Abstract
Plantation forests play a pivotal role in carbon sequestration in terrestrial ecosystems, but enhanced nitrogen (N) deposition in these forests may affect plantation productivity by altering soil N cycling. Hence, understanding how simulated N deposition affects the rate and direction of soil N transformation is critically important in predicting responses of plantation productivity in the context of N loading. This study reports the effects of N addition rate (0, 40, and 120 kg N ha−1 a−1) and form (NH4Cl vs. NaNO3) on net N mineralization and nitrification estimated by in situ soil core incubation and on-soil microbial biomass determined by the phospholipid fatty acid (PLFA) method in a subtropical pine plantation. N additions had no influences on net N mineralization throughout the year. Net nitrification rate was significantly reduced by additions of both NH4Cl (71.5) and NaNO3 (47.1%) during the active growing season, with the stronger inhibitory effect at high N rates. Soil pH was markedly decreased by 0.16 units by NH4Cl additions. N inputs significantly decreased the ratio of fungal-to-bacterial PLFAs on average by 0.28 (49.1%) in November. Under NH4Cl additions, nitrification was positively related with fungal biomass and soil pH. Under NaNO3 additions, nitrification was positively related with all microbial groups except for bacterial biomass. We conclude that simulated N deposition inhibited net nitrification in the acidic soils of a subtropical plantation forest in China, primarily due to accelerated soil acidification and compositional shifts in microbial functional groups. These findings may facilitate a better mechanistic understanding of soil N cycling in the context of N loading.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plantation forests represent a large proportion of the total forest area worldwide (FAO 2006) and play a pivotal role in carbon sequestration in terrestrial ecosystems (Winjum and Schroederb 1997). Atmospheric nitrogen (N) is increasingly deposited on subtropical highly weathered soils where plantation forests predominate (Hansen et al. 2013). N is quantitatively and functionally the most important nutrient for plant growth and N deficiency frequently limits forest productivity (Reich et al. 1997; Burton et al. 2007). However, persistently elevated N deposition may affect forest productivity via altering soil N cycling (Vitousek et al. 1997; Matson et al. 1999, 2002). Hence, understanding how simulated N deposition affects soil N transformations is critically important in predicting the potential responses of productivity of subtropical plantation forests in the context of N loading.
Soil N transformations (e.g., mineralization and nitrification) are highly sensitive to N deposition and mediated by both biotic (microorganisms) and abiotic (acidification) factors (Zhu et al. 2013). Hence, N transformation rates are tightly correlated to microbial biomass, enzymatic activities, and group composition (e.g., fungi to bacteria ratios) (Wallenstein et al. 2006). Additionally, microorganisms generally present varying degrees of sensitivities to acidity (e.g., acid-tolerant vs. acid-adaptive). Evidence has shown that bacterial groups were more affected by soil pH than fungal groups (Fierer et al. 2009). Thus, N deposition induced changes in soil pH will inevitably induce shifts in the relative abundance of different microbial groups and therefore in corresponding N transformations, especially nitrification (Yao et al. 2011).
Different forms of inorganic N addition may have contrasting effects on soil N transformations. Addition of N as ammonium (NH4+) can produce more protons than nitrate (NO3−), regardless of being taken up by plants or being nitrified (Matson et al. 1999). Therefore, microbial groups may respond differentially to inputs of NH4+ and NO3−, which have varying potentials to incur soil acidification. Furthermore, it has been observed that microorganisms preferentially assimilate NH4+ over NO3−, due to the lower energy expenditure of NH4+ assimilation (Recous et al. 1992). However, studies on acidic soils have shown that microorganisms utilize equal or greater amounts of NO3− compared to NH4+ (Zhang et al. 2011a, b; Zhu et al. 2013), which may result from selective NO3− uptake by fungi (Marzluf 1997). To clarify this paradox, further research is needed to unravel the N form effects on microbial functional groups and associated soil N transformations.
