Abstract
The corrosion of copper in 0.25 M sulphuric acid was studied by the addition of aqueous extract of leaves of Morinda tinctoria using weight loss and electrochemical methods. The addition of inhibitor moderately improves the inhibition efficiency. Increase in temperature and acid concentration decreases the performance of the inhibitor. The adsorption of the inhibitor on the copper surface in sulphuric acid medium obeys Freundlich isotherm. The AC impedance studies reveal that the process of inhibition is through charge transfer reaction, and polarization studies indicate the mixed nature of the inhibitor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper exhibits good corrosion resistance properties and hence used in many applications. In spite of the expensive nature of copper involving more capital investment, there is an extended utilization of copper as materials of expression such as constructions, water pipes, in electrical connectors, in decorative roofs, transportation systems, and for pipes in the petroleum and gasoline industries. It is noble to hydrogen in the emf series, and it is thermodynamically inert with no propensity to corrode in water. Among the non-ferrous metals, copper is most often used. Although it exhibits good resistance to corrosion under ordinary conditions, significant metal dissolution arises when exposed to intensive environment. Corrosion can cause a big impact on the safety and dependability of an extremely wide range of articles of commerce, and its economic impact is very great. It takes on a vital function in shaping the life-cycle performance, safety, and cost of engineered products. The successful application of corrosion inhibitor could reduce the cost of corrosion [1–4]. Corrosion inhibitors are of great practical importance, being extensively engaged in minimizing metallic waste in engineering materials. A number of synthetic compounds and their derivatives were reported by many authors for the corrosion inhibition of copper in various media [5–8]. Nevertheless, most of the commercially available inhibitors are harmful to both human beings and the environment and need to be put back with non-toxic, safe, and environmentally friendly green inhibitors. These problems have been resolved by the use of plant extracts, i.e., green inhibitors. This is the reason why the researchers look for eco-friendly, non-toxic, and low cost inhibitors for the erosion protection of metals. A variety of plant extracts have been employed as a corrosion inhibitor for metal and metal-based alloys [9–28]. The bioactive compounds present in the plant extract are as effective as synthetic inhibitors. These bioactive compounds act as inhibitors in acid solution which interact with metals and affect the corrosion reaction in a number of ways. Morinda tinctoria (MT) which belongs to the family of Rubiaceae is a plant which is used extensively in therapeutic applications. It is found to contain phytochemical constituents like alkaloids, flavonoids, terpenoids, steroids, amino acids, etc. [29, 30]. We have used the extracts of this plant successfully for the corrosion prevention of mild steel both in HCl and H2SO4 medium [31, 32], as well as aluminum [33] and copper [34] in HCl medium, and it has been found to be very effective. Hence, in the present work, an effort has been made to scrutinize the inhibition characteristics of aqueous extract (AE) of MT on copper corrosion in sulfuric acid medium.
Experimental
Material Preparations
The copper specimen of dimension 1 × 5 × 0.1 cm with an area of 11.07 cm2 was used for the weight loss study, and specimen with an exposed area of 0.95 cm2 was used for electrochemical study. The surface of the specimens was mechanically polished with different grades (800, 1000, and 2000) of emery papers and then degreased with acetone. The chemical composition (wt.%) of Cu is Si 0.25%, Zn 0.05%, and Mn 0.03%, and the balance is Cu (99.67%). The AE was prepared by refluxing 5 g of dried and powdered leaves of MT for 3 h at 60 °C in 100 ml of distilled water. The solution was allowed to cool to room temperature, filtered, and stored. From the stock solution, the required concentrations of the inhibitor were made by diluting with distilled water.
