Abstract
Phase equilibria in the Cu2Se–GeSe2–SnSe2 quasi-ternary system were studied by differential thermal analysis (DTA) and X-ray powder diffraction analysis. A series of polythermal sections, the 750-K isothermal section of the phase diagram, and the liquid surface projection were plotted. Primary crystallization and homogeneity areas of phases were determined, as well as the characters and types of invariant and monovariant equilibria. Extensive Cu2GeSe3-base and Cu2SnSе3-base solid solutions were found to exist in the system along the Cu2GeSe3–Cu2SnSe3 section.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Copper chalcogenides and their based phases are among the most popular subject matters of research in semiconductor materials science; they have many functional properties showing a potential for use in several fields, such as photoelectrochemical, photocatalytic, and solar cells [1–5]. These phases attract increasing attention as promising thermoelectric materials due to their high efficiency, tunable transport properties, as well as the low toxicity and availability of their constituents [6–11]. In addition, copper chalcogenides are mixed electron–ionic conductors and, due to the high mobility of “liquid-like” copper ions, they exhibit record-breaking values of cationic conductivity (up to ∼3 Ω–1 cm–1) and ion diffusion (~10–5 cm2/s) [12–14]. This makes them promising materials for ion-selective electrodes or solid electrolytes in the development of various types of electric batteries, sensors, etc. [12–19].
It is well known that an efficient strategy for optimizing functional properties of the material is to modify its composition and structure. In order to search for and design new materials and for a better understanding of the relationships among the composition, structure, and properties, one needs to have reliable data on the phase equilibria and thermodynamic properties of the relevant multicomponent systems [19–22].
In our prior works [23–27], we carried out similar diligent studies of complex systems involving copper chalcogenides; we found new phases and determined their primary crystallization and homogeneity areas.
Here, we will present new experimental data on phase equilibria in the Cu2Se–GeSe2–SnSe2 quasi-ternary system (system A). The ternary phases formed in this system (Сu8GeSe6, Cu2SnSe3, and Cu2GeSe3) have extensively been studied as thermoelectric materials [11–13].
The terminal compounds (Cu2Se, GeSe2, and SnSe) and boundary quasi-binary constituents (Cu2Se–GeSe2, GeSe2–SnSe2, and Cu2Se–SnSe2) of the title system have been well characterized.
Cu2Se is a compound melting congruently at 1403 K and undergoing polymorphic transition at 396 K [28]. This compound has a homogeneity area extending toward an excess of selenium, which has the largest extent (33.3–36.6 at % Se) at 800 K.
Germanium diselenide GeSe2 and tin diselenide SnSe2 melt with an open maximum at 1015 [28] and 948 K [29] respectively.
Crystallographic data for the binary and ternary compounds of system A appear in Table 1.
System Cu2Se–GeSe2 forms ternary compounds Cu8GeSe6 and Cu2GeSe3 by a pertectic reaction (at 1083 K) and a dystectic reaction (at 1054 K), respectively [32]. Cu8GeSe6 experiences phase transition at 333 K [32] (or at 328 K [33]). Its low-temperature phase LT-Cu8GeSe6 crystallizes in hexagonal structure [32, 33], while the high-temperature phase HT-Cu8GeSe6 crystallizes in cubic structure [34] (Table 1). Cu2GeSe3 has two polymorphs with the phase transition at 893 K [35–38]. The high-temperature phase forms an orthorhombic lattice, and the low-temperature phase forms a tetragonal lattice. Two eutectics solidify in the system with the coordinates 1033 K, 38 mol % GeSe2 and 973 K, 88 mol % GeSe2 [32].
System Cu2Se–SnSe2 was studied in [39–41], and the results of those studies were summarized in the survey [42]. The system forms one compound, Cu2SnSe3, which melts congruently at 968 K [40, 41] and forms eutectics with the terminal binary components. The eutectic coordinates are 84 mol % SnSe2, 853 K and 22 mol % SnSe2, 983 K [40]. The solubility in the terminal compounds is within 3 mol % (SnSe2) and 10 mol % (Cu2Se) [41]. Cu2SnSe3 was reported to crystallize in a sphalerite-type cubic structure [43, 44]. However, the structural study of a single-crystal sample [45] showed that the compound has a monoclinic structure.
