Abstract
The paper deals with theoretical and experimental study of phase transformation temperatures of steels in high temperature region (above 1000 °C), with focus on the solidus temperature, peritectic transformation temperature and liquidus temperature of multicomponent steels. Experimental data were obtained using Differential Thermal Analysis and “direct” thermal analysis. The experimental data were assessed by basic statistics. The calculations were performed using InterDendritic Solidification software and Thermo-Calc software. Also, selected empirically based models were used for calculations. The study presents the basic principles of theoretical and experimental methods, characteristics, advantages and disadvantages. Both used thermo-analytical methods are set correctly; the results are reproducible, comparable and close to equilibrium temperatures. Furthermore, comprehensive comparisons between the calculated and measured phase transformation temperatures show that the experimental data is satisfactorily accounted for by the present thermodynamic description.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The changing global market of the steel industry requires steelmaking process technologies to be further developed, to provide the steel companies with economically-sustainable steel-making production. To achieve that, it is necessary to know every aspect of the production process that has impact on the final product. One of the most important aspects of steel productions today are the phase transformation temperatures.[1,2]
Phase transformation temperatures are widely used in industrial processing of steels. Among the most important phase transformation temperatures in high temperature region are liquidus temperature (TL), peritectic transformation temperature (TP) and solidus temperature (TS).[3] These phase transformation temperatures are used in modern steel production and processing for better control of the production processes, optimal setting of casting and solidification conditions, and thermal and chemical homogenization of the melt.[4] They are also used for the design of microstructures or alloy development and has significant impact on the understanding of the fundamental properties of steels.[5,6]
Phase transformation temperatures may be investigated by several approaches, but there are two main directions: (1) experimental, using primarily methods of thermal analysis and (2) theoretical, using various models often implemented in software applications.
Thermal analysis is considered among most reliable techniques currently available for study of phase transformation temperatures. The accuracy and reliability was proven over last decades.[7] However, there are many factors affecting the measurement of thermal analysis, such as detection limits of temperature sensors, size of the samples and temperature fields in sample, inhomogeneous chemical composition of the sample, procedure or method bias, where different analytical procedures give different results, environmental influences, type of material, sample mass, sample geometry, heating and cooling rate, atmosphere, temperature range, crucible, evaluation methodology, etc. Therefore it is necessary to check compliance of experimental results with the theoretical model calculation.[8]
Modelling of phase transformation temperatures today presents two main modelling philosophies: (a) the empirical models (black box) or (b) physically-based (fundamental) models (white box). Both concepts were proven to have its merits.[9]
Empirical models are based on extensive study of correlation between chemical composition and its impact on phase transformation temperatures. It can be applied only to relatively simple and specific systems, but are fast and if tested properly, it provides very accurate results.[10] Fundamental models are more general and it is possible to use them for more complex applications. However, in general, fundamental models are not developed enough so they could be used alone for complex industrial applications. The fundamental models also need far more calculation time compared to the empirical models.
Therefore, many fundamental models include also stochastic and empirical features. This approach also uses CALPHAD[11] and Phase Field[12] methods for calculating, among others, the phase transformation temperatures. Thermo-Calc software provides temperatures under equilibrium conditions only (e.g. for phase diagrams). The methods are using databases with the stored assessed information and calculation results are dependent on correct thermodynamic data in the databases. However, accuracy and overall versatility of modern modelling software makes it more competitive to the experimental methods.[13]
Regardless, it is still difficult to find a comprehensive model for phase transformation temperatures calculations that would be consistent with experimental results. One reason is that it is exceptionally difficult to include all the effects and interactions of various elements (and various ranges of element concentrations) in a single computational model. Another reason is that many real experimental systems only approximate to equilibrium, mainly due to the existence of metastable or long lived transient states, and these non-equilibrium states are still difficult to model accurately.
The aim of the paper was to obtain original accurate phase transformation temperatures: liquidus temperature (TL), peritectic transformation temperature (TP) and solidus temperature (TS). Four steel grades were analysed, where steels 1 and 2 are model steel samples, prepared in laboratory conditions. Steel 3 and 4 are commercial steel grades provided by industrial partners. Experimental measurements were conducted by differential thermal analysis (DTA) and direct thermal analysis (TA).[14] The calculations were performed by software Thermo-Calc[15] and IDS.[16] Furthermore, the research of existing empirical models was completed and several empirical models were selected for calculations. The data collected by experimental methods and calculations were assessed in term of comparability and reproducibility.