Weight Loss Method
The pre-cleansed and pre-weighed Cu specimens in triplicate were suspended in 100 ml test solution without and with different concentrations of AE in 0.25 M H2SO4 for a period of 24–96 h. After that, the specimens were taken out, washed with distilled water, dried, and weighed. From the weight loss data, percentage inhibition efficiency (% IE) was calculated and the optimum concentration of the inhibitor was identified from the 24-h immersion study. To predict the effectiveness of the inhibitor at high acid concentration range, the concentration of the acid was increased from 0.25 to 1 M. The synergistic influence of halide ions was studied by adding the halide additives (1% v/v of 0.1 g/L KCl, KBr, and KI) to the test solution. All the above reactions were carried out at 303 ± 1 K. The influence of temperature on the corrosion behavior of Cu in the presence of AE of MT was studied by varying the temperature from 303 to 333 K. The value of percentage inhibition efficiency (% IE) was calculated from the following equation:
where W B and W I are the weight loss of the Cu specimens in the absence and in the presence of inhibitor, respectively. The corrosion rate (CR) was calculated by the following equation:
where W is the weight loss (mg), ρ is the density of the specimen (g cm−3), A is the area of specimen (cm2), and t is the exposure time (h).
Electrochemical Methods
Electrochemical studies were carried out using Electrochemical Analyzer of CH Instruments (Model 608D). The experiments were carried out in a three-electrode cell assembly with a platinum wire mesh electrode and a saturated calomel electrode used as auxiliary and reference electrodes, respectively. Cu specimen was used as the working electrode. AC impendence studies were conducted in the frequency range of 10,000–1 Hz at the rest potential using 0.02 V sine wave as the excitation signal. R ct and C dl values were obtained from the Nyquist plots. The % IE was calculated from the following relationship:
where R 0ct and R ict are the charge transfer resistance values in the absence and in the presence of inhibitor, respectively. Potentiodynamic polarization studies were carried out in the potential range from −0.3 to 0.2 V at a scan rate of 0.01 V Sec−1. The electrochemical parameters such as corrosion current density (I corr), corrosion potential (E corr), and anodic and cathodic slopes (b a and b c) were obtained from Tafel plots, and the % IE was determined using the formula
where I 0corr and I icorr are the corrosion current densities in the absence and in the presence of inhibitor, respectively.
Surface Analysis
In order to investigate the influence of the inhibitor on the corrosion phenomenon of copper, the scanning electron microscope (SEM) coupled with energy dispersive X-ray spectroscopy (EDXS) images of fresh, inhibited, and uninhibited copper samples were recorded using the microscope JOEL-JSM 6390. Further, to confirm the nature of the chemical constituents adsorbed on the metal surface, the fourier transform infra-red (FT-IR) spectra of the dried AE extract and the material obtained by the scrapping of the metal surface after inhibition studies were recorded using Shimadzu spectrophotometer, model-IR Affinity-1.
Results and Discussion
Weight Loss Method
Table 1 shows the calculated values of CR and % IE of the inhibitor at different concentrations for Cu corrosion in 0.25 M H2SO4. The table also gives the data corresponding to the studies on the change in immersion time from 24 to 96 h. These data clearly indicate that the % IE increase with increasing concentration of inhibitor from 5 to 15% v/v. At inhibitor concentration of 19% v/v except for 72-h immersion period, the inhibition efficiency started decreasing and from this the optimum concentration of the inhibitor was fixed. The maximum % IE observed for 24-h study is 52.59% and the efficiency gradually increased up to 96-h immersion time. A maximum % IE of 69.51% was obtained with the inhibitor concentration of 15% v/v at an immersion time of 96 h. Further, it is also clear that at 19% v/v, the % IE reaches a maximum value of about 65.33% at 72-h immersion period and it remains constant (65.37%) with further increase in immersion period (96 h). The increase in % IE with an increase in inhibitor concentration indicates the adsorption of the inhibitor molecules on the copper metal surface in 0.25 M H2SO4 medium. Similarly, the decrease in the efficiency beyond the optimum concentration indicates desorption of the inhibitor molecules from the metal surface.
Table 2 illustrates the data on the corrosion of Cu at different concentrations of H2SO4. The results obtained indicate that the % IE decreases and the CR increases for Cu corrosion in higher concentrations of the acid. This may be attributed to the fact that in increasing the acid concentration from 0.25 to 1 M, the surface coverage by the inhibitor molecules decreases and the corrosion rate increases. The higher concentration of acid also increases the rate of chemical reaction and controls the adsorption power of inhibitor by ionization of active species associated with the inhibitor [35]. The other possibility is that the bond between plant compounds and metal surface may be ruptured by acid molecules at higher concentration to form hydrogen–metal bond. This results in the higher coverage of the active sites by acid molecules which prevent the penetration of inhibitor molecules to the surface of the metal.