However, inconsistent and contradicting results have been reported regarding N transformations and their responses to N deposition or experimental N addition (Rousk et al. 2010; Yao et al. 2011) and may be ascribed to multiple causes, such as temporal variations in edaphic conditions, differences in rates of the added N, and the N status of forest ecosystems before N addition (Gundersen et al. 1998). Previous studies have demonstrated that net N mineralization is low during the growing season relative to the winter, due to the nutrient immobilization of microorganisms during the summer and nutrient release from dying microorganisms during winter (Hobbie and Chapin 1996). Conversely, net N mineralization is greater during the growing season, which has optimal temperatures and moisture conditions in contrast to the non-growing season (Durán et al. 2013). Net N mineralization also generally increases with an increment of inorganic N that is applied (Heitkamp et al. 2009). Additionally, evidence has shown that N deposition generally stimulates net N mineralization in N-limited forest ecosystems (Vourlitis et al. 2007), but may inhibit it as ecosystems become N-saturated (Gundersen et al. 1998).
Globally, China has the largest area of plantations, with more than half in subtropical regions (Department of Forest Resources Management 2010). Recent 15N-labeling studies have shown that subtropical acidic forest soils in south China are naturally N abundant, due primarily to their microorganism-dominated N-retention mechanism (Zhang et al. 2013). Given the important role of soil microorganisms and enhanced N depositions in subtropical China, different rates and forms of inorganic N fertilizers were applied on acidic soils in 2012 to (1) explore the seasonal pattern of net N mineralization and nitrification, and (2) determine the effects of varying rates and forms of N addition on soil N transformations and the relative effects of associated biotic factors (microbial groups) and abiotic factors (soil pH) in these processes. Specifically, we hypothesized that (1) in the acidic soils of subtropical plantation forests, the active growing season (AGS) would have higher soil N transformation rates than the non-active growing season (NAGS) due to optimal temperature and moisture conditions that occur in the AGS, (2) exogenous N additions would suppress N transformations in acidic soils by accelerating acidification and thus altering microbial functional groups, and (3) ammonium has stronger inhibitory effects on soil N transformations than nitrate, since NH4+ is more efficient (higher potential to produce protons) at reducing soil pH.
Materials and methods
Study site and experimental design
The study site is located at the Qianyanzhou (QYZ) Experimental Station of Red Soil and Hilly Land, Chinese Academy of Sciences (CAS), Jiangxi Province, southeastern China (26°44′29.1″N, 115°03′29.2″E, 102 m above sea level). The climate is a subtropical monsoon climate. According to long-term climate records (1989–2008), the annual mean air temperature ranges from 17.4 to 18.9 °C, and the annual precipitation ranges from 945 to 2144 mm, of which approximately 24, 41, 23, and 12% on average falls in spring, summer, autumn, and winter, respectively (Zhang et al. 2011a, b). The lower rainfall and high temperatures in the late summer frequently caused seasonal droughts at the QYZ site (Wen et al. 2010). Based on the USDA soil taxonomy, the soil that weathers from red sandstone and mud stone is classified as Typic Dystrudepts (Wang et al. 2012). The original vegetation on the gently undulating terrain was evergreen broad-leaved forest, but it was heavily cleared by logging and land conversion to agriculture before the 1980s. The vegetation was restored in approximately 1985 by planting slash pine (Pinus elliottii), Masson pine (Pinus massoniana) and Chinese fir (Cunninghamia lanceolata). The background wet N deposition rate at the site is about 33 kg N ha−1 a−1 (Zhu et al. 2015; Kou et al. 2017). More information regarding the soil and stand characteristics of the study site can be found in Table 1 and the description of Kou et al. (2015).
The N addition experiment was carried out in a subtropical P. elliottii plantation in November 2011. A randomized complete block design with three replicates was employed, and each block was divided into five 20 × 20 m plots. The buffer zone between any two plots was more than 10 m. Topographic position and slope (less than 15°) was considered to ensure uniformity among plots. Within each block, one plot served as the control, receiving ambient N deposition only, and the remaining four plots received ambient N deposition plus randomly assigned chronic atmospheric N deposition (40 vs. 120 kg N ha−1 a−1 NH4Cl and 40 vs. 120 kg N ha−1 a−1 NaNO3, respectively.). Fertilizers were weighed (509.5 g, low NH4Cl; 1528.5 g, high NH4Cl; 809.6 g, low NaNO3; 2428.8 g, high NaNO3), fully dissolved in 30 L tap water, and evenly sprayed onto N-addition zone once per month (i.e., 12 equal applications per year), and the control plots were supplied with the equivalent amount of tap water. The understory N addition started on May 1, 2012 and proceeded at 1-month intervals on days without rain. In this study, we divided the year into the active growing season (AGS) from May to October and the non-active growing season (NAGS) from November to April of the following year.
