Abstract
The nature of the isothermal region of the Al–Mo–Gd ternary system at 773 K has been established by analysis of quenched samples, annealed for 6 weeks, by use of x-ray powder diffraction and scanning electron microscopy equipped with energy-dispersive analysis. Two ternary phases, Al43Mo4Gd6 and Al4Mo2Gd were observed. Ten binary phases, including Al17Mo4 rather than Al4Mo, were present at 773 K in the Al–Mo system. The ranges of homogeneity of the AlMo3 and Al8Mo3 phases were 7.5 and 1 at.%, respectively. According to results obtained from the disappearing-phase method, the maximum solubility of Al in Mo is approximately 16 at.%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past three decades, Al-based amorphous alloys have attracted much attention, because of their unique combination of ultrahigh strength, excellent corrosion resistance, and high ductility.[1,2] Unfortunately, poor glass-forming ability (GFA) limits commercial application of Al-based amorphous alloys.[3] Alloying additions are widely used to improve GFA of metallic alloys.[4] High glass formability occurs as a result of negative heat of formation of the alloy components, different atomic size ratios of the components, and use of multicomponent systems.[5,6] Al–TM–RE systems have GF potential. First, the three components of Al–RE–TM alloys have negative heats of mixing with each other.[7] Second, bulk metallic glasses (BMG) can be broadly categorized into two types, the major atom–small atom–large atom (MSL) class and the large atom–small atom or small atom–large atom (LS/SL) class. The Al–TM–RE system can be classified as an MSL class with substantially different atomic size ratios.[6] Third, the appropriate composition for formation of solute-centered quasi-equivalent clusters about rare earth (RE) and transition metal (TM) elements is structurally favorable for retarding formation of α-Al and enhancing the resistance to crystallization of supercooled liquids.[8,9] A series of related studies have been reported.[10,11] Yang et al.[12] were the first to successfully design Al-rich (86 at.% Al) bulk metallic glasses by addition of RE and TM in appropriate proportions. Other research[13–15] has shown that Al–TM–RE alloys of appropriate composition containing more than 80% Al have outstanding ductility and exceptional tensile strength.
Addition of RE elements and transition elements also contributes to amelioration of the mechanical behavior and elevated-temperature applications of traditional aluminium alloys. The strengthening occurs as a result of the distribution of intermetallic dispersoids and the formation of high-melting-point compounds.[16,17]
Although Al–TM–Gd phase diagrams at 773 K have been reported for TM = Cr, Ni, Ti, V, and Cu,[18–22] that of Al–Mo–Gd, also a Al–TM–Gd system, has not been reported, although the presence at 773 K of the ternary compounds Al43Mo4Gd6 and Al4Mo2Gd has been reported.[23,24] In the work discussed in this paper, the Al–Mo–Gd ternary system at 773 K was characterized experimentally to investigate the interactions between the elements Al, Mo, and Gd which may be conducive to the design of new types of Al-based amorphous alloys or new Al alloys.
Literature Data
Phase equilibrium data were obtained for each binary phase diagram of the Al–Mo–Gd ternary system. In 2010, Okamoto[25] updated the Al–Mo phase diagram by summarizing previous literature which confirmed the presence of ten intermetallic phases (AlMo3, AlMo, Al63Mo37, Al8Mo3, Al3Mo, Al4Mo, Al17Mo4, Al22Mo5, Al5Mo, and Al12Mo). Schuster and Ipser[26] emphasized three modifications of Al5Mo, denoted Al5Mo (h), Al5Mo (h′) and Al5Mo (r), respectively. According to Eumann et al.,[27] six compounds, AlMo3, Al8Mo3, Al17Mo4, Al22Mo5, Al5Mo, and Al12Mo were stable at moderate temperatures, although Schuster and Ipser[26] did not confirm the presence of Al22Mo5 at 773 K.
Basic data for the Al–Gd system were obtained by Gschneidner and Calderwood,[28] Saccone et al.,[29] and Elliott and Shunk.[30] Five intermetallic phases (AlGd2, Al2Gd3, AlGd, Al2Gd, and Al3Gd) were identified as stable; two unstable phases, Al4Gd [31] and Al17Gd2,[32] have also been reported.