System GeSe2–SnSe2, which is a boundary quasi-binary system, has a eutectic phase diagram with limited reciprocal solubility in the terminal selenides [46]. The highest solubility in GeSe2 and SnSe2 is ∼9.6 and 6 mol %, respectively, at the eutectic temperature (823 K). The eutectic melt contains 49 mol % SnSe2.
EXPERIMENTAL
The terminal binary compounds (Cu2Se, SnSe2, and GeSe2) and the terminal compounds (Cu2GeSe3, Cu8GeSe6, and Cu2SnSe3) of system A were prepared to be used in experiments.
The constituent elements used in experiments were high-purity samples purchased from Evochem Advanced Materials: granulated copper (Cu-00029, 99.9999%), germanium chips (Ge-00003, 99.9999%), granulated tin (Sn-00005, 99.999%), and granulated selenium (Sе-00002, 99.999%). The binary and ternary compounds were prepared by alloying the constituent elements in stoichiometric proportions in evacuated (to ~10–2 Pa) and then sealed-off silica glass ampoules at temperatures slightly above the melting temperatures of the compounds to be prepared. Cu2Se, GeSe2, Cu2GeSe3, and Cu8GeSe6, whose melting temperatures far exceed the selenium boiling point (958 K [47]), were prepared in two-zone syntheses. An ampoule with the reaction mixture was heated in an inclined tube furnace to a temperature ∼50 K higher than the melting point of the compound to be synthesized (a hot zone). A portion of the ampoule (~8 cm) was outside the furnace and cooled by water to control the selenium vapor pressure and avoid the ampoule explosion (a cold zone). The ampoule was rotated around its longitudinal axis and vibrated for speeding up the reaction. After most selenium was reacted, the ampoule was completely inserted into the furnace, exposed in the hot zone for 1 h, and then slowly cooled. In view of the deviation of Cu2Se at low temperatures [28], the as-synthesized sample was quenched from 1300 K in cold water in order for it acquired a homogeneous stoichiometric composition.
DTA and X-ray powder diffraction were used to verify the identity of every compound prepared. The melting temperature and unit cell parameters for every prepared compound coincided with the above-cited literature data (Table 1) within the measurement error bars (DTA: ±3 K at high temperatures and ±2 at low temperatures; XRD: ±0.0003 Å).
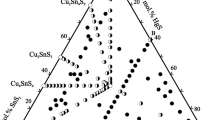
More than 60 alloys to be used in experiments were prepared by direct in vacuo alloying of the terminal compounds; their compositions lie along Cu2GeSe3–Cu2SnSe3, Cu8GeSe6–“Cu8SnSe6,” 0.4Cu8GeSe6–Cu2SnSe3, GeSe2–0.5Cu2SnSe3, 0.5Cu2GeSe3–SnSe2, and Cu2Se–“Ge0.5Sn0.5Se2” sections; some additional alloys were prepared beyond these sections (Fig. 1). In order to provide the conditions as close to equilibrium as possible, cast alloys that were prepared by rapid melt cooling were then annealed at 750 K for 500 h.
DTA experiments were carried out on a 404 F1 Pegasus System (Netzsch) differential scanning calorimeter. The heating rate was 10 K/min. In some cases, cooling curves were measured in order to determine the liquidus temperature. The DTA results were processed in the Netzsch Proteus Software. The temperature measurement accuracy was ±2°.
X-ray powder diffraction experiments were carried out at room temperature on a D8 Advance (Bruker) diffractometer using CuKα1 radiation. X-ray diffraction patterns were indexed in the Topas V3.0 Software (Bruker).
RESULTS AND DISCUSSION
The joint processing of the experimental data set using the literature data on the Cu2Se–GeSe2 [32], Cu2Se–SnSe2 [39–42], and GeSe2–SnSe2 [46] boundary binary systems provided a self-consistent pattern of phase equilibria in the Cu2Se–GeSe2–SnSe2 system.
