Abstract
This work deals with determining temperatures of phase transformations in steel S34MnV in a low-temperature region (below 900 °C). Although S34MnV is a significant tool steel, in the literature, there are only a few works dealing with the study of the thermo-physical properties of this steel. For the study of phase transformation temperatures of steel S34MnV, a differential thermal analysis and dilatometry were used in this study. Both methods are used to determine the phase transformation temperatures of steel. Dilatometry, however, unlike differential thermal analysis, is commonly used to determine the temperature of nonequilibrium phase transformations during cooling. Temperatures of the eutectoid phase transformation (A c1) and temperatures of the end of the ferrite to austenite transformation (A c3) were obtained at heating, and temperatures of the start of the ferrite formation (A r3), the temperature of the start of the pearlite formation (A r1) and the temperature of the start of the bainite formation (B S) were obtained at cooling using these methods. The temperatures obtained using the both methods were compared and discussed. The original thermo-physical data on steel S34MnV were obtained under precisely defined conditions. For the complexity of the study of the steel, a metallographic analysis of samples was also conducted after thermal analysis, which enables determining the phases occurring in the final structure and their quantity. The experimentally obtained data were compared with data calculated by the software QTSteel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phase transformations, occurring in steel during its cooling, are one of the crucial factors that most influence final steel properties. In particular, the transformations of austenite, at different cooling rates and a different degree of undercooling, have great practical importance for the heat treatment of steel [1]. Individual kinds of transformations (peritectic, bainitic, martensitic) differ in the course and products of decomposition of the austenite. Thereby they provide heat-treated steels with various properties. Very often the austenite gradually passes through a series of transformations of a different kind. Transformations diagrams continuous cooling transformation (CCT) and time temperature transformation (TTT) diagrams provide a summary of mutual relationships of undercooling austenite transformations. Diagrams are valid for only one steel (the specific chemical composition) and the specific conditions of austenitization. For this reason, it is difficult to find a diagram (temperatures of phase transformation) in the literature for the particular steel and the required conditions [2].
One possibility of the experimental determination of these diagrams is the use of dilatometry. [3–5]. Dilatometric analysis is commonly used for the experimental study of austenite transformation [6, 7]. An important tool for studying the transformation of austenite is the so-called quenching dilatometers. These dilatometers allow us to study the disintegration of austenite at high cooling rates that correspond to real conditions in the production and subsequent heat/thermo-mechanical treatment of steels. Another advantage of dilatometry is the use of relatively large samples (of the order of grams). In such large samples, the results are not affected by any decarburization of the steel during the experiment. The disadvantage, however, may be a nonuniform temperature field in the samples.
Differential thermal analysis (DTA) commonly used for study of thermo-physical properties of steel also [8–10], e.g. the temperature of phase transformation and latent heat. Differential thermal analysis is used for studying the transformation of austenite during cooling only exceptionally unlike dilatometry. In fact, when using conventional devices, it is not possible to achieve a high cooling rate. Also, the samples used in the DTA analysis are smaller (in the order of tenths of a gram). In such small samples, the decarburization of the steel may occur during analysis, which can influence the results considerably [11]. The advantage of using small samples, however, is achieving a homogeneous temperature field in the samples.
Given the difficulty in experimentally determining the temperatures of phase transformations, computational software such as QTSteel [12], IDS [13], JMatPro [14] is often used to obtain the thermo-physical properties of steels (temperatures of phase transformations, construction of CCT and TTT diagrams). Compared with the experimental determination, using computational software represents a quick and relatively inexpensive way to get data for a particular steel. However, computational software uses various simplifications and the obtained results do not necessarily reflect real values.
This paper deals with the determination of phase transformation temperatures of steel S34MnV during its heating and cooling in the low-temperature region (below 900 °C). Steel S34MnV is a significant tool steel used in the manufacture of crankshafts. Nevertheless, there is not a lot of data in the literature describing the critical temperatures of the steel under various conditions [15].
Temperatures of phase transformation were determined using differential thermal analysis [16] (method exceptionally used for the determination of austenite transformation temperature) and dilatometry [17] subsequently. Original experimental data for real steel and concrete conditions were obtained.
Experimental data (phase transformations temperatures) of steels are one of the crucial thermo-physical parameters used for process behaviour prediction in many applications (thermal and mechanical treatment of steels) [18]. Phase transformations temperatures are input variables for many thermodynamical and kinetic programmes.