Field sampling and measurements
Net N mineralization and nitrification were measured in situ using an intact soil core incubation technique. Monthly (approximately every 4 weeks) measurements started on April 30, 2012 in the AGS, in March and April in the NAGS, and bimonthly (roughly every 8 weeks) during the rest of the NAGS. Five pairs of 5 cm diameter × 15 cm long polyvinyl chloride tubes were inserted (to 10 cm depth) in the soil at each plot when the incubation began. Five tubes were removed immediately prior to the N additions (before incubation), and the soil in the tube was mixed as one composite sample (approximately 300 g) after hand sorting and removing roots and rocks, and then sent to the laboratory for the extraction of NO3− and NH4+ to determine the initial inorganic N content. The other five tubes were capped with parafilm, which is permeable to air but not water, and were left in place for incubation (after incubation). Upon completion of in situ incubation, these five tubes at each plot were removed and processed in the same manner as above to determine soil inorganic N content. For each soil composite sample, a subsample (ca. 30 g) was dried in an oven at 105 °C to determine the soil water content, and 50 mL of 2 mol L−1 KCl was added to approximately 13–15 g soil to extract mineral soil N (NH4+–N and NO3−–N).
Monthly net N mineralization rates (mg kg−1 d−1) were calculated as (Inorganic-NA–Inorganic-NB)/incubation days, and net nitrification rates were calculated as (Nitrate-NA–Nitrate-NB)/incubation days, where Inorganic-NB and inorganic-NA, represents inorganic N contents before and after incubation, and Nitrate-NB and Nitrate-NA represents NO3−–N contents before and after incubation. The amounts of ammonium and nitrate were determined with a Flow Auto Analyzer (Bran Luebbe, Germany). Soil pH values were determined in 1 mol L−1 KCl extracts (soil to KCl ratio of 1:2.5) with a pH meter (Mettler Toledo, Switzerland). The pH value determined for the KCl extract was lower (by approximately 1.0 unit) than it was with the water extract method in this study. Volumetric soil moisture and temperature at 0–10 cm soil depth were automatically monitored by EM50 (Decagon, USA) at a frequency of 30 min.
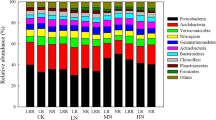
The soil microbial community composition was assessed using phospholipid fatty acids (PLFAs) in June and November 2012. Specifically, lipids were extracted from 8 g soil (sampled from N mineralization composite soils before incubation) using the procedure described by Bååth and Anderson (2003). The abundance of individual fatty acid methyl-esters was expressed as a mole percentage. Nomenclature of fatty acids was made according to Frostegård et al. (1993). PLFAs 16:1ω7c, cy17:0, cy19:0, i15:0, a15:0, i16:0 and i17:0 PLFA are representative of bacteria (Frostegård and Bååth 1996), 18:1ω9 and 18:2ω6 are specific to fungi (Joergensen and Wichern 2008; Frostegård et al. 2011). PLFA 10Me16:0 and 10Me18:0 were chosen to represent actinobacteria (Hossain et al. 2010).
Statistical analyses
Repeated-measures of analysis of variance (RMANOVA) were used to examine effects of N rate, N form, and their interactions on soil N transformations (net N mineralization and nitrification rates). One-way ANOVA was performed to determine the treatment effects on soil pH and microbial functional groups. Where required, data were log (x)-transformed to meet assumptions of normality and homogeneity of variance. Linear regression analyses were conducted on soil microbial groups and soil pH against soil N transformations. Significant differences between means were compared using Tukey’s test. All statistical analyses were conducted using SPSS software version 18.0 (SPSS, Chicago, IL, USA).