Hereinafter in the text, tables, and figures, the following phase notations will be used:
• α and δ stand for HT-Cu2Se-base and HT-Cu8GeSe6-base solid solutions, respectively;
• β1 and β2 stand for GeSe2-base and SnSe2-base solid solutions, respectively; and
• γ1 and γ2 stand for Cu2GeSe3-base and Cu2SnSe3-base solid solutions, respectively.
Quasi-Binary Section Cu2GeSе3–Cu2SnSe3
The DTA and XRD results on the Cu2GeSе3–Cu2SnSe3 system appear in Table 2. This system is a quasi-binary section of the quaternary system and has a peritectic-type phase diagram (Fig. 2). Peritectic equilibrium L ↔ γ1 + γ2 is acquired at 985 K. The peritectic point is at 60 mol % Cu2SnSe3.
The X-ray powder diffraction patterns of homogenized Cu2GeSe3–Cu2SnSe3 alloys verified the formation of extensive substitutional solid solutions. One can see in Fig. 3 that the powder diffraction pattern of a 20 mol % Cu2SnSe3 alloy is qualitatively identical to the diffraction pattern of the compound Cu2GeSе3. This implies that this alloy is a solid solution based on this compound (γ1). The 60 and 80 mol % Cu2SnSe3 alloys have diffraction patterns identiсal to that of pure Cu2SnSe3 with reflection angles slightly shifting in response to changing composition. The diffraction patterns of these alloys are fully indexed in the cubic crystal system (space group F\(\bar {4}\)3m) with reflection angles slightly shifting in response to changing composition. The 40 mol % Cu2SnSe3 alloy is comprised of two phases; its diffraction pattern is the sum of the diffraction lines from the γ1 and γ2 phases. Noticeable is some broadening of the diffraction peaks from the solid solutions compared to those of the parent compounds. This is apparently owing to the crystal distortion caused by nonuniform Ge ↔ Sn substitution despite the long-term annealing. Table 2 lists the unit cell parameters of the alloys and parent compounds of the Cu2GeSe3–Cu2SnSe3 system calculated in the Topas V3.0 program; Fig. 4 displays their concentration dependecies. Figure 4 implies that the unit cell parameters of the γ1 and γ2 phases vary linearly in the range 0–35 and 50–100 mol % Cu2SnSe3, respectively, and in intemediate alloys they remain unchanged regardless of the general alloy composition. This impies that the end-members of mutually saturated γ1 and γ2 phases are 35 ± 1 and 50 ± 1 mol %, respectively.
Having indexed the powder diffraction patterns of Cu2GeSe3 and its base solid solutions and those of Cu2SnSe3 and its solid solutions, we found that the former were fully indexed in the tetragonal crystal system and the latter in the cubic crystal system.
Equilibrium Solid-Phase Diagram at 750 K
The equilibrium solid-phase diagram at 750 K was plotted using XRD data for some equilibrium alloys inside the Cu2Se–GeSe2–SnSe2 concnetration triangle and the phase diagrams of boundary quasi-binary systems [32, 39–42, 46]. Figure 5 shows that the system features extensive solid solutions based on the terminal ternary compounds along the Cu2GeS3–Cu2SnSe3 quasi-binary section. The solid-solution homogeneity areas are shaped as bands with a width of ∼1–2 mol % (for the γ1 phase) and ∼3–5 mol % (for the γ2 phase). This correlates with the Cu2Se–GeSe2 and Cu2Se–SnSe2 phase diagrams [32, 42].
Limited solid solutions based on GeSe2 (β1), SnSe2 (β2), and НТ-Cu8GeSe6 (δ) also exist in the system at 750 K. The β1 and β2 phases form narrow bands extending along the GeSe2–SnSe2 boundary quasi-binary system ∼1 mol % wide and 8 and 6 mol % long, respectively. The δ phase homogeneity area extends to Cu8Ge0.9Sn0.1Se6 along the Cu8GeSe6–Cu8SnSe6 section. The solubility in the low-temeprature Cu2Se phase is insignificant.
