Abstract
Phase equilibria in the quasi-ternary system Cu2S-HgS-SnS2 were studied by physico-chemical analysis methods on 152 alloys that were synthesized by direct single-temperature method. Phase diagrams of the quasi-binary system Cu2S-HgS, six vertical sections (Cu2S-Cu2HgSnS4, Cu2SnS3-HgS, Cu2HgSnS4-SnS2, Cu2Sn4S9-Cu2HgSnS4, Cu4SnS4-Cu2HgSnS4, A–B (A—40 mol.% HgS, 60 mol.% Cu2S; B—40 mol.% HgS, 60 mol.% SnS2), liquidus surface projection, and isothermal section at 670 K were investigated. The coordinates of eutectic points were determined: 59 mol.% HgS, 983 K (in the Cu2S-HgS system); 73 mol.% Cu2S, 1060 K (at the Cu2S-Cu2HgSnS4 section); 18 mol.% HgS, 1113 K and 88 mol.% HgS, 1035 K (at the Cu2SnS3-HgS section); 83 mol.% SnS2, 1021 K (at the Cu2HgSnS4-SnS2 section).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Investigation of phase equilibria in the Cu2S-HgS-SnS2 system is part of the systematic study of quasi-ternary systems Cu2X-BIIX-DIVX2 (BII-Zn, Cd, Hg; DIV-Si, Ge, Sn; X-S, Se) and the crystal structure of compounds which formed in the systems. Binary system components Cu2S, HgS and SnS2 melt congruently at 1401 K,[1] 1098 K,[2] and 1143 K,[3] respectively, and have narrow homogeneity regions near the stoichiometric composition.
The Cu2S-HgS-SnS2 system features the Cu2HgSnS4compound which is a direct-band semiconductor. Cu2HgSnS4 has properties suitable for optoelectronic devices and absorbing layer of thin-film solar cells[4] but any possible use would be severely limited due to the toxicity of mercury.
2 Quasi-binary Systems
2.1 Cu2S-SnS2 Systems
The results of the studies of the Cu2S-SnS2 system were published in Ref 5,6,7,8,9,10. According to Ref 10, three ternary compounds form in the system, Cu4SnS4, Cu2SnS3 and Cu2Sn4S9. The Cu2SnS3 compound has a natural analog, the mochite mineral[9].
According to Ref 6, in addition to the above compounds, the ternary phase Cu4Sn3S8 was found in the Cu2S-SnS2 system. Cu2SnS3 melts congruently at 1123 K. Compounds Cu4SnS4 and Cu2Sn4S9 formed in solid-state reactions Cu2SnS3 + Cu2S ⇔ Cu4SnS4 and Cu4Sn3S8 + 5SnS2 ⇔ 2Cu2Sn4S9 at 1083 and 938 K, respectively. Cu4Sn3S8 is formed by the peritectic reaction L + Cu2SnS3 ⇔ Cu4Sn3S8 and exists in the temperature range 658–1063 K. There are three invariant points in the system, two eutectics and one peritectic, with the coordinates 31 mol.% SnS2 and 1093 K, 77 mol.% SnS2 and 1043 K, 70 mol.% SnS2 and 1063 K, respectively.
According to Ref 5, three phases were found in the Cu2S-SnS2 system, Cu2SnS3, Cu2Sn4S9 and Cu8SnS6. The formation of the first two compounds occurs as described in Ref 6. The difference is in the value of the melting point of Cu2SnS3 which according to Ref 5 is 1173 K. Cu8SnS6 is formed by the solid-state reaction 3Cu2S + Cu2SnS3 ⇔ Cu8SnS6 at 1083 K. The solid solutions range of Cu2S, SnS2 and ternary compounds are 2 mol.% or less.
Given existing contradictions in the literature, the authors of Ref 11 re-investigated the Cu2S-SnS2 system in detail. The authors report the existence of three compounds, Cu2SnS3 which melts congruently at 1133 K, Cu4SnS4, formed by the solid-state reaction of Cu2SnS3 + Cu2S ⇔ Cu4SnS4 at 1083 K, and Cu2Sn4S9 formed by the reaction Cu2SnS3 + 3SnS2 ⇔ Cu2Sn4S9 at 943 K (closely agreeing in this regard to Ref 6). The existence of Cu4Sn3S8 and CuSn3.75S8 compounds was not confirmed. The solid solubility based on the starting components does not exceed 2 mol.%. Polymorphous transformations of Cu2S result in solid-state processes at 656 and 381 K.