Results
Net N mineralization and nitrification rates
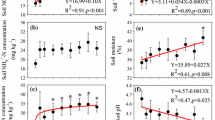
Monthly N transformation rates presented significant seasonal variations (P < 0.001, Table 2), ranging from − 0.16 to 0.52 mg kg−1 d−1 for net N mineralization rate (Fig. 1a) and − 0.03 to 0.18 mg kg−1 d−1 for nitrification rate across all sampling months in the control plots (Fig. 1b). Net N mineralization and nitrification rates peaked in June and September and were minimal in December (Fig. 1). Over the sampling period, both net N mineralization and nitrification rates were higher in the AGS than in the NAGS (Fig. 1). Mean net N mineralization rates in the control plots were 0.38 mg kg−1 d−1 during the AGS and − 0.02 mg kg−1 d−1 during the NAGS (Fig. 1a). Mean net nitrification rates in the control plots were 0.12 mg kg−1 d−1 during the AGS and almost negligible during the NAGS (Fig. 1b).
Temporal variation in net N mineralization (a) and nitrification (b) rates in control and N-addition (low N: 40 kg N ha−1 a−1, high N: 120 kg N ha−1 a−1) plots during the study. Months without gray shadows represent AGS (mean monthly soil temperature > 20 °C). Months with gray shadows represent NAGS (mean monthly soil temperature < 20 °C). N + D: November + December, J + F: January + February. Data are mean ± standard error (n = 3)
Exogenous N input significantly inhibited nitrification, and the inhibitory effect was more dependent on rate (P = 0.032, Table 2; Fig. 1b) than form (marginally significant, P = 0.054, Table 2; Fig. 1b) of N. Specifically, net nitrification rate significantly decreased with addition of N as NH4Cl by 71.5% and NaNO3 by 47.1% during the AGS. The inhibitory effect of N input on nitrification was stronger under the low rate (73.3%) than high rate of N (45.3%), especially under addition of NaNO3. However, exogenous N additions slightly reduced net N mineralization throughout the year (Fig. 1a).
N transformations and microbial community
Microbial PLFA biomass (in nmol g−1 dry mass) and the PLFA ratio of fungi to bacteria differed among sampling times (Fig. 2). Although N addition showed a trend of inhibition for all microbial groups, it was not statistically significant (Fig. 2a, b). Specifically, N input slightly decreased the PLFA biomass of all microbial groups in June and fungal PLFA in November. Although N input slightly decreased the fungal PLFA biomass, the ratio of fungi to bacteria decreased significantly by 0.28 (49.1%) on average compared with the control in November (P = 0.008, Fig. 2d).
Phospholipid fatty acid (PLFA) biomass (nmol g−1) of bacteria, fungi, and actinobacteria and ratio of fungi to bacteria in control and N-addition plots in June (a, c) and November (b, d). CK control, LA low NH4Cl, HA high NH4Cl, LN low NaNO3, HN high NaNO3, respectively. Data are means + standard error (n = 3). Different letters indicate significant differences (P < 0.05) between treatments
Varying forms of N addition exerted contrasting influences on the relationships between soil N transformations and microbial groups (Table 3). Specifically, net N mineralization exhibited significant negative relationships with fungal biomass (P = 0.018, Table 3), and nitrification presented positive relationships with fungal biomass (P = 0.038, Table 3) after ammonium-based N addition. Net N mineralization exhibited significant negative relationships with all microbial groups except for fungal biomass (Table 3), and nitrification had a significant positive relationship with all microbial groups (Table 3) except for bacterial biomass when nitrate-based N was added.
N transformations and soil pH
The response of soil pH to N additions fluctuated little during the entire study. Varying rates and forms of N addition decreased soil pH, and a significant difference was observed after 10 months of N input (Fig. 3). Irrespective of the rate (40 vs. 120 kg N ha−1 a−1) applied, ammonium-based N additions caused a significant decline in soil pH by 0.16 units (P < 0.05, Fig. 3).
The effects of varying forms of N addition on the relationships between soil N transformations and soil pH were divergent (Table 3). Specifically, net N mineralization (P = 0.041, Table 3) and nitrification (P = 0.029, Table 3) exhibited significant positive relationships with soil pH under ammonium-based N additions. However, no significant relationship between soil N transformations and soil pH were observed with nitrate-based N additions (Table 3).
Discussion
Seasonal variations in net N mineralization and nitrification
Supporting our first hypothesis, net N mineralization and nitrification in both the control and the N addition plots were higher during the AGS than during the NAGS (Fig. 1). This result is consistent with previous studies, suggesting higher mineralization and nitrification rates during the growing season than in the non-growing season (Durán et al. 2013). It has been reported that competition between roots and microorganisms for inorganic N has temporal variations (Xu et al. 2011), which may be associated with soil temperature and moisture. Higher temperatures during the growing season may intensify soil respiration and competition for N between fine roots and microorganisms. Consequently, greater N transformation rates help meet the demand for N by fine roots and/or microorganisms. Conversely, lower N transformation rates were observed in the winter, because both plants and microorganisms are inactive or dormant.