The interaction of coexisting phases in the system gives rise to two-phase areas (β1 + γ1, β2 + γ2, β2 + γ1, γ1 + γ2, γ1 + δ, γ2 + δ, γ2 + α, and α + δ) and three-phase areas (β1 + β2 + γ2, β1 + γ1 + γ2, γ1 + γ2 + δ, α + γ2 + δ). The phase compositions of samples in these fields were verified by XRD.
Figure 6 displays exemplary powder diffraction patterns of three alloys chosen from various phase areas (alloys 1, 2, and 3 in Fig. 5). An inspection of these diffraction patterns shows that they are the sums of diffraction lines from phases that are in equilibrium according to Fig. 5.
Liquids Surface Projection
Figure 7 images the liquidus surface projection of the Cu2Se–GeSe2–SnSe2 system consisting of six primary crystallization fields of solid solutions based on binary compounds (α, β1, and β2) and ternary compounds (γ1, γ2, and δ). These fields are demarcated by eutectic and peritectic curves. On curve р2U1 at point K, peritectic equilibrium L + γ1 ↔ γ2 transforms to eutectic equilibrium L ↔ γ1 + γ2. These equilibria involve solid solutions. Therefore, in principle this transition must be accompanied by the formation of some surface in the relevant three-phase area, where three-phase equilibrium would become two-phase equilibrium with a passive role of the third phase [48, 49]. Since the γ1 and γ2 homogeneity areas almost do not go beyond the Cu2GeSe3–Cu2SnSe3 quasi-binary section, this surface is most likely degenerates into a point (K).
The types and coordinates of invariant equilibria and the types and temperature ranges of monovariant equilibria appear in Tables 3 and 4, respectively.
The Cu2GeSe3–Cu2SnSe3 quasi-binary section divides the Cu2Se–GeSe2–SnSe2 concnetration triangle into a pair of independent subsystems: Cu2Se–Cu2GeSe3–Cu2SnSe3 and GeSe2–Cu2GeSe3–Cu2SnSe3. The former features two invariant transition reactions (U2 and U3), and the latter features one invariant transition equilibrium (U1) and one eutectic equilibrium (Е). Noteworthy, the latter can be regarded the reciprocal system 1.5GeSe2 + Cu2SnSe3 ↔ 1.5SnSe2 + Cu2GeSe3. Figure 7 implies that it is a reversibly reciprocal system, that is, it has not a quasi-binary diagonal. This is owing to the fact that the terminal compounds do not play a decisive role in the distrobution of phase areas, but their base extensive solid-solutions areas do.
Polythermal Sections of the Phase Diagram
Below we will consider some polythermal sections of the phase diagram of the title system in the context of the liquidus surface projection (Fig. 7; Tables 3 and 4) and the 750-K solid-phase equilibrium diagram (Fig. 5).
Section Cu8GeSe6–“Cu8SnSe6” (Fig. 8) entirely lies in the α phase primary crystallization area. The primary crystallization of the α phase is followed by crystallization of the HT-Cu8GeSe6-base δ phase by peritectic reaction L + α ↔ δ and the crystallziation of α + γ2 two-phase mixtures by eutectic reaction L ↔ α + γ2 (Table 4; Fig. 7, curves p1U3 and U3e3).
The specificity of this section is in the δ phase homogeneity area lying on its plane, so that peritectic reaction L + α ↔ δ fully consumes both initial phases simultaneously. It is for this reason that this peritectic reaction in Cu8GeSe6-rich compositions ends by the formation of δ solid solutions, which immediately border the L + α + δ three-phase area (Fig. 8). In 15–85 mol % compositions, crystallization ends by invariant transition reaction L + δ ↔ α + γ2 (U3) to form the α + δ + γ2 three-phase subsolidus area.