2.2 HgS-SnS2 System
The HgS-SnS2 system is a quasi-binary section of the ternary system Hg-Sn-S[12] and belongs to the eutectic type. The eutectic coordinates are 920 K and 48 mol.% HgS. The solid solution ranges of the binary compounds at 700 K are 0–2 and 99–100 mol.% SnS2.
2.3 Cu2S-HgS Systems
The Cu2S-HgS system was studied in Ref 13, 14. According to Ref 13, the Cu2S-HgS system is a quasi-binary section of the ternary system Cu-Hg-S and exhibits eutectic type of interaction. The eutectic coordinates are 963 K and 58 mol.% HgS[13] or 976 K and 74 mol.% HgS.[14]
Crystallographic characteristics of binary, ternary and quaternary chalcogenides of the quasi-ternary system Cu2S-HgS-SnS2 are gathered in Table 1.
3 Experimental
The compounds and alloys of the studied system were synthesized from semiconductor-purity elements (Cu, Ge and S) and pre-synthesized HgS. Sulfur and mercury were further purified by vacuum distillation before use. Due to the high vapor pressure of the components, the synthesis of HgS was performed in an evacuated quartz container with thickened walls. Stoichiometric amounts of starting elements were used for the synthesis. At the first stage the ampoule was heated to 473 K at the rate of 30–40 K/h. The heating to the maximum temperature of 873 K was held at the rate of 5–10 K/h. After annealing for 48 hours, the container with synthesized HgS was cooled to room temperature at the rate of 10–15 K/h.
The calculated amounts of starting components were loaded into quartz ampoules that were evacuated to residual pressure of 10–2 Pa and soldered.
Based on the p-T diagrams of the starting materials, single-temperature method was selected for the synthesis of alloys. The synthesis was performed in commercial programmable furnaces. The temperature was raised at the rate of 20–30 K/h to the maximum of 1400 K, with 4 h stays at the melting points of the batch components. The alloys were then cooled at the rate of 10–20 K/h to 670 K where homogenizing annealing at was held for 500 h. Annealed alloys were quenched into 25% aqueous NaCl solution.
Differential thermal analysis utilized a Paulik–Paulik–Erdei derivatograph, with Pt/Pt-Rh thermocouple and Al2O3 as a standard. All static parameters were stable during the experiment. X-ray phase analysis using WinCSD software package[24] was performed on diffraction patterns recorded at a DRON 4-13 diffractometer (CuKα-radiation). Microstructural analysis was performed using an MMU-3 metal microscope.
3.1 Quasi-ternary System Cu2S-HgS-SnS2
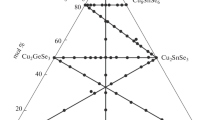
Phase equilibria in the quasi-ternary system Cu2S-HgS-SnS2 were studied on 152 alloys the chemical and phase composition of which is shown in Fig. 1.
3.2 Characteristics of Quasi-binary Boundary Systems of the Quasi-ternary System
Ambiguous data regarding the melting point and coordinates of the eutectic point led to reinvestigation of the phase equilibria in the Cu2S-HgS system.
The phase diagram of this system in the entire concentration range is shown in Fig. 2. The Cu2S-HgS system is a quasi-binary section of the ternary system Cu-Hg-S. The eutectic of the section components has the coordinates of 59 mol.% HgS (δ′) and 983 K. The solid solution range of HT-modification of Cu2S (γ′′) extends to 52 mol.% HgS at the eutectic temperature and decreases with decreasing temperature.
The presence of three polymorphous modifications of Cu2S and one polymorphous transition of HgS determines the complex nature of phase formation in the sub-solidus part of the diagram where there are two eutectoid (δ′ ⇔ γ′′ + δ at 587 K and γ′′ ⇔ γ′ + δ at 524 K) and one peritectoid (γ′ + δ ⇔ γ at 386 K) processes. Literature data on the investigation of the Cu2S-SnS2 and HgS-SnS2 systems were used in the construction of the liquidus surface projection and isothermal section of the quasi-ternary system Cu2S-HgS-SnS2 at 670 K.
3.3 Quasi-binary System Cu2S-Cu2HgSnS4
The Cu2S-Cu2HgSnS4 section shown in Fig. 3 is a quasi-binary section of the quasi-ternary subsystem Cu2SnS3-HgS-Cu2S and belongs to the eutectic type. The eutectic process L ⇔ Cu2HgSnS4 + γ′′ takes place at 1060 K, and the eutectic point has composition of 73 mol.% Cu2S.