Many studies have shown that N mineralization significantly increases with temperature (Rustad et al. 2001), while warming may stimulate (Verburg et al. 1999) or have no influence on nitrification (Niboyet et al. 2011). We observed that soil temperature rather than moisture had a dominant effect on net N mineralization (Table S1), which confirms that soil temperatures exert greater impacts on soil N transformations than moisture during most of the year. However, in our study, net N mineralization peaked at the beginning of the summer (June), rather than when temperatures were the highest (Fig. 1a), perhaps due to the seasonal drought that occurred later in the summer (Wen et al. 2010), which generally inhibited N mineralization and its sensitivity to warming (Auyeung et al. 2013).
Nitrification rates are positively related with soil moisture, but may progressively decrease as the water content exceeds a specific threshold (Kiese et al. 2008). We noticed that nitrification rates were lowest in June during the AGS, but peaked 3 months later than net N mineralization rates (Fig. 1), probably due to the excessive precipitation for nitrifiers in June and relatively favorable hydrothermal conditions in September (Figure S1).
Effects of N addition on net N mineralization and nitrification
N additions slightly reduced N mineralization throughout the year, but significantly inhibited nitrification in the AGS (Table 2; Fig. 1). This partially supports the first part of our second hypothesis that N additions would reduce net N mineralization and strongly supports the second part of the hypothesis that N additions would reduce nitrification. N transformations are mediated and influenced by the biomass, enzymatic activities, and functional group composition of the soil microorganisms (Wallenstein et al. 2006). In our study, N inputs slightly decreased the biomass of all microbial groups in June and of fungal groups in November (Fig. 2a, b). These decreases may, to some extent, be responsible for the slight declines in N mineralization (Fig. 1a).
Recent studies have revealed that humid subtropical acidic soils in China have low autotrophic and relatively high heterotrophic nitrification rates due to low soil pH (Zhang et al. 2011a, b, 2013). Heterotrophic nitrification is primarily carried out by fungi in the acidic forest soils of subtropical China (Zhu et al. 2013). Our study shows that the ratio of fungal to bacterial PLFAs decreased after the N additions (Fig. 2d), and the fungi PLFA had a positive relationship with nitrification (Table 3), which might partially explain the decreased nitrification rates (P = 0.031, Fig. 1b). In addition, accumulated NH4+ and NO3− in the soil due to the addition of NH4+ and NO3− (Table S2) may promote the reverse processes of N transformation, thereby suppressing the transformation of organic N to NH4+ (ammonification), the transformation of organic N to NO3− (heterotrophic nitrification) and the oxidation of NH4+ to NO3− (autotrophic nitrification).
Unexpectedly, the inhibitory effect of N addition on net nitrification was much higher under low than high N rates (P = 0.032, Table 2; Fig. 1b). Relative to the low N rate, the high N rate is theoretically more capable of increasing the N to C ratio of soil organic matter or organic N (the base of heterotrophic nitrification), which may facilitate heterotrophic nitrification. This facilitation may, to some extent, offset the negative effects of high N rate on biomass and activities of some heterotrophic nitrifiers. Additionally, we found that NO3− contributed primarily to the stronger inhibitory effects of low N rate (Fig. 1b). In planted soils, NO3− generally has a greater stimulation on root-derived respiration (Gavrichkova and Kuzyakov 2008). A recent study based on our N-manipulative experiment shows that low level of NO3− input significantly increased soil CO2 flux during the AGS, which is possibly due to the increased root autotrophic respiration (Wang et al. 2015). Hence, we speculate that low rates of N supply may stimulate autotrophic respiration and N uptake of roots, while adding N at high rate has a neutral or inhibitory effect on root-associated variables.