Theoretical calculation
At present, several software programs exist which enable the calculation of phase transformations temperatures of steel. Compared to experimental determination, using computational software represents a quick and inexpensive way to obtain the necessary transformation temperatures of steel. With these software programs, it is possible to calculate the equilibrium phase transformations temperatures (e.g. ThermoCalc [19]) as well as temperatures at different conditions (nonequilibrium) of heating or cooling (e.g. QTSteel [12], IDS [13], JMatPro [14]). In this paper, the software QTSteel, for the calculation of phase transformation temperatures and the phase composition of steel, was used.
QTSteel
The software QTSteel is designated for the calculations of shares of the secondary structural components, arising from the decomposition of the cooled austenite, and the final mechanical properties of heat-treated carbon, alloy and tool steels [3].
Based on entering the input data (the chemical composition of steel, temperature and time of austenitizing treatment, the type of the body and its starting temperature, conditions of cooling), the software QTSteel calculates and creates images of cooling curves of the selected body. Besides that, it also determines the decomposition CCT diagrams for the given steel. Based on the interaction between the cooling curve and the decomposition diagram, the software determines the resulting microstructure and mechanical properties of the investigated steel.
The calculated model of the CCT diagram consists of the set of lines (significant temperatures A c3, A c1, M S) and curves specified by time and the temperature coordinates of their significant points (nose, upper and lower branch). The position of lines and curves in the CCT diagram for specified chemistry was acquired from published diagrams processed separately for each steel group with the use of regression analysis [12].
Experimental
Samples characterization
The steel S34MnV is intended for production of crankshaft [20]. Samples for analysis were mechanically cut from the heavy steel forging ingot. The chemical composition of the analysed sample of steel is given in Table 1. The samples for DTA analysis were processed into the form of cylinders with a diameter of 3.5 mm and a height of approx. 3 mm. The mass of the cylinders was 230 ± 5 mg. The samples for dilatometry were processed into the form of cylinders with a diameter of 6 mm and a height of approx. 17 mm. The mass of the cylinders was approx. 2500 ± 10 mg. Dimensions of samples for both methods are summarized in Fig. 1.
The samples were polished (removal of possible oxidation layer) and cleaned by ultrasonic impact in acetone before analysis.
Experimental conditions
Setaram SETSYS 18TM (TG/DTA/DSC) laboratory system for thermal analysis and DTA “S” type (thermocouple Pt/PtRh 10 %) measuring rod were used for the obtaining of phase transformations temperatures by the DTA method. The samples were analysed in corundum crucibles with a volume of 100 µl. An empty corundum crucible served as a reference sample. A dynamic atmosphere of argon was maintained in the furnace during analysis in order to protect the sample against oxidation. The purity of argon was higher than 99.9999 %. Steel samples were heated up to the temperature of 880 °C with a rate of 5 °C min−1 and held for 15 min and then cooled down with a rate of 5 or 20 °C min−1 to ambient temperature.
Dilatometric measurement was carried out on the dilatometer DIL 402 C. The type “B” (PtRh 6 %/PtRh 30 %) thermocouple was used for measuring of the sample temperature. Dilatometry of the investigated samples was carried out in a protective inert atmosphere of argon with purity 99.9999 %. Steel samples were analysed according to the same temperature programme as in differential thermal analysis.
Results and discussion
Heating
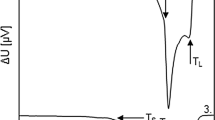
Temperatures of phase transformations were obtained on the basis of DTA curves (Fig. 2) evaluation. Figure 2 shows the DTA curve obtained for the analysed steel at the heating rate of 5 °C min−1. Two overlapping thermal effects (peaks) are observed on the DTA curve. The first peak (bold thermal effect) corresponds to eutectoid phase transformation (α + Fe3C → γ). The temperature of eutectoid phase transformation corresponds to the temperature of the start of austenite formation. This temperature is marked as A c1 (Fig. 2).
On the basis of the characteristic form of the curve before the start of the peak, it is possible to assume that part of the peak was the peak, corresponding rather to the Curie transformation (a complete overlapping of these two peaks took place). Due to overlapping of the peaks, it is, however, impossible to exactly determine the temperature of the Curie transformation. For this reason, the temperature was not evaluated.