Table 3 presents the influence of halide additives on the corrosion inhibition of Cu in 0.25 M H2SO4 medium using AE. The effect of halide ions on the inhibition efficiency of various organic compounds and natural products on metal corrosion was studied by many authors and they have observed both synergistic and antagonistic effects for various reaction systems [36–42]. In the present study, addition of halide ions has improved the inhibiting action of inhibitor in 0.25 M H2SO4 medium and decreased the amount of inhibitor usage. Data in Table 3 reveal that the addition of halide ions increases the inhibition efficiency in the order Cl− < Br− < I−. The increase in % IE in the presence of halide ions is due to the co-operative reaction between the inhibitor molecules and the additives. Here the halide ions act as a bridge between the metal surface and the inhibitor.
Effect of Temperature
Table 4 provides details about the effect of temperature on the corrosion of Cu in free acid and in the presence of different concentrations of AE in the temperature range of 303 to 333 K at 1-h immersion time. Stronger acids and higher temperatures are used in the acid pickling process to provide faster and efficient pickling process. Although a variety of chemicals are used in metal pickling, more specific data are available only for hydrochloric and sulfuric acids. Table 4 clearly shows that on increasing the temperature from 303 to 333 K, there is an increase in the weight loss of the metal. The decrease in % IE at higher temperatures could be due to desorption of the inhibitor molecules from metal surface. The activation energy (E a) for Cu corrosion reaction at different inhibitor concentrations was found out from the slope of the Arrhenius plot (log CR vs. 1/T) (Fig. 1), where the slope is E a/2.303 R, CR is corrosion rate, R is gas constant, and T is temperature in absolute scale. These values are given in Table 5 and it is clear from the table that the calculated values of E a for the inhibited system are found to be higher than the uninhibited system. This is an indication of spontaneous adsorption of the inhibitor molecules on Cu surface and is attributed to physisorption [43]. The increase in the activation energy of inhibited system is due to the adsorption of the inhibitor molecules on active sites.
Adsorption Isotherm
The nature of interaction between the inhibitor and metal surface can be clearly explained by the adsorption isotherm. This plays an important role in determining the adsorption process. The surface coverage values obtained from the weight loss method were tested with different adsorption isotherms. Among various adsorption isotherms tested, Freundlich isotherm gave the best fit in the temperature range of 303–333 K. There is a deviation from the Langmuir equation because of the fact that the surface may not be uniform and there could have been interactions between the adsorbed molecules. Further, there may be an adsorption in more than one layer that is multilayer adsorption. These deviations when occur lead in an adsorption process, obeying Freundlich isotherm. According to this isotherm, the amount of a substance adsorbed (θ) is related to the concentration (C) by the equation,
The plot of log θ versus log C is a straight line with “n” as slope value and is shown in Fig. 2. The free energy of adsorption (∆G 0ads ) for various concentrations of inhibitor at different temperatures was calculated using the following equation:
where K = θ/C inh (1 − θ), θ is surface coverage, C inh is the concentration of inhibitor, and the constant value of 55.5 represents the concentration of water in solution. Generally, a value of ∆G 0ads less negative than −20 kJ mol−1 signifies physisorption and a value more negative than about −40 kJ mol−1 designates chemisorption [31, 32, 44]. Analysis of Table 5 shows that the values of ∆G 0ads are less negative than −20 kJ mol−1 suggesting that the process of inhibition is through physisorption. Further, the negative values of ∆G 0ads point out the stability of the adsorbed layer and the spontaneity of adsorption. Table 5 also points out that ∆H 0ads values are negative and ∆S 0ads are positive. The negative value of ∆H 0ads and the positive value of ∆S 0ads particularize the feasibility of the reaction. Thus, the ∆G 0ads , ∆H 0ads , and ∆S 0ads values indicate that the process of adsorption of AE is spontaneous and exothermic in nature.