Contrasting effects of N form on microbial groups and pH and thus nitrification
Soil pH was more markedly reduced by ammonium-based than nitrate-based N inputs (Fig. 3), concurring with with previous studies that ammonium-based N contributed greatly to soil acidification (Matson et al. 1999). Microorganisms are considered to present differential sensitivities to acidity (Fierer et al. 2009). In our study, microbial groups exhibited close relationships with net nitrification as nitrated-based N was added (Table 3). However, only fungal groups presented a strong relationship with net nitrification (P = 0.038, Table 3) when ammonium-based N was applied. Hence, relative to other microbial groups, fungal groups play a dominant role in soil N transformations when ammonium-based N is applied. This finding indicates that soil N transformation is mediated by compositional shifts in microbial groups due to inorganic N additions, and in particular, that this mediation is also strongly N-form dependent.
Reduced soil pH may affect N transformations by altering soil chemical and biological properties (Fu et al. 1987). Accumulating evidence shows that soil pH is positively related to nitrification (Cheng et al. 2011; Persson and Wiren 1995). For instance, Nugroho et al. (2007) found that the low nitrification rates in acidic soils of Scots pine (Pinus sylvestris L.) forest are due to low pH. We observed a significantly positive relationship between soil pH and net nitrification rate (P = 0.029, Table 3) with ammonium-based rather than nitrate-based N inputs. Consequently, different forms of N inputs may exert contrasting influences on soil pH and therefore on soil N transformations.
Despite varying responses of microbial groups and soil pH to different forms of N, the N form effects on nitrification were only marginally significant (P = 0.054, Table 2; Fig. 1b). The reduced pH in our study occurred only 10 months after ammonium-based N was added (Fig. 3). Two factors may be responsible for this observation. First, the N-retention mechanism in the acidic forest soils, which can efficiently immobilize inorganic N ions (NH4+ and NO3−) into organic N pool, reduces the release of hydrogen ions (H+) (Zhang et al. 2013). Second, the seasonal drought from early July to late October (Wen et al. 2010) may reduce the risk of leaching loss of NO3−. Therefore, the lag effect of soil acidification may contribute to explaining the lack of contrasting effects of the N form (Fig. 1).
Heterotrophic nitrification is the dominant mode of nitrification in humid subtropical acidic soils in China (Zhang et al. 2011a, b, 2013) and primarily carried out by fungi (Zhu et al. 2013). Given the decreased ratio of fungi to bacteria, enhanced acidification, and inhibited nitrification after N addition, our findings imply that enhanced N deposition on the subtropical acidic soils may promote the accumulation of soil organic matter (the basis of heterotrophic nitrification), increase the ratio of NH4+ to NO3−, and decelerate N cycling. All these effects may therefore influence plant growth and plantation productivity via altering soil N supply and plant uptake. Soil N transformations are extremely complex and are affected by multiple factors. Our results based on a year-long manipulative experiment provide only a “snapshot” of short-term soil N transformations in response to N additions. Further studies are therefore needed to examine the long-term effects of N deposition on soil N cycling.
Conclusions
In our field N-manipulation experiment, net N mineralization and nitrification were higher during the active growing season (AGS) than during the non-active growing season (NAGS). Both ammonium- and nitrate-based N inputs significantly reduced net nitrification rates during the AGS. Ammonium-based N inputs exerted larger effects on microbial functional groups and on soil pH than did nitrate-based N inputs. The inhibitory effects on soil N transformations are better explained by changes in the fungal groups and soil pH when ammonium-based N was applied. However, bacteria and/or actinobacteria groups contributed more than fungal groups to the inhibitory effects when nitrate-based N was applied. In summary, accelerated soil acidification (abiotic factor) together with compositional shifts in microbial groups (biotic factor) was largely responsible for the inhibited nitrification by N addition. These results may contribute to mechanistically understanding soil N cycling in the context of N loading.