The solid solubility in HT-Cu2S modification (γ′′-solid solutions) at 1060 K does not exceed 18 mol.% Cu2HgSnS4 and decreases with decreasing temperature. At the annealing temperature, the solid solubility of Cu2HgSnS4 in γ′′ does not exceed 3 mol.% Cu2HgSnS4. The solid solubility based on Cu2HgSnS4 is less than 2 mol.% Cu2S.
3.4 Quasi-binary System Cu2SnS3-HgS
Phase diagram of the Cu2SnS3-HgS section plotted from the results of physico-chemical analysis is shown in Fig. 4. The system is a quasi-binary section of the quasi-ternary system Cu2S-HgS-SnS2. The Cu2HgSnS4 (ε) compound which melts congruently at 1122 K is formed in the system at the 1:1 ratio of the section components. The maximum melting point of the quaternary compound is shifted towards the ternary phase Cu2SnS3 (χ).
The diffraction pattern of Cu2HgSnS4 was indexed well in the tetragonal symmetry (stannite structural type, SG \({\text{I}}\overline{4}2{\text{m}}\)) with unit cell parameters a = 0.5580(2) nm: c = 1.0895(3) nm. The ternary compound Cu2SnS3 crystallizes in the sphalerite structure (SG, a = 0.54276(2) nm).
The interaction of Cu2HgSnS4 with the section components is eutectic. The eutectics melt at 1113 K and 1035 K and have the composition of 18 and 88 mol.% HgS, respectively. The solid solubility in the section components at the annealing temperature is less than 2 mol.%.
3.5 Quasi-binary System Cu2HgSnS4-SnS2
Phase diagram of the Cu2HgSnS4-SnS2 section based on the results of DTA, XRD and microstructure analysis is shown in Fig. 5. The section is quasi-binary, with the eutectic nature of interaction. The eutectic reaction L ⇔ ε + SnS2 takes place at 1021 K, the composition of the eutectic point is 83 mol.% SnS2. The solid solubility based on the section components is negligible.
3.6 Vertical Section Cu2Sn4S9-Cu2HgSnS4
The section liquidus consists of two curves of the primary crystallization of the ternary Cu2SnS3 and quaternary Cu2HgSnS4 compounds (Fig. 6). The secondary crystallization is represented by the binary eutectics Cu2SnS3 + SnS2 (field 4) and Cu2HgSnS4 + Cu2SnS3 (field 5).
The horizontal line at 1015 K corresponds to the ternary invariant eutectic process L ⇔ SnS2 + Cu2SnS3 + ε which has at this section an excess of SnS2 and Cu2SnS3. The solid-state process SnS2 + Cu2SnS3 ⇔ Cu2Sn4S9 at 933 K results in all alloys of the section becoming two-phase at the annealing temperature 670 K except end components of the section Cu2Sn4S9 and Cu2HgSnS4.
3.7 Vertical Section Cu4SnS4-Cu2HgSnS4
The liquidus of the Cu4SnS4-Cu2HgSnS4 section consists of two lines of the primary crystallization of the ternary Cu2SnS3 and quaternary Cu2HgSnS4 phases (Fig. 7). The horizontal line at 1083 K corresponds to the four-phase peritectic process L + Cu2SnS3 + γ′′ ⇔ Cu4SnS4.
3.8 Vertical Section A–B (A—60 mol.% Cu2S; 40 mol.% HgS; B—60 mol.% SnS2; 40 mol.% HgS)
The A–B section (Fig. 8) crosses two subsystems, Cu2S-HgS-Cu2HgSnS4 and HgS-SnS2-Cu2HgSnS4. The section liquidus consists of three lines of the primary crystallization of γ′′-solid solution range of HT-Cu2S modification (part a–b), Cu2HgSnS4 (part b–d), and SnS2 (part d–e).
