024.
Sun, S. W., Liang, H. F., Cross, A. H., & Song, S. K. (2008). Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. NeuroImage, 40(1), 1–10. doi:10.1016/j.neuroimage.2007.11.049.
Sun, S. W., Liang, H. F., Le, T. Q., Armstrong, R. C., Cross, A. H., & Song, S. K. (2006). Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. NeuroImage, 32(3), 1195–1204. doi:10.1016/j.neuroimage.2006.04.212.
Teipel, S. J., Meindl, T., Wagner, M., Stieltjes, B., Reuter, S., Hauenstein, K. H., & Hampel, H. (2010). Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. Journal of Alzheimer’s Disease, 22(2), 507–522. doi:10.3233/JAD-2010-100234.
Tomasi, D., & Volkow, N. D. (2012). Aging and functional brain networks. Mol Psychiatry, 17(5), 471, 549–458. doi: 10.1038/mp.2011.81.
Vernooij, M. W., Ikram, M. A., Vrooman, H. A., Wielopolski, P. A., Krestin, G. P., Hofman, A., & Breteler, M. M. (2009). White matter microstructural integrity and cognitive function in a general elderly population. Archives of General Psychiatry, 66(5), 545–553. doi:10.1001/archgenpsychiatry.2009.5.
Wakefield, D. B., Moscufo, N., Guttmann, C. R., Kuchel, G. A., Kaplan, R. F., Pearlson, G., & Wolfson, L. (2010). White matter hyperintensities predict functional decline in voiding, mobility, and cognition in older adults. Journal of American Geriatrics Society, 58(2), 275–281. doi:10.1111/j.1532-5415.2009.02699.x.
Wang, L., Goldstein, F. C., Levey, A. I., Lah, J. J., Meltzer, C. C., Holder, C. A., & Mao, H. (2011). White matter hyperintensities and changes in white matter integrity in patients with Alzheimer’s disease. Neuroradiology, 53(5), 373–381. doi:10.1007/s00234-010-0806-2.
Wang, R., Fratiglioni, L., Laukka, E. J., Lovden, M., Kalpouzos, G., Keller, L., & Qiu, C. (2015). Effects of vascular risk factors and APOE epsilon4 on white matter integrity and cognitive decline. Neurology. doi:10.1212/WNL.0000000000001379.
Westlye, L. T., Walhovd, K. B., Dale, A. M., Bjørnerud, A., Due-Tønnessen, P., Engvig, A., & Fjell, A. M. (2010). Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex, 20(9), 2055–2068.
Zhang, Y., Du, A. T., Hayasaka, S., Jahng, G. H., Hlavin, J., Zhan, W., & Schuff, N. (2010). Patterns of age-related water diffusion changes in human brain by concordance and discordance analysis. Neurobiology of Aging, 31(11), 1991–2001. doi:10.1016/j.neurobiolaging.2008.10.009.
Acknowledgements
The present work was supported by SwitchBox-FP7-HEALTH-2010-grant 259772–2 and co-financed by the Portuguese North Regional Operational Program (ON.2 – O Novo Norte) under the National Strategic Reference Framework (QREN), through the European Regional Development Fund (FEDER). Paulo Marques, José M. Soares and Nadine C. Santos are supported by Switchbox fellowships. Ricardo Magalhães is supported by a fellowship from the project FCT-ANR/NEU-OSD/0258/2012 funded by FCT/MEC (www.fct.pt) and by FEDER. The authors would like to thank the study participants and acknowledge Carlos Portugal-Nunes and Teresa Castanho for assisting with participant recruitment and evaluation, and Pedro Moreira and Patrício Costa for aiding in manuscript preparation.
Disclosures
Paulo César Gonçalves Marques, José Miguel Montenegro Soares, Ricardo Joséda Silva Magalhães, Nadine Correia Santos, and Nuno Jorge Carvalho Sousa declare that they have no conflicts of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marques, P.C.G., Soares, J.M.M., Magalhães, R.J.d. et al. Macro- and micro-structural white matter differences correlate with cognitive performance in healthy aging. Brain Imaging and Behavior 10, 168–181 (2016). https://doi.org/10.1007/s11682-015-9378-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-015-9378-4