Electrochemical Methods
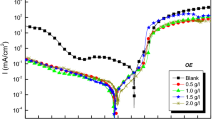
Impedance studies were carried out by varying the concentration of inhibitor, the concentration of acid, temperature, and the concentration of added halide ions. The impedance data obtained from Nyquist plots for the above-mentioned systems are given in Tables 6, 7, 8, 9, and 10. The impedance diagrams obtained for all the studied systems are not perfect semicircles. The imperfect semicircle (depressed semicircle) is attributed to the frequency dispersion as a result of the roughness and inhomogeneity of the electrode surface. In order to get a more accurate fit for the experimental data, various circuits have been tried and the best accuracy is found with the simple Randles equivalent circuit. The Randles equivalent circuit used for impedance studies is given in Fig. 3, where R s is solution resistance, C dl is the double-layer capacitance, and R ct is the charge transfer resistance. The representative Nyquist plots for various concentrations of inhibitor AE is given in Fig. 4. The values of R ct increased with an increase in concentrations of the inhibitor AE. The size of the loop is increased upon increasing the concentration of inhibitor, and the shape is maintained throughout the experiment suggesting the protective layer formation on the metal surface and corrosion of Cu is controlled by charge transfer process.
Tables 6, 7, 8, 9, and 10 also show the electrochemical parameters associated with polarization behavior for Cu in H2SO4 under different experimental conditions. It is clear from the Tables 6, 7, 8, 9, and 10 that the corrosion current (I corr) decreases and the % IE increases with an increase in the concentration of inhibitor which is due to the increase in the surface coverage by the inhibitor molecules on the metal surface. It is clear from the data obtained from Tables 6, 7, and 8, with the increase in concentration of acids, the % IE decreases. It is also clear from the tables at all the concentrations of acids, the b a, b c, and E corr values are altered suggesting that the corrosion of copper in sulfuric acid medium is reduced by controlling both anodic and cathodic reactions. Table 9 illustrates the effect of halide ions on the % IE of the inhibitor at 0.25 M H2SO4. It is clear from the table that the addition of the halide ions improve the % IE of the inhibitor at all the concentrations studied. Further, it is also evident from the table that though all the three halide ions improve the efficiency of the inhibitor, the increase in efficiency follows the order Cl− < Br− < I−. The results indicating the effect of temperature on % IE are given in Table 10 and the observation of the results show that the E corr values are shifted toward more active direction indicating that the corrosion is enhanced at higher temperatures. The shift in E corr values shows the prevention of both anodic and cathodic reactions. It is further confirmed by the variation of b a and b c values of the blank solution from that of the inhibited solutions.
Figure 5 shows the typical cathodic and anodic Tafel polarization curves for Cu in 0.25 M H2SO4 in the absence and presence of different concentrations of inhibitor. Similar curves were obtained (not shown here) for the other systems (varying the acid medium, the addition of halide ions, and varying the temperature). The presence of inhibitor produces a marked increase in the inhibition efficiency. It shifts the anodic curves to more positive potentials and the cathodic curves to more negative potentials. Comparison of the results in Tables 6, 7, 8, 9, and 10 shows that the % IE calculated from I corr values of polarization studies is not equal to that obtained from impedance studies but the trend was similar. This is due to the fact that the real polarization resistance or the charge transfer resistance is lower than polarization resistance obtained from EIS method. This deviation is the result of the resistance of the diffuse layer [32, 45, 46]