No intermetallic compounds have been reported for the binary Gd–Mo system. Low terminal solubilities have been reported for this system.[33,34]
Only two ternary aluminides have been reported for the Al–Mo–Gd system. Fornasini and Palenzona[24] reported the crystal structure of the Al4Mo2Gd phase in 1976, and the structure of Al43Mo4Gd6 was determined by Niemann and Jeitschko.[23]
Details of these phases, and of the stable phases discovered in this work are listed in Table 1.
Experimental Procedure
All the samples were prepared in an arc-melting furnace. The nominal compositions of the alloys were determined on the basis of the predicted three-phase regions. High-purity metals, aluminium pieces (99.99 wt.%), molybdenum rods (99.9 wt.%), and gadolinium pieces (99.9 wt.%), were used as starting materials to ensure the accuracy of the experiments. One-hundred and seventy samples were prepared. The weight of each sample was 2 g. Electric argon-arc welding with a tungsten electrode was performed with the sample in a water-cooled copper vessel. Each sample was remelted at least five times (weight loss <1%) to ensure compositional uniformity. The samples were then placed in quartz tubes (10 mm diameter), which were sealed under high vacuum, and annealed at 773 K for 6 weeks in an electric resistance furnace. After heat-treatment, the samples were quenched in liquid nitrogen, so the rapid cooling preserved the composition of the material obtained at 773 K. All the equilibrated samples were subjected to x-ray diffraction (XRD) analysis with a Rigaku D/Max 2500 V diffractometer operated at 40 kV and 200 mA with a copper target. XRD data were analyzed by use of Jade 6.5 software and Pearson’s Handbook on Crystallographic Data. Scanning electron microscopy (SEM) with energy-dispersive analysis (EDS) (Hitachi S-3400) was used to examine the microstructure of samples.
Results and Discussion
Binary Phase Analysis
Five binary Al–Mo phases were observed at 773 K: AlMo3, Al8Mo3, Al17Mo4, Al5Mo, and Al12Mo. Five binary Al–Gd phases were observed at 773 K: Al3Gd, Al2Gd, AlGd, Al2Gd3, and AlGd2. No binary Mo-Gd compound was found.
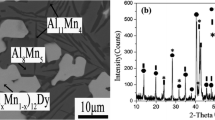
On the basis of the crystallographic data of Grin et al.[35] and Fornasini and Palenzona,[24] XRD patterns of Al17Mo4 and Al4Mo2Gd were identified by the powder cell software. Brewer et al.[36] reported five phases (Al12Mo, Al5Mo, Al4Mo, Al8Mo3, AlMo3) at 773 K. However, the Al4Mo phase was replaced by Al17Mo4 in results published by EuMann et al.[27] and Schuster and Ipser.[26] Moreover, in the study by Potzschke et al.,[37] the Al4Mo phase decomposed below 993 K. In our work we identified five phases, Al12Mo, Al5Mo, Al17Mo4, Al8Mo3, and AlMo3, at 773 K, in good agreement with the results of EuMann et al.[27] and Schuster and Ipser.[26] In Fig. 1(a), black triangles, black squares, and solid black circles are used to mark the peaks of Al8Mo3, Al17Mo4, and Al43Mo6Gd4, respectively. The PDF cards of the other phases, i.e. Al4Mo and Al5Mo, did not match the XRD result. It is obvious that each phase has unique peaks which unequivocally prove the presence of the corresponding phase. When the peaks of relevant phases overlap, these are marked, in order, from high to low in accordance with the diffraction peak intensities on the PDF cards of corresponding phases. The XRD pattern in Fig. 1(a) and the SEM micrograph of the ternary alloy of composition 78 at.% Al, 20 at.%, 2 at.% Gd in Fig. 1(b) indicate the presence of the equilibrium phase Al8Mo3 + Al17Mo4 + Al43Mo4Gd6, which confirms the occurrence of the binary phase Al17Mo4 at 773 K. Moreover, the XRD pattern of the equilibrated alloy containing 82 at.% Al, 15 at.% Mo, and 3 at.% Gd is indicative of the equilibrium of three phases Al5Mo + Al17Mo4 + Al43Mo4Gd6 at 773 K, as shown in Fig. 2. The EDS results for relevant samples are shown in Table 2. The EDS data were acquired by scanning small areas; each measurement was performed three times and the average value was calculated. The EDS results also confirm the presence of Al17Mo4. The uncertainty of some measurements can be attributed to the presence of solid solutions. These still provide effective references for the XRD patterns, however. By analysis of related results, the presence of Al17Mo4, rather than Al4Mo, was confirmed at 773 K.