Section 0.4Cu8GeSe6–Cu2SnSe3 (Fig. 9) crosses the primary crystallization fields of the α phase (0–15 mol % Cu2SnSe3), δ phase (15–50 mol % Cu2SnSe3), and γ2 phase (50–100 mol % Cu2SnSe3). The α phase primary crystallization is followed by peritectic monovariant reaction L + α ↔ δ, which gives rise to the L + α + δ three-phase area. This reaction ends at ∼1040 K by an excess of the liquid phase and the formation of the L + δ two-phase area. In this area, crystallization ends by reaction L + δ ↔ γ2 (Table 4, U2U3), and the δ + γ2 two-phase area appears in the subsolidus.
In the 45–80 mol % Cu2SnSe3 range, transition reaction L + δ ↔ α + γ2 (Table 3, U3) ends with an excess of the δ phase to form the α + δ + γ2 three-phase area. In Cu2SnSe3-rich areas, crystallization ends by L ↔ γ2 and L ↔ α + 훾2 equilibria (Table 4, U3e3).
Section GeSe2–0.5Cu2SnSe3 (Fig. 10) has a liquidus of three branches that correspond to the primary crystallization of the β1, γ1, and γ2 phases. Their intersection points correspond to the onset of a monovariant peritectic reaction (p2K) and a eutectic reaction (e1Е). The L + β1 + γ1 and L + γ1 + γ2 three-phase areas are formed as a result of these reactions. In the 80–95 mol % GeSe2 area, crystallization ends by peritectic reaction р2K and to form the β1 + γ1 two-phase area.
The γ2 phase primary crystallization field continues by the eutectic monovariant scheme L ↔ β2 + γ2 (е4U1) and ends in the 5–20 mol % GeSe2 range to form the β2 + γ2 two-phase area. In the 20–25 mol % GeSe2 narrow concentration range, crystallization ends by transition reaction U1 (845 K) to form the β2 + γ1 + γ2 three-phase area.
The 805-K horizontal (Е) corresponds to the invariant solidification of the β1 + β2 + γ1 ternary eutectic.
Section 0.5Cu2GeSe3–SnSe2 (Fig. 11) crosses the primary crystallization fields of the γ1, γ2, and β2 phases. Below liquidus, there are monovariant equilibria (е1Е, U1Е, KU1, and е4U1) and invariant equilibria (U1 and Е). When the crystallization is over, a series of two- and three-phase mixtures are formed by these reactions.
Section Cu2Se–Ge0.5Sn0.5Se2 (Fig. 12) passes through the primary crystallization fields of the α, δ, γ1, and γ2 phases; in the subsolidus, it crosses four three-phase areas. Phase equilibria along this section can easily be recognized by comparing Fig. 11 with Figs. 4 and 6. The Т–х diagram of this section clearly features transition invariant equilibria U1, U2, U3, and Е (horizontals at 845, 965, 950, and 805 K, respectively). The 398-K horizontal corresponds to the α ↔ LТ-Cu2Se polymorphic transition.
CONCLUSIONS
We have elucidated the full pattern of phase equilibria in the Cu2Se–GeSe2–SnSe2 quasi-ternary system, involving the solid-phase diagram at 750 K, the liquidus surface projection, and a series of polythermal sections of the phase diagram. Extensive solid solutions have been found to exist in the system along the Cu2GeSe3–Cu2SnSe3 quasi-binary section. Primary crystallization and homogeneity areas of phases have been determined, as well as the characters and types of invariant and monovariant equilibria in the title system. The prepared new phases of variable composition are of interest as environmentally safe candidate thermoelectric materials.
REFERENCES
X. L. Sanghoon, L. J. Tengfei, and L. Zhang, Y-H. Chalcogenide: From 3D to 2D and Beyond (Elsevier, 2019). https://doi.org/10.1016/C2017-0-03585-1.