Surface Analysis
The SEM images of the fresh Cu specimen, specimen immersed in acid, and specimen immersed in inhibited acid are shown in Fig. 6. Analysis of Fig. 6 reveals that the Cu surface is damaged more in the absence of inhibitor and less in the presence of inhibitor and the image shows good protective film formation by the adsorbed bioactive species on the metal surface. Also, the EDXS images of the Cu specimens obtained after immersion experiments with uninhibited acids (Fig. 7a) show the presence of Copper, Aluminum, Silicon, Zinc, Oxygen, Sulfur, and Carbon signals, whereas the surface of the inhibited sample (Fig. 7b) shows the presence of Copper, Silicon, Zinc, Oxygen, Sulfur, Nitrogen, and Carbon signals. The results are given in Table 11; it is clear from the table that the percentage of copper in inhibited sample has decreased by about 14.67% indicating that the Cu sample is covered by the inhibitor molecules. In addition, the presence of nitrogen signal in the inhibited sample and the increase in the % of oxygen, sulfur, and carbon indicate that the inhibitor molecules containing these elements are adsorbed on the Cu metal surface. Figure 8a shows the FT-IR spectrum of the dried AE extract. The strong band at 3334 cm−1 is attributed to O–H and/or N–H stretching vibrations. The bands at 2983 and 2901 cm−1 are due to aliphatic and aromatic C–H vibrations, respectively. The bands at 1722 and 1640 cm−1 could be assigned to C=O stretching and N–H bending vibrations, respectively. The band at 1402 cm−1 indicates the presence of coupled C–O stretching and O–H in plane bending vibration. The bands at 1072 and 1050 cm−1 are ascribed to C–N and C–O stretching vibrations, respectively. The bands at 945 and 873 cm−1 indicate the C=S and S–O stretching vibrations. Further, the C–S and S–S stretching vibrations were observed at 602 and 522 cm−1 [47]. Comparison of the above (Fig. 8a) with the spectrum of the AE extract of MT obtained from the surface of copper after immersion experiments (Fig. 8b) clearly points out the shift in the frequencies of various functional groups indicating the adsorption of these chemical constituents on copper surface. Hence, from the FT-IR and SEM-EDXS studies, it can be reasonably concluded that various organic constituents containing N, S, O atoms and groups with π electrons are effectively adsorbed on the copper surface.
Conclusion
-
1)
Increasing concentration of inhibitor increases the inhibition efficiency and the efficiency decreased with increasing the concentration of acid as well as temperature.
-
2)
The inhibitor gives maximum efficiency at 96-h immersion time.
-
3)
In general, the addition of halide additives increases the inhibition efficiency.
-
4)
The calculated values of Ea are found to be higher for inhibited system than that for uninhibited system advocating the physisorption of the inhibitor molecules on metal surface. The adsorption of AE on Cu follows Freundlich isotherm.
-
5)
Negative ΔG 0ads values indicate the spontaneous adsorption of inhibitor molecules on Cu surface. The negative values of ΔH 0ads and the positive values of ΔS 0ads predict that the reaction is exothermic in nature and the process of adsorption of the inhibitor is through physisorption.
-
6)
Even though the aqueous extract of Morinda tinctoria forms a protective layer on the copper surface and acted as an inhibitor in sulfuric acid medium, its performance as an inhibitor on the copper surface is not satisfactory. It could thus be concluded that aqueous extract of Morinda tinctoria is not very effective for the protection of copper in sulfuric acid medium.
References