Vertical section A–B: (A—60 mol.% Cu2S; 40 mol.% HgS; B—60 mol.% SnS2; 40 mol.% HgS): 1 – L, 2 – L + γ′′, 3 – L + Cu2HgSnS4, 4 – L + SnS2, 5 – γ′′, 6 – L + γ′′ + δ′, 7 – L + γ′′ + Cu2HgSnS4, 8 – L + Cu2HgSnS4 + δ′, 9 – L + SnS2 + Cu2HgSnS4, 10 – L + SnS2 + δ′, 11 – γ′′ + δ′, 12 – γ′′ + Cu2HgSnS4 + δ′, 13 – Cu2HgSnS4 + SnS2 + δ′, 14 – SnS2
The section solidus is formed by the boundary compositions of γ′′- and δ′-solid solutions above the temperature of invariant processes and by the horizontal lines at 965 K and 888 K which belong to the eutectic processes L ⇔ Cu2HgSnS4 + γ′′ + δ′ and L ⇔ Cu2HgSnS4 + SnS2 + δ′.
The space between the liquidus and solidus lines, along with the fields of the primary crystallization volumes, contains the fields of the secondary crystallization L ⇔ γ′′ + Cu2HgSnS4, L ⇔ Cu2HgSnS4 + SnS2, and L ⇔ Cu2HgSnS4 + δ′. Of all investigated alloys, three alloys with the content of 0, 50 and 100 mol.% B are two-phase in the subsolidus region; the remaining alloys contain three phases.
3.9 Liquidus Surface Projection of the Quasi-ternary System Cu2S-HgS-SnS2
Liquidus surface projection of the Cu2S-HgS-SnS2 system on the concentration triangle was plotted from the results presented above (Fig. 9). The liquidus consists of six fields of the primary crystallization of Cu2S (γ′′-solid solutions), HgS (δ′-solid solutions), SnS2, Cu2SnS3, Cu4SnS4, and Cu2HgSnS4. They are separated by fourteen monovariant lines and fourteen invariant points, of which eight correspond to binary and six to ternary invariant processes. The nature and temperature of invariant processes are gathered in Table 2.
The Cu2S-HgS-SnS2 system is divided by the quasi-binary sections Cu2SnS3-HgS, Cu2HgSnS4-Cu2S and Cu2HgSnS4-SnS2 into four subsystems. The sub-systems Cu2S-HgS-Cu2HgSnS4, HgS-SnS2-Cu2HgSnS4 and SnS2-Cu2SnS3-Cu2HgSnS4 are of the eutectic type.
The crystallization of alloys in the Cu2S-Cu2HgSnS4-Cu2SnS3 system is more complex, due to the solid-state process of the formation of the ternary compound Cu4SnS4 (γ′′ + Cu2SnS3 ⇔ Cu4SnS4) in the boundary system Cu2S-SnS2 at 1083 K. This is higher than the temperature of the eutectic process in the Cu2S-Cu2HgSnS4 system (1060 K). Therefore, the ternary compound Cu4SnS4 has its own field of the primary crystallization on the liquidus surface, caused by the peritectic process L + Cu2SnS3 + γ′′ ⇔ Cu4SnS4 which takes place at 1083 K.
3.10 Isothermal Section of the Quasi-ternary System Cu2S-HgS-SnS2 at 670 K
Isothermal section of the quasi-ternary system Cu2S-HgS-SnS2 at 670 K was plotted based on obtained results (Fig. 10). The quasi-binary systems Cu2SnS3-HgS, Cu2HgSnS4-SnS2, Cu2S-Cu2HgSnS4, and the Cu2Sn4S9-Cu2HgSnS4 and Cu4SnS4-Cu2HgSnS4 sections which are quasi-binary in the sub-solidus part, separate the quasi-ternary system Cu2S-SnS2-HgS at 670 K into six subsystems. The quaternary compound Cu2HgSnS4 at the annealing temperature is in equilibrium with the components of the quasi-ternary system Cu2S, HgS and SnS2, as well as the ternary phases Cu4SnS4 and Cu2SnS3. The γ′′-solid solution range of HT-Cu2S modification is stretched at 670 K along the Cu2S-HgS side. The solid solubility in Cu2HgSnS4, Cu4SnS4, Cu2SnS3, Cu2Sn4S9, SnS2, and HgS is negligible and does not exceed 2-3 mol.% at 670 K. Solid-state processes involving the Cu2Sn4S9 compound should be noted in the Cu2SnS3-Cu2HgSnS4-SnS2 subsystem. All alloys of this subsystem complete their crystallization in the ternary eutectic process L ⇔ SnS2 + Cu2SnS3 + Cu2HgSnS4 at 1015 K. The quasi-binary section Cu2S-SnS2 features at 933 K the peritectoid process of the formation of the ternary phase Cu2Sn4S9 (Cu2SnS3 + SnS2 ⇔ Cu2Sn4S9) which is stable at 670 K. This process takes also place in all alloys of the Cu2SnS3-Cu2HgSnS4-SnS2 subsystem. The process ends with an excess of the ternary compound Cu2SnS3 in the Cu2SnS3-Cu2HgSnS4-Cu2Sn4S9 part, with an excess of the binary compound SnS2 in the Cu2Sn4S9-Cu2HgSnS4-SnS2 part, and only at the Cu2Sn4S9-Cu2HgSnS4 section the process is completed stoichiometrically. The above solid-state processes lead to the emergence of the binary equilibrium Cu2HgSnS4-Cu2Sn4S9 at the isothermal section.