2 Experiment
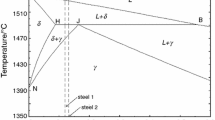
Steel samples 1 and 2 with graded carbon and chromium content were prepared in the laboratory. Samples were prepared by vacuum melting of electrochemically produced iron (99.9 wt.%) with the addition of graphitic carbon and pieces of chromium (99 wt.%).
The composition of the samples is shown in Table 1. Steel 3 is alloy steel grade with marginally increased content of Mn, Cr and Mo. Steel 4 is tool steel designed for special machine components with substantial alloying element content (e.g. Ni, Cr, Mo, V …).
Chemical composition of the samples was determined using a spectrometer with spark discharge. Carbon, oxygen, sulphur and nitrogen content in the samples were determined using combustion analysers.
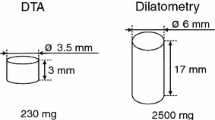
Two thermal analysis methods were used for experimental investigation. The experiments were performed in high temperature region, this means in temperature region above 1000 °C. The experiments were performed in corundum crucibles in inert atmosphere of argon with purity higher than 99.9999 mol.%. Such high purity gas is accomplished by using Getter-gas purifier (MicroTorr Canister Purifier MC200). The samples were machined to a desired shape for each equipment and method, then polished and cleaned by ultrasound in acetone. Description of the equipment and adjustment of experimental conditions is described, e.g. Ref 17 and 18.
Temperature calibration was performed using melting temperature of pure palladium (99.999 wt.%) and pure nickel (99.999 wt.%). Furthermore, the liquidus temperature results of DTA analysis were corrected on influence of the heating rate and sample mass[19,20] The evaluation of the DTA and TA curves was carried out by the tangent interception method.
The Setaram SETSYS 18™ was used for Differential Thermal Analysis method (DTA). The laboratory system for thermal analysis and DTA ‘S’ type (Pt/PtRh 10%) thermocouple were used for obtaining the phase transformations temperatures. The samples were analysed in crucibles with volume of 100 µl. The mass of the samples was approximately 200 mg. Heating rate was 10 °C min−1. An empty corundum crucible served as reference. The result of the measurement is a DTA curve. DTA analysis was performed with at least three different pieces of samples to improve statistical analysis.
The Netzsch STA 449 F3 Jupiter was used for Direct Thermal Analysis method (TA). The laboratory system uses sensor S-type (mono-couple). Specifically, it is one thermocouple inserted in the crucible with a sample. The mass of sample was approximately 22 g. Heating and cooling rate was 5 °C min−1. The result of the measurement is either a Heating curve (TAH) or a Cooling curve (TAC). The TA analysis was performed on two different pieces of samples. The TA analysis was performed using two cycle measurement. A sample was heated to set temperature and cooled twice, when the second cycle was following the first cycle immediately. The bottom temperature after first cycle was set approximately bellow the solidus temperature.
3 Empirical Models
Empirical models are generally obtained on the basis of the Fe-i binary phase diagrams. The different effects of elements on the melting point of pure iron are implemented. The value of phase transformation temperatures decreases or increases together with the content of element i in Fe-i binary phase diagram. The data are fitted to obtain the mathematical formula that is normally represented by linear or quadratic equation. The model of phase transformation temperature calculation is established, introducing the mathematical formula of each element into one (or more) equations. The general calculation model for phase transformation temperature calculation is as follows[21]:
where TPTT is the general phase transformation temperature of steel, T0 is the melting point of pure iron; ∂TPTT/∂Ci is the changing rate of isotherm with the element content i on the Fe-i binary phase diagram and [%Ci] is the percentage content of the element i. Similarly, the model can be modified to fit the quadratic function. Furthermore, there can be found more complex equations that includes interactions of elements between themselves.[22] The Table 2 comprehends empirical models obtained by extensive research. 13 models for liquidus temperature, 1 model for peritectic transformation temperature and 6 models for solidus temperature are presented and further used for calculations.