R.W. Revie, H.H. Uhlig, Corrosion and Corrosion Control, An Introduction to Corrosion Science and Engineering, 4th edn. (A John Wiley & Sons, Inc., Hoboken, NJ, 2008). (in English)
D. Landolt, Corrosion and Surface Chemistry of Metals, 1st edn. (EPFL Press, A Swiss academic publisher distributed by CRC Press, Taylor and Francis Group, LLC, Boca Raton, FL, 2007). (in English)
W.L. Harris, Chair, Committee on Assessing Corrosion Education, Assessment of Corrosion Education (The National Academies Press, Washington, DC, 2009). (in English)
E. McCafferty, Introduction to Corrosion Science (Springer, Alexandria, VA, 2009). (in English)
S. Li, S. Chen, S. Lei, H. Ma, R. Yu, D. Liu, Investigation on some Schiff bases as HCl corrosion inhibitors for copper. Corros. Sci. 41(7), 1273–1287 (1999). (in English)
A. Igual Munoz, J. Garcia Anton, J.L. Guinon, V. Perez Herranz, Comparison of inorganic inhibitors of copper, nickel and copper–nickels in aqueous lithium bromide solution. Electrochimica Acta 50(4), 957–966 (2004). (in English)
E.M. Sherif, S.-M. Park, Effects of 2-amino-5-ethylthio-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in aerated acidic pickling solutions. Electrochim. Acta 51(28), 6556–6562 (2006). (in English)
E.M. Sherif, Effects of 2-amino-5-(ethylthio)-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in 3% NaCl solutions. Appl. Surf. Sci. 252(24), 8615–8623 (2006). (in English)
L. Valek, S. Martinez, Copper corrosion inhibition by Azadirachta indica leaves extract in 0.5 M sulphuric acid. Mater. Lett. 61(1), 148–151 (2007). (in English)
A.M. Shah, A.A. Rahim, S.A. Hamid, S. Yahya, Green inhibitors for copper corrosion by mangrove tannin. Int. J. Electrochem. Sci. 8, 2140–2153 (2013). (in English)
B.A. Abd-El-Nabey, A.M. Abdel-Gaber, M.E.S. Ali, Khamis E, S. El-Housseiny, Inhibitive action of cannabis plant extract on the corrosion of copper in 0.5 M H2SO4. Int. J. Electrochem. Sci. 8, 5851–5865 (2013). (in English)
R. Senthooran, N. Priyantha, Inhibition of corrosion of copper in hcl by tea leaves extracts: I. Corrosion rate measurements. Ann. Res. J. SLSAJ 12, 1–10 (2012). (in English)
E.A. Noor, Potential of aqueous extract of Hibiscus sabdariffa leaves for inhibiting the corrosion of aluminum in alkaline solutions. J. Appl. Electrochem. 39(9), 1465–1475 (2009). (in English)
A. Minhaj, P.A. Saini, M.A. Quraishi, I.H. Farooqi, A study of natural compounds as corrosion inhibitors for industrial cooling systems. Corros. Preven. Control 46(2), 32–38 (1999). (in English)
M.A. Quraishi, A. Singh, V.K. Singh, D.K. Yadav, A.K. Singh, Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Mater. Chem. Phys. 122(1), 114–122 (2010). (in English)
I.E. Uwah, P.C. Okafor, V.E. Ebiekpe, Inhibitive action of ethanol extracts from Nauclea latifolia on the corrosion of mild steel in H2SO4 solutions and their adsorption characteristics. Arab. J. Chem. 6(3), 285–293 (2013). (in English)
P.C. Okafor, E.E. Ebenso, U.J. Ekbe, Azadirachta indica extracts as corrosion inhibitor for mild steel in acidic medium. Int. J. Electrochem. Sci. 5(7), 978–993 (2010). (in English)
M. El-Sayed, O.Y. Mansour, I.Z. Selim, M.M. Ibrahim, Identification and utilization of banana plant juice and its liquor as anti-corrosive materials. J. Sci. Ind. Res. 60(9), 738–747 (2001). (in English)
J.C. da Rocha, J.A. da Cunha Ponciano Gomes, E. D’Elia, Corrosion inhibition of carbon steel in hydrochloric acid solution by fruit peel aqueous extracts. Corros. Sci. 52(7), 2341–2348 (2010). (in English)
P.C. Okafor, M.E. Ikpi, I.E. Uwah, E.E. Ebenso, U.J. Ekpe, S.A. Umoren, Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 50(8), 2310–2317 (2008). (in English)
P.C. Okafor, E.E. Ebenso, Inhibitive action of Carica papaya extracts on the corrosion of mild steel in acidic media and their adsorption characteristics. Pigm. Resin Technol. 36(3), 134–140 (2007). (in English)