Ternary Compounds
Two ternary compounds, Al43Mo4Gd6 and Al4Mo2Gd, were detected in this work, in good agreement with the literature. Figure 3(a) shows that the equilibrated sample Al86Mo12Gd2 annealed at 773 K consists of three phases, Al5Mo, Al43Mo4Gd6, and Al12Mo. The microstructure of the sample examined by SEM and EDS clearly indicates the presence of these three phases: the light gray phase is Al5Mo, the white phase is Al43Mo4Gd6, and the dark grey phase is Al12Mo, as indicated in Fig. 3(b). Figure 4 clearly indicates that the Al4Mo2Gd phase is located in the AlMo3 + Al8Mo3 + Al4Mo2Gd three-phase region.
Solid Solubility
Solid solubility in this isothermal region was determined by use of the disappearing-phase method, each experiment being performed in duplicate. Figure 5 shows that the binary compound AlMo3 has a distinct homogeneity range from 21 to 28.5% Al at 773 K. When the aluminium content was outside this range the peak arising from the other phase was observed. In this way the maximum solubility of Al in Al8Mo3 was confirmed to be approximately 1 at.% (approx. 72–73% Al) at 773 K, as shown in Fig. 6; this was consistent with the reported Al–Mo binary phase diagram.[27] The solid solubility of Al in Mo was determined to be approximately 16 at.%.
Isothermal Region
The composition of the isothermal region of the Al–Mo–Gd system at 773 K was determined by analysis of all the samples (Fig. 7). The isothermal region consists of 15 single-phase regions, 29 binary-phase regions, and 15 ternary-phase regions. The solubility of Al in the AlMo3, Al8Mo3, and Mo phases was measured to be approximately 7.5, 1.0, and 16.0 at.% respectively. All the details of three-phase regions of the Al–Mo–Gd ternary system at 773 K are shown in Table 3.
Conclusions
As part of the ternary Al–TM–RE system with excellent potential in GFA and outstanding mechanical properties, the Al–Mo–Gd ternary phase diagram at 773 K was investigated. The results show that the isothermal region consists of 15 single-phase regions, 29 binary-phase regions, and 15 ternary-phase regions. The main results are summarized as follows:
-
1.
The presence of ten binary phases (AlMo3, Al8Mo3, Al17Mo4, Al5Mo, Al12Mo, Al3Gd, Al2Gd, AlGd, Al2Gd3, AlGd2) and two ternary phases (Al43Mo4Gd6, Al4Mo2Gd) was confirmed at 773 K. No binary Mo–Gd phases were found at 773 K.
-
2.
At 773 K, the homogeneity ranges of the AlMo3 and Al8Mo3 phases are 7.5 and 1 at.%, respectively, and the solid solubility of Al in Mo is approximately 16 at.%.
-
3.
The presence at 773 K of the controversial phase Al17Mo4 was confirmed experimentally.