References
Auyeung DS, Suseela V, Dukes JS (2013) Warming and drought reduce temperature sensitivity of nitrogen transformations. Glob Change Biol 19:662–676
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963
Burton J, Chen CR, Xu ZH, Ghadiri H (2007) Gross nitrogen transformations in adjacent native and plantation forests of subtropical Australia. Soil Biol Biochem 39:426–433
Cheng Y, Cai ZC, Zhang JB, Chang SX (2011) Gross N transformations were little affected by 4 years of simulated N and S depositions in an aspen-white spruce dominated boreal forest in Alberta, Canada. For Ecol Manag 262:571–578
Department of Forest Resources Management, SFA (2010) The 7th National forest inventory and status of forest. For Resour Manag 1:3–10
Durán J, Rodriguez A, Morse JL, Groffman PM (2013) Winter climate change effects on soil C and N cycles in urban grasslands. Glob Change Biol 19:2826–2837
FAO (2006) Global forest resources assessment 2005. Forestry paper 147, Rome
Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC (2009) Global patterns in belowground communities. Ecol Lett 12:1238–1249
Frostegård Å, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fert Soils 22:59–65
Frostegård A, Bååth E, Tunlid A (1993) Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty-acid analysis. Soil Biol Biochem 25:723–730
Frostegård Å, Tunlid A, Bååth E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43:1621–1625
Fu MH, Xu XC, Tabatabai MA (1987) Effect of pH on nitrogen mineralization in crop-residue-treated soils. Biol Fert Soils 5:115–119
Gavrichkova O, Kuzyakov Y (2008) Ammonium versus nitrate nutrition of Zea mays and Lupinus albus: effect on root-derived CO2 efflux. Soil Biol Biochem 40:2835–2842
Gundersen P, Emmett BA, Kjonaas OJ, Koopmans CJ, Tietema A (1998) Impact of nitrogen deposition on nitrogen cycling in forests: a synthesis of NITREX data. For Ecol Manag 101:37–55
Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavina A, Thau D, Stehman SV, Goetz SJ, Loveland TR, Kommareddy A, Egorov A, Chini L, Justice CO, Townshend JRG (2013) High-resolution global maps of 21st-century forest cover change. Science 342:850
Heitkamp F, Raupp J, Ludwig B (2009) Impact of fertilizer type and rate on carbon and nitrogen pools in a sandy Cambisol. Plant Soil 319:259–275
Hobbie SE, Chapin FSIII (1996) Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochemistry 35:327–338
Hossain MZ, Okubo A, Sugiyama S (2010) Effects of grassland species on decomposition of litter and soil microbial communities. Ecol Res 25:255–261
Joergensen RG, Wichern F (2008) Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biol Biochem 40:2977–2991
Kiese R, Hewett B, Butterbach-Bahl K (2008) Seasonal dynamic of gross nitrification and N2O emission at two tropical rainforest sites in Queensland, Australia. Plant Soil 309:105–117
Kou L, Guo DL, Yang H, Gao WL, Li SG (2015) Growth, morphological traits and mycorrhizal colonization of fine roots respond differently to nitrogen addition in a slash pine plantation in subtropical China. Plant Soil 391:207–218
Kou L, McCormack ML, Chen WW, Guo DL, Wang HM, Gao WL, Yang H, Li SG (2017) Nitrogen ion form and spatio-temporal variation in root distribution mediate nitrogen effects on lifespan of ectomycorrhizal roots. Plant Soil 411:261–273
Marzluf GA (1997) Genetic regulation of nitrogen metabolism in the fungi. Microbiol Mol Biol Rev 61:17–32
Matson PA, McDowell WH, Townsend AR, Vitousek PM (1999) The globalization of N deposition: ecosystem consequences in tropical environments. Biogeochemistry 46:67–83
Matson PA, Lohse KA, Hall SJ (2002) The globalization of nitrogen deposition: consequences for terrestrial ecosystems. Ambio 31:113–119
Niboyet A, Le Roux X, Dijkstra P, Hungate BA, Barthes L, Blankinship JC, Brown JR, Field CB, Leadley PW (2011) Testing interactive effects of global environmental changes on soil nitrogen cycling. Ecosphere 2:1–24
Nugroho RA, Roling WFM, Laverman AM, Verhoef HA (2007) Low nitrification rates in acid scots pine forest soils are due to pH-related factors. Microb Ecol 53:89–97
Persson T, Wiren A (1995) Nitrogen mineralization and potential nitrification at different depths in acid forest soils. Plant Soil 168:55–65
Recous S, Machet JM, Mary B (1992) The partitioning of fertilizer-N between soil and crop-comparison of ammonium and nitrate applications. Plant Soil 144:101–111