4 Conclusions and Future Work
A total of 152 alloys were investigated by DTA, x-ray diffraction and MCA methods in the quasi-ternary system Cu2S-HgS-SnS2. Phase diagrams of the quasi-binary system Cu2S-HgS, six vertical sections HgS-Cu2SnS3, Cu2HgSnS4-SnS2, Cu2S-Cu2HgSnS4, Cu2Sn4S9-Cu2HgSnS4, Cu4SnS4-Cu2HgSnS4, A–B (A—40 mol.% HgS, 60 mol.% Cu2S; B—40 mol.% HgS, 60 mol.% SnS2), liquidus surface projection onto the concentration triangle and isothermal section of the quasi-ternary system Cu2S-HgS-SnS2 at 670 K were investigated. The sections Cu2HgSnS4-SnS2 and Cu2S-Cu2HgSnS4 are quasi-binary systems of eutectic type with eutectic points coordinates 1021 K, 17 mol.% Cu2HgSnS4 and 1060 K, 27 mol.% Cu2HgSnS4, respectively. The existence of the quaternary compound Cu2HgSnS4 which melts congruently at 1122 K was found in the HgS-Cu2SnS3 system at the component ratio 1:1. The interaction of Cu2HgSnS4 with the section components is eutectic. The eutectics melt at 1113 K and 1035 K, eutectic points have composition of 18 and 88 mol.% HgS, respectively. The sections Cu2Sn4S9-Cu2HgSnS4 and Cu4SnS4-Cu2HgSnS4 are quasi-binary only in the sub-solidus part due to the solid-state formation of ternary compounds. The Cu2S-HgS-SnS2 system is triangulated by quasi-binary sections into four subsystems. The coordinates of invariant points and positions of monovariant lines were established.
Presented results of the study of phase equilibria in the Cu2S-HgS-SnS2 system expand the database in the field of semiconductor materials science. Obtained results can be used to predict phase equilibria in analogous systems and in the development of technology for obtaining ternary and quaternary chalcogenides in the single-crystalline or polycrystalline state.
References
D.J. Chakrabarti, and D.E. Laughlin, The Cu-S (Copper-Sulfur) System, Bull. Alloy Phase Diagr., 1983, 4(3), p 254–271
R.C. Sharma, Y.A. Chang, and C. Guminski, The Hg-S (Mercury-Sulfur) System, J. Phase Equilib., 1993, 14(1), p 100–109
R.C. Sharma, and Y.A. Chang, The S-Sn (Sulfur-Tin) System, Bull. Alloy Phase Diagr., 1986, 7(3), p 269–273
T.V. Vu, A.A. Lavrentyev, B.V. Gabrelian, H.D. Tong, V.A. Tkach, O.V. Parasyuk, and O.Y. Khyzhun, A Theoretical and Experimental Study of the Valence-Band Electronic Structure and Optical Constants of Quaternary Copper Mercury Tin Sulfide, Cu2HgSnS4, A Potential Material for Optoelectronics and Solar Cells. Optical Materials, 2019, 96, p 109296
T.V. Zolotova, Y.A. Karagodin, Investigation of Phase Equilibria in the Cu-Ge(Sn)-S Systems at the Ge(Sn)S2-Cu2S sections, Sb. nauch. tr. po probl. mikroelektron, vol. XXVIII. MIET, Moscow, 1975, p 174–181
M. Khanafer, J. Rivet, J. Flahaut, Étude du ternaire Cu-Sn-S. Diagrammes d’équilibre des systèmes: Cu2S-SnS, Cu2S-Sn2S3 et Cu2S-SnS2. Étude cristallographique des composés Cu4SnS4, Cu2SnS3, Cu2Sn4S9 et Cu4Sn3S8, Bull. Soc. Chim. France, 1974, 12, p 2670–2676
N. Wang, The Three Ternary Phases in the System Cu-Sn-S, N. Jb. Miner. Monatsh., 1977, 9, p 424–431
G.H. Moh, Tin-Containing Mineral Systems. Pt. II. Phase Relations and Mineral Assemblages in the Cu-Fe-Zn-Sn-S System, Chem. Erde. 1975, 34, p 142–146
V.A. Kovalenker, B.C. Malov, T.L. Evstigneeva, and L.N. Vyalsov, Mokhit Cu2SnS3-novyj sulfid olova i medi. Zap. VMO, Moscow, 1975.