4 Software Calculations
The calculations were performed using two software codes. The Thermo-Calc software (TC) uses the CALPHAD approach. Thermo-Calc calculations were performed with TC v. 2015b, using TCFE8 database. TCFE covers the assessments of many important binary and ternary systems, as well as the iron-rich corner of some higher order systems, within the 28-element framework. It can be used with satisfactory results for a range of different alloy types: e.g. stainless steels, tool steels, cast iron, etc..[15]
The InterDendritic Solidification software (IDS) is thermodynamic–kinetic–empirical tool. IDS includes two main modules, the IDS module and the ADC module. IDS module simulates the solidification phenomena from liquid down to 1000 °C and ADC the austenite decomposition down to room temperature. Both modules have their own recommended composition ranges shown in Table 3. The IDS module is based on the so-called sharp interface concept. The ADC is mainly statistical based on empirical CCT (Continuous Cooling Transformation) diagrams. IDS is valid for simulation of the solidification of low-alloyed steels and stainless steels.[16]
5 Results and Discussion
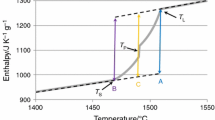
Examples of original DTA curves that were used for the phase transformation temperatures determination are shown in the Fig. 1. For accurate evaluation, it was necessary to use extrapolation and numerical derivation. These methods were particularly necessary for the TA method, where the phenomena are generally less clear on the curve.
Heating curves (TAH, Fig. 2) and cooling curves (TAC, Fig. 3) provided in some cases substantial differences of results. Therefore the results of direct thermal analysis were discussed separately. Also, the direct thermal analysis was not used for study of samples 1 and 2. As a default, comparison of experimental methods and calculations is conducted using only steels 3 and 4. Statistic evaluation of obtained experimental results was performed by mean value, standard deviation and variation coefficient. All measurements, in general, show high level of consistency and low level of variability (Table 4).
The values obtained by thermal analysis measurements were determined based on standardized methodology. For this work, it can be stated that measured results are the most accurate to the real phase transformation temperatures of studied steels. Therefore to determine final phase transformation temperatures, the mean values were calculated from DTA, TAH and TAC results. This mean values of thermal analysis results are determined as final valid real phase transformation temperatures.
The calculations were performed using empirical models and software. The Table 5 comprehends results of 13 models for liquidus temperature, 1 model for peritectic transformation temperature and 6 models for solidus temperature. The evaluation of empiric models was challenging due to the relatively high amount of results the empirical models provided. Therefore, the mean values were calculated for each phase transformation temperature, using all the empiric models presented in in the Table 2. Also, the standard deviations and variation coefficients were calculated, results are presented in the Table 6. For further comparisons, the abbreviation EMP will be used to stand for mean value calculated out of empiric models.
The software calculations were performed using Thermo-Calc (TC) v. 2015b with TCFE8 database and InterDendritic Solidification software (IDS). The results are shown in the Table 7. All elements shown in the Table 1 were included to the calculations except Sn, As, Sb and O. Sn, As and Sb are not defined in the databases of both software codes. Also, the three elements, in such low amount, would have had insignificant impact on the calculation results.
For Thermo-Calc calculations all phases and components were allowed for calculations. The software developers recommend to exclude only the phases and components that certainly cannot be created at given conditions. However, after detailed investigation it was concluded, that no phase or component restriction has impact on the calculation results, with exception of main phases and components (FCC, BCC, Liquid, Cementite, etc.).
The implementation of oxygen in the calculations was not successful. The oxygen caused instability of the calculation. The software IDS always crashed and had to be rebooted. Thermo-Calc either crashed or finished calculation when the temperature reached 1300–1350 °C region. However the results of TS temperature obtained from such a finished calculations were showing significant error (over 100 °C).
Regardless, relevant issue was not found in the available publications. Furthermore, extensive testing was conducted, where various concentrations of oxygen were used, or phase and compositions allowed. The stability of the software was tested on multiple platforms (different hardware and operational systems) unsuccessfully.
The issue is being further investigated. However, the oxygen content in presented steel grades is very low and insignificant influence on calculated phase transformation temperatures is expected.
5.1 Liquidus Temperature
The experimental and calculated results of liquidus temperature are considerably more consistent compared to solidus and peritectic transformation temperatures.
The results of measurement are reliable, with standard deviation close to zero and variation coefficient below 0.05%. The average difference between DTA and TAH (considering only steels 3 and 4) is within ± 5 °C and the average difference between DTA and TAC is within ± 1 °C. This is particularly interesting given the fact that the DTA method has been evaluated only during heating. Therefore, there is a greater consistency between heating and cooling results than heating–heating results. The determined liquidus temperatures are for steel 1 TL = 1493 °C, steel 2 TL = 1490 °C, steel 3 TL = 1500 °C, steel 4 TL = 1477 °C.