Reich P, Grigal D, Aber J, Gower S (1997) Nitrogen mineralisation and productivity in 50 hardwood and conifer stands on diverse soils. Ecology 78:335–347
Rousk J, Brookes PC, Bååth E (2010) The microbial PLFA composition as affected by pH in an arable soil. Soil Biol Biochem 42:516–520
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J, News G (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Verburg PSJ, Van Loon WKP, Lukewille A (1999) The CLIMEX soil-heating experiment: soil response after 2 years of treatment. Biol Fert Soils 28:271–276
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman D (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Vourlitis GL, Zorba G, Pasquini SC, Mustard R (2007) Chronic nitrogen deposition enhances nitrogen mineralization potential of semiarid shrubland soils. Soil Sci Soc Am J 71:836–842
Wallenstein MD, McNulty S, Fernandez IJ, Boggs J, Schlesinger WH (2006) Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. For Ecol Manag 222:459–468
Wang YD, Wang ZL, Wang HM, Guo CC, Bao WK (2012) Rainfall pulse primarily drives litterfall respiration and its contribution to soil respiration in a young exotic pine plantation in subtropical China. Can J For Res 42:657–666
Wang YS, Cheng SL, Fang HJ, Yu GR, Xu XF, Xu MJ, Wang L, Li XY, Si GY, Geng J, He S (2015) Contrasting effects of ammonium and nitrate inputs on soil CO2 emission in a subtropical coniferous plantation of southern China. Biol Fert Soils 51:815–825
Wen XF, Wang HM, Wang JL, Yu GR, Sun XM (2010) Ecosystem carbon exchanges of a subtropical evergreen coniferous plantation subjected to seasonal drought, 2003–2007. Biogeosciences 7:357–369
Winjum JK, Schroederb PE (1997) Forest plantations of the world: their extent, ecological attributes, and carbon storage. Agric For Meteorol 84:153–167
Xu XL, Ouyang H, Richter A, Wanek W, Cao GM, Kuzyakov Y (2011) Spatio-temporal variations determine plant-microbe competition for inorganic nitrogen in an alpine meadow. J Ecol 99:563–571
Yao HY, Campbell CD, Qiao XR (2011) Soil pH controls nitrification and carbon substrate utilization more than urea or charcoal in some highly acidic soils. Biol Fert Soils 47:515–522
Zhang JB, Zhu TB, Cai ZC, Müller C (2011a) Nitrogen cycling in forest soils across climate gradients in Eastern China. Plant Soil 342:419–432
Zhang WJ, Wang HM, Yang FT, Yi YH, Wen XF, Sun XM, Yu GR, Wang YD, Ning JC (2011b) Underestimated effects of low temperature during early growing season on carbon sequestration of a subtropical coniferous plantation. Biogeosciences 8:1667–1678
Zhang JB, Cai ZC, Zhu TB, Yang WY, Müller C (2013) Mechanisms for the retention of inorganic N in acidic forest soils of southern China. Sci Rep 3:1–8
Zhu TB, Meng TZ, Zhang JB, Yin YF, Cai ZC, Yang WY, Zhong WH (2013) Nitrogen mineralization, immobilization turnover, heterotrophic nitrification, and microbial groups in acid forest soils of subtropical China. Biol Fert Soils 49:323–331
Zhu JX, He NP, Wang QF, Yuan GF, Wen D, Yu GR, Jia YL (2015) The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci Total Environ 511:777–785
Acknowledgements
Special thanks are due to the Qianyanzhou Experimental Station of Red Soil and Hilly Land, Chinese Academy of Sciences, Jiangxi Province, China, for permission to work in their permanent slash pine plantation experiment plots. Thanks to all the staff and students of the Qianyanzhou Experimental Station for their assistance in the fieldwork. The authors acknowledge the contributions of the anonymous reviewers. This research is financially supported by the Grants from the National Key Research and Development Plan (No. 2016YFD06000202), and the National Natural Science Foundation of China (Nos. 31570443, 31130009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Project funding: This research is financially supported by the Grants from the National Key Research and Development Plan (No. 2016YFD06000202), and the National Natural Science Foundation of China (Nos. 31570443, 31130009).
The online version is available at http://www.springerlink.com
Corresponding editor: Chai Ruihai.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kou, L., Zhang, X., Wang, H. et al. Nitrogen additions inhibit nitrification in acidic soils in a subtropical pine plantation: effects of soil pH and compositional shifts in microbial groups. J. For. Res. 30, 669–678 (2019). https://doi.org/10.1007/s11676-018-0645-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0645-2