D. Wu, C. Knowles, and L.Y. Chang, Coppertin Sulphides in the System Cu-Sn-S, Miner. Mag., 1986, 50(6), p 323–325
I.D. Olekseyuk, I.V. Dudchak, and L.V. Piskach, Phase Equilibria in the Cu2S-ZnS-SnS2 System, J. Alloys Compd., 2004, 368(1–2), p 135–143
S.F. Motrya, E.E. Semrad, Yu.V. Voroshilov, and I.I. Yaczkovich, Physico-Chemical Study of Systems HgS-SnS, HgS-SnS2, Zhurn. Neorgan. Khimii., 1988, 33(8), p 2103–2105
R. Ollitrault-Fichet, J. Rivet, and J. Flahaut, Diagramme de Phase du Système Hg-Cu-S: Étude du Triangle HgS-Cu2S-S, J. Less-Common Met., 1984, 96, p 49–62
M.I. Kulakov, Zh.D. Sokolovskaya, and V.I. Sorokin, System HgS-Cu2-xS, Izvestiya Akademii Nauk SSSR Neorg. Materialy, 1985, 21(4), p 551–555
H.T. Evans, H.T. Evans, The Crystal Structures of Low Chalcocite and Djurleite, Z. Kristallogr, 1979, 150, p 299–320
P. Lukashev, W.R.L. Lambrecht, T. Kotani, and M. van Schilfgaarde, Electronic and Crystal Structure of Cu2−xS: Full-Potential Electronic Structure Calculations, Phys. Rev. B, 2007, 76(19), p 195202
D.-W. Fan, W.-G. Zhou, C.-Q. Liu, F. Wan, Y.-S. Xing, J. Liu, Y.-C. Li, and H.-S. Xie, Phase Transition and EOS of Cinnabar (alpha-HgS) at High Pressure and High Temperature, Chin. Phys. Lett., 2009, 26(4), p 046402
T. Ohmiya, Thermal Expansion and the Phase Transformation in Mercury Sulphide, J. Appl. Cryst., 1974, 7, p 396–397
S.K. Arora, D.H. Patel, and M.K. Agarwal, Microtopographical Characterization of Vapour-Grown SnS2 Single Crystals, Cryst. Res. Technol., 1993, 28, p 623–627
S. Jaulmes, J. Rivet, and P. Laruelle, Cuivre–Etain–Soufre Cu4SnS4, Acta Cryst. B., 1977, 38, p 540–542
X. Chen, H. Wada, A. Sato, and M. Mieno, Synthesis, Electrical Conductivity, and Crystal Structure of Cu4Sn7S16 and Structure Refinement of Cu2SnS3, J. Solid State Chem., 1998, 139, p 144–151
M. Onoda, X.A. Chen, A. Sato, and H. Wada, Crystal Structure and Twinning of Monoclinic Cu2SnS3, Mater. Res. Bull., 2000, 35, p 1563–1570
V.S. Gruzdev, V.Iu. Volgin, E.M. Spiridonov, L.N. Kaplunnik, E.A. Pobedimskaya, N. Chvileva, and N.M. Chernitsova, Velikite Cu2HgSnS4—The Mercury Member of the Stannite Group, Doklady Akademii Nauk SSSR, 1988, 300(2), p 432–435
L. Akselrud, and Yu. Grin, WinCSD: Software Package for Crystallographic Calculations (Version 4), J. Appl. Cryst., 2014, 47, p 803–805
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marchuk, O.V., Smitiukh, O.V. & Kogut, Y. Quasi-Ternary System Cu2S-HgS-SnS2. J. Phase Equilib. Diffus. 42, 245–253 (2021). https://doi.org/10.1007/s11669-021-00873-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-021-00873-1