The empirical models show good maximal standard deviation 7 °C in case of steel 4. The difference between the mean value of empirical models (EMP) and the DTA results (DTA–EMP) is 4 °C, TAH–EMP is 3 °C and TAC–EMP is 3 °C. This is very good conformity of empirical models with experiments, considering the different nature of each empirical model.
It is difficult to recommend the best one model for TL determination. However, L8 and L12 seems the most reliable. On the other hand, the L9 is not suitable for used steel grades with relatively high deviation.
The software TC and IDS show difference from each other 7 °C. Considering different approaches of the software, it is very good compliance between software but also if comparing software to experimental results. It is not possible to determine better software, although the IDS software show slightly better results compared to TC.
It is interesting to notice that steel grade 2 show highest overall deviations for both empiric models and software. It is exceptionally difficult to include all the effects and interactions of various elements (or not existing interactions in case of model steels) in a single computational model.
Although the deviations (from experimental results) of separate empirical model varies from 1–9 °C, the TL deviation of empirical models is similar software deviations. This is inconsistent with presumption of publication[16] that empirical models can be applied only to relatively simple and specific systems. Steel grades 1–4 are laboratory and commercial steel grades, with various element concentrations and yet the results of empirical models mean value is consistent. It is therefore assumed, that use of several empirical models for TL calculations provides solid robustness of results.
5.2 Peritectic Transition Temperature
The average difference between DTA and TAH (steel 3 and 4) is within ± 2 °C and average difference between DTA and TAC is within ± 25 °C. The overall consistency of results is good. The highest average standard deviation (8 °C) and variation coefficient (0.59%) presents the TAC method. Compared to DTA (1 °C) and TAH (0 °C), the deviation of TAC method is relatively high and suggest issue with reproducibility of results. Also, in a peritectic reaction kinetics play an important role.[36] The literature[37,38] suggests that nucleation in cooling regime is energetically more demanding rather than during heating. The issue of nucleation of primary solid phase exists in this case from melt to delta ferrite. This can lead to minor distortion of the results. The determined peritectic transformation temperatures are for steel 1 TP = 1478 °C, steel 2 TP = 1478 °C, steel 3 TP = 1476 °C, steel 4 TP = 1432 °C.
Only 1 empirical model for calculation of peritectic transformation temperature was used. Relatively low conformity with experiments has been obtained, particularly in case of TAC. Considering the deviation from experimental results, the empirical model is not suitable for determination of peritectic transformation temperature of analysed steel grades. It can be used only for the approximation of peritectic transformation temperatures.
The software TC and IDS show difference from each other 2 °C. Excluding TAC deviations, the results are good not only with very good compliance between software but also if comparing software to experimental results. Compared to TL results, in case of peritectic transformation temperature it is recommended in this case to use software preferably to empirical model. As well as in TL case, it is not possible to determine better software, although the IDS software shows slightly lower deviations compared to TC.
5.3 Solidus Temperature
The average difference between DTA and TAH is within ± 5 °C and average difference between DTA and TAC is within ± 8 °C. As well as TP results, the overall consistency of results is good. The highest standard deviation (8 °C) and variation coefficient (0.56%) show TAC method. It can be seen that some variability between obtained phase transformation temperatures exists.
Determination of the start of melting by both methods (DTA and TA), in the case of investigated steel grades, is strongly depended on deflection of the base line. Therefore it was difficult to determine the initiation of melting. In case of cooling, the temperature of solidus was strongly dependant on the creation (nucleation) of secondary phase (austenite), and temperature of solidus can be more affected by nucleation process (shifted towards lower values).[39]
Compared to DTA (1 °C) and TAH (4 °C), the TAC method is charged with relatively high deviations connected with high probability with experimental arrangement alone. The determined solidus temperatures are for steel 1 TS = 1425 °C, steel 2 TS = 1430 °C, steel 3 TS = 1449 °C, steel 4 TS = 1404 °C.
The standard deviation of empirical models is several times higher compared to TL models. It is obvious that empiric models are not consistent, the calculated results are not comparable. Only 6 models are used for TS calculations compared to 13 models for TL, however such variability is not acceptable for practical use. Mean value of empirical models compared to DTA (DTA–EMP) is 12 °C, TAH–EMP 9 °C and TAC–EMP 7 °C. This is relatively good conformity with experiments and above expectations considering high standard deviations of empirical models.