X. Chen, J. Yang, T. Wu, et al., Nanoscale 10, 15130 (2018). https://doi.org/10.1039/C8NR05558
E. Peccerillo and K. Durose, MRS Energy Sustainability 5, 1 (2018). https://doi.org/10.1557/mre.2018.10
B. Yun, H. Zhu, J. Yuan, et al., J. Mater. Chem. B 8, 4778 (2020). https://doi.org/10.1039/D0TB00182A
Z. Xia, H. Fang, X. Zhang, et al., Chem. Mater. 30, 1121 (2018).https://doi.org/10.1021/acs.chemmater.7b05104
T. R. Wei, Y. Qin, T. Deng, et al., Sci. China Mater. 62, 8 (2019). https://doi.org/10.1007/s40843-018-9314-5
A. Mikula, K. Mars, P. Nieroda, and P. Rutkowski, Materials 14, 2635 (2021). https://doi.org/10.3390/ma14102635
T. Deng, P. Qiu, and T. Xing, J. Mater. Chem. A 9, 7946 (2021). https://doi.org/10.1039/D0TA12042A
B. Jiang, P. Qiu, E. Eikeland, et al., J. Mater. Chem. C 5, 943 (2017). https://doi.org/10.1039/c6tc05068a
M. Siyar, J. -Y. Cho, Y. Youn, et al., J. Mater. Chem. C 6, 1780 (2018). https://doi.org/10.1039/C7TC05180H
R. Chetty, D. S. Prem-Kumar, M. Falmbigl, et al., Intermetallics 54, 1 (2014). https://doi.org/10.1016/j.intermet.2014.05.006
A. K. Ivanov-Shchits and I. V. Murin, Solid State Ionics, vol. 1 (Izd-vo St. Peterb. Univ., St. Petersburg, 2000) [in Russian].
V. M. Berezin and G. P. Vyatkin, Superionic Semiconductor Chalcogenides (Chelyabinsk, 2001) [in Russian].
H. Liu, X. Shi, F. Xu, et al., Nature Materials 11, 422 (2012). https://doi.org/10.1038/nmat3273
M. B. Babanly, Yu. A. Yusibov, and V. T. Abishev, Three-Component Chalcogenides Based on Copper and Silver (Baku, 1993) [in Russian].
M. B. Babanly, Yu. A. Yusibov, and N. B. Babanly, in Electromotive Force and Measurement in Several Systems, Ed. by S. Kara (IntechOpen, 2011). https://doi.org/10.5772/28934
X. Liang, Appl. Phys. Lett. 111, 133902 (2017). https://doi.org/10.1063/1.4997501
P. Qiu, M. T. Agne, Y. Liu, et al., Nature Commun. 9 (2910) (2018). https://doi.org/10.1038/s41467-018-05248-8
M. B. Babanly, L. F. Mashadiyeva, D. M. Babanly, et al., Russ. J. Inorg. Chem. 64, 1134 (2019). https://doi.org/10.1134/S0036023619130035
M. B. Babanly, E. V. Chulkov, Z. S. Aliev, et al., Russ. J. Inorg. Chem. 62, 1703 (2017). https://doi.org/10.1134/S0036023617130034
V. P. Zlomanov, A. M. Khoviv, and A. Yu. Zavrazhnov, in Materials Science – Advanced Topics (IntechOpen, 2013). https://doi.org/10.5772/56700
S. Z. Imamaliyeva, D. M. Babanly, D. B. Tagiev, and M. B. Babanly, Russ. J. Inorg. Chem. 13, 1703 (2018). https://doi.org/10.1134/S0036023618130041
I. J. Alverdiyev, Z. S. Aliev, S. M. Bagheri, et al., J. Alloys Compd. 691, 255 (2017). https://doi.org/10.1016/j.j
L. F. Mashadiyeva, Z. T. Gasanova, Yu. A. Yusibov, and M. B. Babanly, Russ. J. Inorg. Chem. 62, 598 (2017). https://doi.org/10.1134/S0036023617050151
Z. T. Gasanova, L. F. Mashadiyeva, Yu. A. Yusibov, and M. B. Babanly, Russ. J. Inorg. Chem. 62, 591 (2017). https://doi.org/10.1134/S0036023617050126
L. F. Mashadieva, Z. T. Gasanova, Yu. A. Yusibov, and M. B. Babanly, Inorg. Mater. 54, 8 (2018). https://doi.org/10.1134/S0020168518010090
E. N. Ismailova, L. F. Mashadieva, I. B. Bakhtiyarly, and M. B. Babanly, Russ. J. Inorg. Chem. 64, 801 (2019). https://doi.org/10.1134/S0036023619060093
Binary Alloy Phase Diagrams, Ed. by T. B. Massalski T. B. (ASM International, Materials Park, Ohio, 1990). https://doi.org/10.1002/adma.19910031215