The second peak (featureless thermal effect) corresponds to the ferrite to austenite transformation. Ferrite starts transform to austenite at the temperature of eutectoid phase transformations (A c1). The temperature of the second peak top corresponds to the temperature of the end of the ferrite to austenite transformation. The temperature of the end of the ferrite to austenite transformation is marked as A c3 (Fig. 2).
Temperatures A c1 and A c3 were also obtained on the basis of dilatometric curves (Fig. 3) evaluation for comparison. The transformation temperatures were determined with the assistance of the first derived function. The characteristic transformation temperatures for heating are given in the Table 2.
It is possible to consider the acquired temperatures A c1 and A c3 as the equilibrium due to low heating rate (5 °C min−1) [21].
In the case of temperature A c1, a very good agreement between the temperatures determined by both methods was achieved, which is evident from Table 2. The maximal temperature difference is 2 °C.
The biggest temperature difference (17 °C) was observed in the case of temperature A c3. Temperature A c3 provided by DTA is 765 °C and by dilatometry is 782 °C.
This difference can be caused by the difficult evaluation of the temperature on DTA and dilatometric curves or the various sizes of the analysed samples. Samples analysed by dilatometry are ca. ten times bigger than samples analysed by DTA. (The larger sample requires more heat for the phase transformation, and that the phase transformation takes place over a longer period of time, i.e. that the phase transformation of the larger sample will be terminated at higher temperature).
Cooling
Various phase transformations may occur in samples during cooling depending on the cooling rate (ferrite, pearlite, bainite and martensite formation). The different transformations that occur within similar temperatures can often overlap in the thermoanalytical curves (DTA and dilatometric curves). This evaluation of thermoanalytical curves then becomes difficult. It is difficult to assign the effect of the appropriate phase transformation solely on the basis of the shape of thermoanalytical curves.
Microstructural characterization on samples after thermal analysis (at different cooling rates) helped to better interpret effects on thermoanalytical curves. All samples after analysis were prepared by metallographic techniques and microstructures by optical microscopy were observed.
Only the ferrite–pearlite structure was observed in the sample after DTA analysis at a cooling rate of 5 °C min−1 (Fig. 4). The presence of ferrite, pearlite and bainite was observed in all other samples (after DTA at a cooling rate of 20 °C min−1 and after dilatometry at a cooling rate of 5 and 20 °C min−1). This type of structure is shown in Fig. 5 (the structure of sample after DTA at a cooling rate of 20 °C min−1). The bainite morphology for the individual specimens did not differ, and it was always the upper bainite. The amount of each phase was also determined using metallographic analysis. The amount of each phase in samples is given in Table 3 in vol%. (Due to the structure in homogeneity, the results exhibit a higher standard of deviation in some cases). Although the samples were analysed using both methods at the same temperature conditions, the presence and the amount of phases differ significantly in the samples after analysis.
DTA curves obtained at cooling and their evaluation are shown in Fig. 6. Figure 7 shows dilatometric curves for cooling. The following phase transformations temperatures were evaluated on the basis of the thermoanalytical curves shape and on the basis of metallographic analysis: the start of ferrite formation A r3, the start of pearlite formation A r1 and the start of bainite formation B S. Although the microstructure of the sample after dilatometry at a cooling rate of 5 °C min−1 appears bainite, bainitic transformation on the dilatometric curve was not detected. The resulting temperatures are given in Table 4.
The temperature A r3 determined by the DTA method at a cooling rate of 5 °C min−1 is lower by 3 °C than the temperature determined by dilatometry at the same cooling rate. On the contrary, the temperature A r3 determined at a cooling rate of 20 °C min−1 is 5° C higher than the temperature determined by dilatometry.
The temperature A r1 determined by the DTA method at a cooling rate of 5 °C min−1 is 6 °C lower and at a cooling rate of 20 °C min−1 is 7 °C higher than the temperature determined by dilatometry.
The biggest difference (18 °C) was observed in the case of the bainite start temperature. This difference may be caused by the complicated determining of the bainite start temperature (B S) on thermoanalytical curves.
Comparison with accessible data
As mentioned above, there are not many works in the literature dealing with the critical temperatures of S34MnV steel. In [15], the authors present an experimentally determined CCT diagram of steel S34MnV. In the work, the authors do not specify the experimental conditions (method, sample size), under which the results were obtained. They only state the austenitizing temperature of 950 °C and a holding time of 15 min.