TS results calculated by empirical models show orderly higher deviations from experimental values and cannot be recommended for TS determination separately. However, the S2 equation provided the best results compared to all three experimental results, with deviation approximately half of the second lowest deviation (average deviation under 10 °C). Nevertheless, the deviation from experimental results is relatively high for steels 1–4 and therefore use of empirical models is not recommended for TS determination. Although it can be used for solid estimations.
Considering high deviations of separate models, the average results showed good compliance with measurement results. This confirms the premise mentioned above, that more empirical models provides robustness of the calculations, mitigating impact of systematic and random errors in separate models. This behaviour should be further studied in followed up research. The software show considerably better compliance with experimental results than empirical models and it is recommended for TS determination. The software TC show better conformity of results compared to IDS, so on the contrary to TL and TP, usage of TC is preferable.
One thing that needs to be highlighted is, that all the comparisons were conducted only on steel 3 and 4, because steel 1 and 2 was not analysed by the TAH and TAC methods. In general, liquidus temperature is easier to evaluate and calculate, and most models reflect that. Contrarily solidus temperature is highly dependent on the data and particularly on kinetics as the segregation of elements and their combination in an alloy. Therefore, there have been found extreme deviations in case of solidus temperatures of software-experimental results of steel 1 and 2, exceeding 100 °C.
The issue could be caused by the fact, that software calculations are not including kinetics and the measurements and the interpretation of the curves have their own issues. Moreover, some authors[32] state that the microstructure (phases) may also play a role on the solidus measurement. The steel 2 showed in general highest calculated—experimental deviations, but the increase is more or less similar for all phase transformation temperatures.
The difference between experimental methods is caused mainly due to the arrangement of the equipment alone, sample mass and sensitivity of the used sensors. The TA heating and cooling curve is also affected by inhomogeneous temperature field, release and absorption of latent heat during ongoing phase transition, possible decarburisation, and contact of sample with sensor or crucible. Furthermore, the evaluation of obtained curves can be difficult in cases, where heat effects overlap or there is not sharp deviation from the base line. Also faster cooling because of a smaller sample could alter the solidification behaviour of the steel, affecting the undercooling.
6 Conclusions
The average difference between DTA results and TAH results is higher than DTA results and TAC results. Considering the fact that the DTA method has been evaluated only during heating, there is a greater consistency between heating - cooling results than heating–heating results.
Steel 2 shows the highest overall deviations for both empiric models and software. The deviation is several times higher than rest of the steel grades. It is exceptionally difficult to include all the effects and interactions of various elements (or not existing interactions) in a single computational model.
Some empirical models provide results with deviations far exceeding expectations and possibilities of both software. Thus, it can be argued that empirical equations are an interesting low-cost alternative for calculating phase transformation temperatures.
Although the deviations of separate empirical models varies from experimental results, the phase transformation temperatures determined by mean value of group of empirical models is consistent with deviations software provides. It is therefore assumed, that use of several empirical models for phase transformation temperatures calculations provides solid robustness of results. This behaviour should be further studied in followed up research.
Compared to the measured values, the theoretical calculations by IDS software provided slightly more consistent results than TC results. TC and IDS software are providing good calculation results, except there have been found extreme deviations of software-experimental results of model steel 1 and 2 in case of solidus temperatures. The issue was not explained. In general, the software are reliable tool for verification of measured data. However it is always vital to check the calculated data with an experiment.
All experimental values, in general, show high level of consistency and low level of variability. It was shown that both thermo-analytical methods used are set correctly; the results are reproducible, comparable and close to the equilibrium. Obtained experimental temperatures by the thermal analysis can be used to optimize production and processing of analysed steel grades.