N. Kh. Abrikosov, in Semiconductor Physics (Springer, 2013). https://doi.org/10.1007/978-1-4899-6373-4.
L. Gulay, M. Daszkiewicz, O. Strok, and A. Pietraszko, Chem. Met. Alloys 4, 200 (2011). https://doi.org/10.30970/cma4.0184
G. Dittmar and H. Schafer, Acta Crystallogr. 32, 2726 (1976). https://doi.org/10.1107/s0567740876008704
V. Tomashik, Copper-Germanium-Selenium, Ternary Alloys VCH 2, 288 (2015).
M. Onoda, M. Ishii, P. Pattison, et al., J. Solid State Chem. 146, 355 (1999). https://doi.org/10.1006/jssc.1999.8362
V. N. Moroz, Izv. Akad. Nauk SSSR, Neorg. Mater. 26, 1830 (1990).
G. Marcano and L. Ieves, J. Appl. Phys. 87, 1284 (2000). https://doi.org/10.1063/1.372010
O. V. Parasyuk, L. D. Gulay, Ya. E. Romanyuk, and L. V. Piskach, J. Alloys Compd. 329, 202 (2001). https://doi.org/10.1016/s0925-8388(01)01606-1
O. S. Lychmanyuk, L. D. Gulay, I. D. Olekseyuk, et al., Pol. J. Chem. 81, 353 (2007).
B. B. Sharma, Phys. Status Solidi A 2, 13 (1970). https://doi.org/10.1002/pssa.19700020125
J. Rivet, P. Laruelle, and J. Flahaut, Bull. Soc. Chim. Fr. 5, 1667 (1970).
L. I. Berger and E. K. Kotina, Inorg. Mater. (Engl. Trans.) 9, 330 (1973).
T. V. Zotova and Yu. A. Karagodin, Sb. Nauchn. Trudov Probl. Mikroel. 21, 57 (1975).
I. D. Olekseyuk, O. V. Parasyuk, L. V. Piskach, et al., Quasi-Ternary Systems of Chalcogenides (Vega Publish, Lutsk, 1999).
L. S. Palatnik, Yu. F. Komnik, E. K. Belova, and L. V. Atroshchenko, Kristallografiya 6, 960 (1961).
B. B. Sharma, R. Ayyar, and H. Singh, Phys. Status Solidi A 40, 691 (1977). https://doi.org/10.1002/pssa.2210400237
G. Marcano, L. M. de Chalbaud, C. Rincon, and G. Sanchez-Perez, Mater. Lett. 53, 151 (2002). https://doi.org/10.1016/S0167-577X(01)00466-9
M. I. Karakhanova, L. P. Sokolova, and A. V. Novoselova, Izv. Akad. Nauk SSSR, Neorgan. Mater. 12, 1221 (1976).
J. Emsley, The Elements (Clarendon Press, Oxford, 1991).
V. I. Lutsyk, V. P. Vorob’eva, and S. Ya. Shodorova, Russ. J. Phys. Chem. 89, 2331 (2015). https://doi.org/10.1134/S0036024415130245
V. I. Lutsyk and V. P. Vorob’eva, Russ. J. Phys. Chem. A 91, 2593 (2017). https://doi.org/10.1134/S0036024417130131
Funding
The work was supported by the Science Development Foundation under the President of the Azerbaijan Republic (project No. EİF-BGM-4-RFTF-1/2017-21/11/4-M-12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Fedorova
Rights and permissions
About this article
Cite this article
Mashadieva, L.F., Alieva, Z.M., Mirzoeva, R.D. et al. Phase Equilibria in the Cu2Se–GeSe2–SnSe2 System. Russ. J. Inorg. Chem. 67, 670–682 (2022). https://doi.org/10.1134/S0036023622050126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622050126