References
Y. He, S.J. Poon, and G.J. Shifle, Synthesis and Properties of Metallic Glasses that Contain Aluminum, Science, 1988, 241(4873), p 1640-1642
A. Inoue, K. Ohtera, A.P. Tsai, and T. Masumoto, Aluminum-Based Amorphous Alloys with Tensile Strength Above 980 MPa (100 kg/mm2), Jpn. J. Appl. Phys., 1988, 27(4), p 479
L.C. Zhou, S.J. Pang, H. Wang, and T. Zhang, Ductile Bulk Aluminum-Based Alloy with Good Glass-Forming Ability and High Strength, Chin. Phys. Lett., 2009, 26(6), p 066402
W.H. Wang, Roles of Minor Additions in Formation and Properties of Bulk Metallic Glasses, Prog. Mater. Sci., 2007, 52(4), p 540-596
A. Inoue, Stabilization of Metallic Supercooled Liquid and Bulk Amorphous Alloys, Acta Mater., 2000, 48(1), p 279-306
S.J. Poon, G.J. Shiflet, F. Guo, and V. Ponnambalam, Glass Formability of Ferrous- and Aluminum-Based Structural Metallic Alloys, J. Non Cryst. Solids, 2003, 317(1), p 1-9
J. Wang, Y. Liu, S. Imhoff, N. Chen, D. Louzguine-Luzgin, A. Takeuchi, M. Chen, H. Kato, J. Perepezko, and A. Inoue, Enhance the Thermal Stability and Glass Forming Ability of Al-Based Metallic Glass by Ca Minor-Alloying, Intermetallics, 2012, 29, p 35-40
Z.P. Chen, J.E. Gao, Y. Wu, H.X. Li, H. Wang, and Z.P. Lu, Role of Rare-Earth Elements in glass Formation of Al-Ca-Ni Amorphous Alloys, J. Alloys Compd., 2012, 513, p 387-392
H. Yang, J. Wang, and Y. Li, Glass Formation and Microstructure Evolution in Al-Ni-RE (RE = La, Ce, Pr, Nd and Misch Metal) Ternary Systems, Philos. Mag., 2007, 87(27), p 4211-4228
K. Song, X. Bian, J. Guo, S. Wang, B.A. Sun, X. Li, and C. Wang, Effects of Ce and Mm Additions on the Glass Forming Ability of Al-Ni-Si Metallic Glass Alloys, J. Alloys Compd., 2007, 440(1), p L8-L12
B. Yang, J. Yao, Y. Chao, J. Wang, and E. Ma, Developing Aluminum-Based Bulk Metallic Glasses, Philos. Mag., 2010, 90(23), p 3215-3231
B.J. Yang, J.H. Yao, J. Zhang, H.W. Yang, J.Q. Wang, and E. Ma, Al-Rich Bulk Metallic Glasses with Plasticity and Ultrahigh Specific Strength, Scripta Mater., 2009, 61(4), p 423-426
R.A. Dunlap, V. Srinivas, G. Beydaghyan, and M.E. McHenry, Magnetic and Thermal Properties of Amorphous Al–Gd-TM (TM = Fe, Cu) Alloys, J. Mater. Sci., 1993, 28(11), p 2893-2897
A. Inoue, K. Ohtera, A.-P. Tsai, and T. Masumoto, New Amorphous Alloys with Good Ductility in Al-YM and Al-La-M (M = Fe Co, Ni or Cu) Systems, Jpn. J. Appl. Phys, 1988, 27(3A), p 280
L. Chaosan, Z. Yongchang, and S. Changxu, New Amorphous Al-La-Y-Ni (Fe) Quaternary Alloys with Superior Strength and Ductility, Acta Metall. Sin., 1993, 6(6), p 442-446
C.B. Fuller, D.N. Seidman, and D.C. Dunand, Mechanical Properties of Al (Sc, Zr) Alloys at Ambient and Elevated Temperatures, Acta Mater., 2003, 51(16), p 4803-4814
O. Sitdikov, T. Sakai, E. Avtokratova, R. Kaibyshev, K. Tsuzaki, and Y. Watanabe, Microstructure Behavior of Al-Mg-Sc Alloy Processed by ECAP at Elevated Temperature, Acta Mater., 2008, 56(4), p 821-834
M. Ling, Y. Liang, S. Wei, Y. Liu, M. Pang, Y. Zhan, and Y. Du, Experimental Investigation of the Al-Cr-Gd Ternary System at 773 K, J. Phase Equilib. Diffus., 2012, 33(3), p 203-209