References
V.I. Lakshmanan, R. Roy, and M.A. Halim, Innovative Process for the Production of Titanium Dioxide, Innovative Process Development in Metallurgical Industry, 2016, p. 359-383
E. Karakaya, C. Nuur, and L. Assbring, Potential Transitions in the Iron and Steel Industry in Sweden: Towards a Hydrogen-Based Future?, J. Clean. Prod., 2018, 195, p 651-663
E. Pereloma, Phase Transformations in Steels: Diffusionless Transformations, High Strength Steels, Modelling and Advanced Analytical Techniques, Woodhead Publishing, 2012, 53
K. Gryc, B. Smetana, M. Tkadlečková, M. Žaludová, K. Michalek, P. Machovčák, L. Socha, J. Dobrovská, and K. Janiszewski, Determination of Solidus and Liquidus Temperatures for S34MnV Steel Grade by Thermal Analysis and Calculations, Metalurgija, 2014, 53(3), p 295-298
A. Tiwari, and B. Raj, Reactions and Mechanisms in Thermal Analysis of Advanced Materials, Scrivener Publishing, 2015
A. Hoffmann, and W.R. Sponholz, Direct Thermal Analysis of Solids: A Fast Method for the Determination of Halogenated Phenols and Anisols in Cork, 2004
A.Ş. Hakan, E. Erişir, and S. Gümüş, Modeling and Thermal Analysis of Solidification in a Low Alloy Steel, J. Therm. Anal. Calorim., 2013, 114(1), p 179-183
R. Ferro and A. Saccone, Thermal Analysis and Alloy Phase Diagrams, Thermochim. Acta, 2004, 418(1-2), p 23-32
I. Steinbach, B. Böttger, J. Eiken, N. Warnken, and S.G. Fries, CALPHAD and Phase-Field Modeling: A Successful Liaison, J. Phase Equilibria Diffus., 2007, 28(1), p 101-106
O. Martiník, B. Smetana, J. Dobrovská, A. Kalup, S. Zlá, M. Kawuloková, K. Gryc, P. Dostál, Ľ. Drozdová, and B. Baudišová, Prediction and Measurement of Selected Phase Transformation Temperatures of Steels, J. Min. Metall. Sect. B: Metall., 2017, 53(3), p 391-398
N. Saunders, and P. Miodownik, CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide, 1998, 1
I. Steinbach, Phase-field models in materials science, Modelling and Simulation in Materials Science and Engineering. 2009, 17(7)
A. Kroupa, Modelling of Phase Diagrams and Thermodynamic Properties Using Calphad Method: Development of Thermodynamic Databases, Comput. Mater. Sci., 2013, 66, p 3-13
T. Hatakeyama and Z. Liu, Handbook of Thermal Analysis, Wiley, London, 1998
J.O. Andersson, T. Helander, L. Höglund, P. Shi, and B. Sundman, Thermo-Calc & DICTRA, Computational Tools for Materials Science, Calphad, 2002, 26(2), p 273-312
J. Miettinen, S. Louhenkilpi, H. Kytönen, and J. Laine, IDS: Thermodynamic-Kinetic-Empirical Tool for Modelling of Solidification, Microstructure and Material Properties, Math. Comput. Simul., 2010, 80(7), p 1536-1550
K. Gryc, B. Smetana, M. Žaludová, K. Michalek, P. Klus, M. Tkadlečková, L. Socha, J. Dobrovská, P. Machovčák, L. Válek, R. Pachlopnik, and B. Chmiel, Determination of the Solidus and Liquidus Temperatures of the Real-Steel Grades with Dynamic Thermal-Analysis Methods, Mater. Tehnol., 2013, 47(5), p 569-575
B. Smetana, M. Žaludová, S. Zlá, S. Rosypalová, A. Kalup, J. Dobrovská, K. Michalek, M. Strouhalová, P. Dostál, and L. Válek, Important Aspects of Phase Transformations Temperatures Study of Steels by use oF Thermal Analysis Methods, METAL 2014—23rd International Conference on Metallurgy and Materials, Conference Proceedings, 93-98.