It was determined that the A c1 temperature was 735 °C and the A c3 temperature 825 °C. The A c1 temperature is close to the experimentally determined temperatures. The difference is less than 10 °C. However, the given A c3 temperature is significantly different from the experimentally determined temperature. The minimum difference is 40 °C and the maximum 60 °C.
The temperature readings from the CCT diagram shown in [15] are summarized in Table 5. The temperatures stated by the authors in [15] are higher than the experimentally determined temperatures. It varies in the tens of °C.
Significant differences between the temperatures specified in [15] and determined by differential thermal analysis and dilatometry may be caused by differing austenitizing conditions, different chemical composition (even small changes in chemical composition can significantly affect the results) or the difficult reading of the critical temperatures from the diagram. These large differences illustrate how difficult it is to find the thermodynamical data for the specific chemical composition and the required conditions.
Experimentally obtained temperatures of phase transformations and the amount of phases were compared with the data calculated by the software QTSteel also.
For the calculation, the same conditions as in the experiments were entered. Chemical composition, isothermal holding at temperature 880 °C for 15 min and cooling rates 5 and 20 °C min−1 were entered.
The calculated temperatures A c1 and A c3 are given in Table 6. The software does not state for which heating rate the temperatures A c1 and A c3 were calculated. The software QTSteel calculated the temperature A c1 as 717 °C. This temperature is about 9–11 °C lower than the temperature determined experimentally by DTA and dilatometry. The calculated temperature A c3 was 784 °C. This temperature corresponds to the temperature obtained by dilatometry and is 19 °C higher than the temperature obtained by DTA.
Using the software QTSteel, a CCT diagram (continuous cooling transformation) was also constructed, phase transformations temperatures for cooling were calculated and amount of the secondary structural components arising from decomposition of the cooled austenite was calculated as well. The calculated CCT diagram is shown in Fig. 8. The calculated phase transformations temperatures for cooling are given in Table 7. The calculated amount of the individual phases is listed in Table 8.
Neither of the calculated cooling curves pass through the bainitic region (Fig. 8). However, it can be observed in the CCT diagram that the curve calculated for the cooling rate 20 °C min−1 is very close to the bainite region. (The lowest calculated cooling rate at which bainite will arise is 22 °C min−1). For this reason, the bainite is not in the calculated resulting structures (Table 8) and the temperature of the bainite formation (B S) is not calculated.
Temperatures A r3 and A r1 calculated by the software QTSteel for cooling rate 5 °C min−1 are lower than experimentally obtained temperatures (comparison of Tables 4, 7). The difference between the calculated and experimentally obtained A r3 temperature is 13 °C (DTA) resp. 16 °C (dilatometry). The calculated temperature A r1 is lower by 15 °C (DTA) resp. 21 °C (dilatometry). In addition, the temperatures calculated by the software for the cooling rate 20 °C min−1 are lower than the experimentally obtained temperatures. The calculated temperature A r3 is lower by 10 °C (DTA) resp. 5 °C (dilatometry), and temperature A r1 is lower by 15 °C (DTA) resp. 8 °C (dilatometry). Generally the calculated temperatures A r3 and A r1 are in relatively good agreement with the experimentally determined temperature.
The calculated amount of phases in the resulting structure differs significantly from the amount of phases determined by the metallographic analysis (comparison of Tables 3, 8).
Conclusions
The steel S34MnV was investigated by the DTA method and dilatometry. The temperatures of the eutectoid phase transformation (A c1) and the temperatures of the end of the ferrite to austenite transformation (A c3) were obtained at heating, and the temperatures of start of the ferrite formation (A r3), the temperature of the start of the pearlite formation (A r1) and the temperature of the start of the bainite formation (B S) were obtained at cooling. Differential thermal analysis is not commonly used for determination of temperatures of nonequilibrium phase transformations during cooling unlike dilatometry. Nevertheless, temperatures determined by both methods are in good agreement.
The experiments were supported by theoretical calculations using the QTSteel software. The temperature of bainite start was not calculated. The other calculated temperatures are lower than experimentally obtained. These results demonstrate that due to their simplicity, the calculations are an effective tool for obtaining the required data, but they can be considered as indicative only. The calculated results should always be verified by the experimental determination.
The phase transformations temperatures of steels are one of the crucial thermo-physical parameters used for process behaviour prediction in many applications (thermal treatment of steels). Phase transformations temperatures are input variables for many thermodynamical and kinetic programmes. Experimental data of phase transformations temperatures can be found in the literature, but it is difficult to find data for concrete steel (with exact chemical composition).