S. Delsante and G. Borzone, The Gd-Ni-Al System: Phases Formation and Isothermal Sections at 500 °C and 800°C, Intermetallics, 2014, 45, p 71-79
V. Raghavan, Al–Gd-Ti (Aluminum-Gadolinium-Titanium), J. Phase Equilib. Diffus., 2005, 26(2), p 182-183
V. Raghavan, Al–Gd-V (Aluminum-Gadolinium-Vanadium), J. Phase Equilib. Diffus., 2012, 33(1), p 62-63
V. Raghavan, Al-Cu-Gd (Aluminum-Copper-Gadolinium), J. Phase Equilib. Diffus., 2007, 28(6), p 547-548
S. Niemann and W. Jeitschko, Ternary Aluminides A6T4Al43 with A = Y, Nd, Sm, Gd-Lu, Th, U and T = Cr, Mo, W, Z. MetaIlkd., 1994, 85(5), p 345-349
M.L. Fornasini and A. Palenzona, Crystal Structure of the Ternary REMo2Al4 Phases (RE = Gd, Er, Yb), J. Less-Common Met., 1976, 45(1), p 137-141
H. Okamoto, Al–Mo (Aluminum-Molybdenum), J. Phase Equilib. Diffus., 2010, 31(5), p 492-493
J.C. Schuster and H. Ipser, The Al-Al8Mo3 Section of the Binary System Aluminum-Molybdenum, Metall. Trans. A, 1991, 22(8), p 1729-1736
M. Eumann, G. Sauthoff, and M. Palm, Re-evaluation of Phase Equilibria in the Al–Mo System, Z. MetaIlkd., 2006, 97(11), p 1502-1511
K.A. Gschneidner and F.W. Calderwood, The Al–Gd (Aluminum-Gadolinium) System, Bull. Alloy Phase Diagrams, 1988, 9(6), p 680-683
A. Saccone, A.M. Cardinale, S. Delfino, and R. Ferro, Gd-Al and Dy-Al Systems: Phase Equilibria in the 0 to 66.7 at.% Al Composition Range, Z. MetaIlkd., 2000, 91(1), p 17-23
R.P. Elliott and F.A. Shunk, The Al–Gd (Aluminum-Gadolinium) System, Bull. Alloy Phase Diagrams, 1981, 2(2), p 215-217
O.J.C. Runnalls and R.R. Boucher, Phase Equilibria in Aluminium-Rich Alloys of Aluminium-Gadolinium and Aluminium-Terbium, J. Less Common Met., 1967, 13(4), p 431-442
I. Pop, N. Dihoiu, and M. Coldea, Magnetic Behaviour of the Inter metallic System Gd2Ni17-xAlx, Philos. Mag. B, 1979, 39(3), p 245-252
L. Brewer and R.H. Lamoreaux, The Gd-Mo System (Gadolinium-Molybdenum), J. Phase Equilib., 1980, 1(2), p 78-79
M. Zinkevitch, N. Mattern, and K. Wetzig, Experimental Study of Ternary Fe-Gd-Mo Phase Diagram, J. Phase Equilib., 1999, 20(2), p 119-124
Y.N. Grin, M. Ellner, K. Peters, and J.C. Schuster, The Crystal Structures of Mo4Al17 and Mo5Al22, Z. Kristal., 1995, 210(2), p 96-99
L. Brewer, R.H. Lamoreaux, R. Ferro, R. Marazza, and K. Girgis, Molybdenum: Physico-chemical Properties of Its Compounds and Alloys, Vol 7, International Atomic Energy Agency, Vienna, 1980, p 123-127
M. Potzschke and K. Schubert, Towards the Synthesis of Several T4-B3 Homologous and Quasi-Homologous System. II. The Systems Ti-Al, Zr-Al, Hf-Al, Mo-Al and Several Ternary Systems, Z. Metallkd., 1962, 53, p 548-561
Acknowledgments
This research work was jointly supported by the Guangxi Science and Technology Development Project (12118001-2B, 2013AA01013), and the Science and Technology Project of Guangxi Education Department (2013ZL010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H., Pan, Y., Tang, C. et al. Experimental Phase Diagram of the Al–Mo–Gd Ternary System at 773 K. J. Phase Equilib. Diffus. 36, 218–223 (2015). https://doi.org/10.1007/s11669-015-0375-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-015-0375-5