M. Žaludová, B. Smetana, S. Zlá, J. Dobrovská, A. Watson, J. Vontorová, S. Rosypalová, J. Kukutschová, and M. Cagala, Experimental Study of Fe-C-O Based System Above 1000 °C, J. Therm. Anal. Calorim., 2013, 112(1), p 465-471
M. Žaludová, B. Smetana, S. Zlá, J. Dobrovská, V. Vodárek, K. Konečná, V. Matějka, and P. Matějková, Experimental Study of Fe-C-O Based System Below 1000 °C, J. Therm. Anal. Calorim., 2013, 111(2), p 1203-1210
Z. Liu, Y. Kobayashi, and K. Nagai, Effect of Phosphorus on Sulfide Precipitation in Strip Casting Low Carbon Steel, Mater. Trans., 2005, 46(1), p 26-33
X. Wang, X. Wang, B. Wang, B. Wang, and Q. Liu, Differential Calculation Model for Liquidus Temperature of Steel, Steel Res. Int., 2011, 82(3), p 164-168
J.M. Cabrera-Marrero, V. Carreno-Galindo, R.D. Morales, and F. Chavez-Alcala, Macro-Micro Modeling of the Dentritic Microstructure of Steel Billets Processes by continuous Casting, ISIJ Int., 1998, 38(8), p 812-821
Z. Han, K. Cai, and B. Liu, Prediction and Analysis on Formation of Internal Cracks, ISIJ Int., 2001, 41(12), p 1473-1480
D. Kalisz and S. Rzadkosz, Modeling of the Formation of AlN Precipitates During Solidification of Steel, Archives Foundry Eng., 2013, 13(1), p 63-68
J. Bažan, O. Salva, Z. Michalík, and J. Milatová, Calculation of the Melting and Solidification Temperatures of Steels, Sborník vědeckých prací Vysoké školy báňské v Ostravě, 1993, 39(1), p 19-26
R. Diederichs and W. Bleck, Modelling of Manganese Sulphide Formation During Solidification, Part I: Description of MnS Formation Parameters, Steel Res. Int., 2006, 77(3), p 202-209
R.J. Fruehan, The Making, shaping, and Treating of Steel, AISE Steel Foundation, 1998
Q. Liu, X. Zhang, B. Wang, and B. Wang, Control Technology of Solidification and Cooling in the Process of Continuous Casting of Steel, in Science and Technology of Casting Processes, 2012, p 169-204.
M. Wolf, Proceedings of Concast Metallurgical Seminar, 1982, 1
E. Kivineva, and N. Suutuala, Ruostumattomien terästen likviduslämpötilojen riippuvüüs koostumuksesta, 1987, 87, p 5397-109
J.P. Aymard and P. Détrez, Fonderie, 1974, 330, p 11-24
T. Elbel, Výpočet intervalu teplot tuhnutí u uhlíkových a nízkolegovaných ocelí, Slévárenství, 1980, 28, p 318
Unpublished information from the industrial partner (2018)
R. Sarkar, A. Sengupta, V. Kumar, and S.K. Choudhary, Effects of Alloying Elements on the Ferrite Potential of Peritectic and Ultra-Low Carbon Steels, ISIJ Int., 2015, 55(4), p 781-790
J. Štětina, Dynamický model teplotního pole plynule odlévané bramy, Dissertation thesis, Faculty of Metallurgy and Materials Engineering, VŠB-Technical University of Ostrava.Ostrava, 2007
A.A. Howe and J. Miettinen, Estimation of Liquidus Temperatures for Steels Using Thermodynamic Approach, Ironmak. Steelmak., 2000, 27(3), p 212-227
A. Kalup, B. Smetana, M. Kawuloková, S. Zlá, H. Francová, P. Dostál, and J. Dobrovská, Liquidus and Solidus Temperatures and Latent Heats of Melting of Steels, J. Therm. Anal. Calorim., 2017, 127(1), p 123-128. https://doi.org/10.1007/s10973-016-5942-4
Q. Wu, J. Wang, Y. Gu, Y. Guo, G. Xu, and Y. Cui, Experimental Diffusion Research on BCC Ti-Al-Sn Ternary Alloys. J. Phase Equilib. Diffus. 2018, p 1-7
Acknowledgments
This work was supported by GAČR Project No. 17-18668S, TAČR Project No. TA03011277, student Project SP2018/93, and the “Support of talented students of doctoral studies at VŠB-TUO” Project No. 04766/2017/RRC.
Author information
Authors and Affiliations
Corresponding author
Additional information
This invited article is part of a special issue of the Journal of Phase Equilibria and Diffusion in honor of Prof. Jan Vrestal’s 80th birthday. This special issue was organized by Prof. Andrew Watson, Coventry University, and Dr. Ales Kroupa, Institute of Physics of Materials, Brno, Czech Republic.
Rights and permissions
About this article
Cite this article
Martiník, O., Smetana, B., Dobrovská, J. et al. Experimental and Theoretical Assessment of Liquidus, Peritectic Transformation, and Solidus Temperatures of Laboratory and Commercial Steel Grades. J. Phase Equilib. Diffus. 40, 93–103 (2019). https://doi.org/10.1007/s11669-019-00707-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-019-00707-1