References
Gomez M, Medina SF, Caruana G. Modelling of phase transformation kinetics by correction of dilatometry results for a ferritic Nb-microalloyed steel. ISIJ Int. 2003;. doi:10.2355/isijinternational.43.1228.
Ryš P, Cenek M, Mazanec K, Hrbek A. Material science I., Metal science 4. 1st ed. Praha: Academia; 1975.
Kawulok P, et al. Deformation behaviour of low-alloy steel 42CrMo4 in hot state. In: Proceedings paper, METAL 2011: 20th anniversary international conference on metallurgy and materials, p. 350–356. ISBN 978-80-87294-24-6.
Kawulok R, et al. Effect of deformation on the continuous cooling transformation (CCT) diagram of steel 32CRB4. Metalurgija. 2015;54:473–6.
Gomez M, et al. Phase transformation under continuous cooling conditions in medium carbon microalloyed steels. J Mater Sci Technol. 2014;. doi:10.1016/j.jmst.2014.03.015.
Garcia de Andres C, et al. Application of dilatometric analysis to the study of solid–solid phase transformations in steels. Mater Charact. 2002;48:101–11.
Grajcar A, et al. Dilatometric study of phase transformations in advanced high-strength bainitic steel. J Therm Anal Calorim. 2014;. doi:10.1007/s10973-014-4054-2.
Smetana B, et al. Application of high temperature DTA to microalloyed steels. Metalurgija. 2012;51:121–4.
Atapek SH, et al. Modelling and thermal analysis of solidification in a low alloy steel. J Therm Anal Calorim. 2013;. doi:10.1007/s10973-012-2930-1.
Gojic M, et al. Thermal analysis of low alloy Cr–Mo steel. J Therm Anal Calorim. 2004;. doi:10.1023/B:JTAN.0000027188.58396.03.
Žaludová M, et al. Experimental study of Fe–C–O based system above, 1000 °C. J Therm Anal Calorim. 2013;. doi:10.1007/s10973-012-2847-8.
Šimeček P. Software QTSteel 3.1—user’s manual. 1st ed. Ostrava: ITA s.r.o.; 2010.
Mittinen J. Solidification analysis package for steels—user’s manual of DOS version. 1st ed. Helsinki: University of Technology; 1999.
Prasanthi TN, et al. Explosive cladding and post-weld heat treatment of mild steel and titanium. Mater Des. 2016;. doi:10.1016/j.matdes.2015.12.120.
Sun M, et al. Prediction of mechanical properties and microstructure distribution of normalized large marine crank throw. Adv Mater Processes. 2007;. doi:10.4028/www.scientific.net/AMR.26-28.1037.
Boettinger WJ, et al. DTA and heat-flux DSC measurements of alloy melting and freezing. 1st ed. Washington: National Institute of Standards and Technology; 2006.
Gallagher PK. Handbook of thermal analysis and calorimetry: principles and practice. 2nd ed. Oxford: Elsevier; 2003.
Smetana B, et al. Experimental verification of hematite ingot mould heat capacity and its direct utilisation in simulation of casting process. J Therm Anal Calorim. 2013;. doi:10.1007/s10973-013-2964-z.
ThermoCalc Software TCFE7 Steels/Fe-alloys database version 7. Accessed 23 Aug 2013.
Gryc K, et al. Determination of solidus and liquidus temperatures for S34MnV steel grade by thermal analysis and calculations. Metalurgija. 2014;53:295–8.
Žaludová M, et al. Influence of experimental conditions on data obtained by thermal analysis methods. In: Proceedings paper, METAL 2013: 22nd anniversary international conference on metallurgy and materials, p. 585–591. ISBN 978-80-87294-41-3.
Acknowledgements
This paper was created on the Faculty of Metallurgy and Materials Engineering in the Project No. LO1203 “Regional Materials Science and Technology Centre-Feasibility Program” funded by Ministry of Education, Youth and Sports of the Czech Republic, TAČR projects No. TA04010035 and No. TA03011277 and student project SP2016/90.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawuloková, M., Smetana, B., Zlá, S. et al. Study of equilibrium and nonequilibrium phase transformations temperatures of steel by thermal analysis methods. J Therm Anal Calorim 127, 423–429 (2017). https://doi.org/10.1007/s10973-016-5780-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5780-4