C.A. Loto, The effect of mango bark and leaf extract solution additives on the corrosion inhibition of mild steel in dilute sulphuric acid. Corros. Prev. Control 48(2), 59–64 (2001). (in English)
C.A. Loto, The effect of mango bark and leaf extract solution additives on the corrosion inhibition of mild steel in dilute sulphuric acid. Corros. Prev. Control 48(1), 38–41 (2001). (in English)
I.B. Obot, N.O. Obi-Egbedi, Ginseng root: a new efficient and effective eco-friendly corrosion inhibitor for aluminium alloy of type AA 1060 in hydrochloric acid solution. Int. J. Electrochem. Sci. 4(9), 1277–1288 (2009). (in English)
S.A. Umoren, I.B. Obot, E.E. Ebenso, N.O. Obi-Egbedi, The Inhibition of aluminium corrosion in hydrochloric acid solution by exudate gum from Raphia hookeri. Desalination 247(1–3), 561–572 (2009). (in English)
Xianghong Li, Shuduan Deng, Inhibition effect of Dendrocalamus brandisii leaves extract on aluminum in HCl, H3PO4 solutions. Corros. Sci. 65, 299–308 (2012). (in English)
E.E. Oguzie, Corrosion inhibition of aluminium in acidic and alkaline media by Sansevieria trifasciata extract. Corros. Sci. 49(3), 1527–1539 (2007). (in English)
P.B. Raja, M.G. Sethuraman, Inhibitive effect of black pepper extract on the sulphuric acid corrosion of mild steel. Mater. Lett. 62(17–18), 2977–2979 (2008). (in English)
K. Nisha, V. Priscillapushparani, R. Yogeshwari, P. Subashree, M. Chandran, S. Hariram, IJPI’s J. Pharmacogn. Herbal Formul. 1(6), 47–50 (2011). (in English)
G. Shanthi, D. Saridha, V. Mariappan, Pharmacognostical studies on morinda tinctoria.roxb. Int. J. Pharm. Pharm. Sci. 4(2), 636–638 (2012). (in English)
K. Krishnaveni, J. Ravichandran, A. Selvaraj, Effect of Morinda tinctoria leaves extract on the corrosion inhibition of mild steel in acid medium. Acta Metall. Sin (Engl. Lett. 26(3), 321–327 (2013). (in English)
K. Krishnaveni, J. Ravichandran, A. Selvaraj, Inhibition of mild steel corrosion by Morinda tinctoria leaves extract in sulphuric acid medium. Ionics 20(1), 115–126 (2014). (in English)
K. Krishnaveni, J. Ravichandran, Effect of aqueous extract of leaves of Morinda tinctoria on corrosion inhibition of aluminium surface in HCl medium. Trans. Nonferrous Met. Soc. China 24, 2704–2712 (2014). (in English)
K. Krishnaveni, J. Ravichandran, Influence of aqueous extract of leaves of Morinda tinctoria on Copper corrosion in HCl medium. J. Electroanal. Chem. 735, 24–31 (2014). (in English)
D.U. Omo-Dudu, N.C. Oforka, Inhibition of the corrosion of mild steel in tri oxonitrate (v) acid. Niger. J. Phys. 2, 148–153 (1999). (in English)
E.E. Oguzie, A.I. Onuchukwu, P.C. Okafor, E.E. Ebenso, Corrosion inhibition and adsorption behaviour of Ocimum basilicum extract on aluminium. Pigm. Resin Technol. 35, 63–70 (2006). (in English)
E.E. Oguzie, Inhibition of acid corrosion of mild steel by Telfaria occidentalis extract. Pigment Resin Technol. 34(6), 321–326 (2005). (in English)
N.O. Eddy, S.A. Odoemelam, A.O. Odiongenyi, Joint effect of halides and ethanol extract of Lasianthera africana on inhibition of corrosion of mild steel in H2SO4. J. Appl. Electrochem. 39, 849–857 (2009). (in English)
S.A. Umoren, M.M. Solomon, I.I. Udosoro, A.P. Udoh, Inhibition of aluminium and mild steel corrosion in acidic medium using Gum Arabic. Cellulose 17, 635–648 (2010). (in English)
D.Q. Zhang, L.X. Gao, G.-D. Zhou, Synergistic effect of 2-mercapto benzimidazole and KI on copper corrosion inhibition in aerated sulfuric acid solution. J. Appl. Electrochem. 33(5), 361–366 (2003). (in English)
Y.C. Wu, P. Zhang, H.W. Pickering, D.L. Allara, Effect of KI on improving copper corrosion inhibition efficiency of benzotriazole in sulfuric acid electrolytes. J. Electrochem. Soc. 140(10), 2791–2800 (1993). (in English)
S. Golfomitsou, J.F. Merkel, Synergistic effects of corrosion inhibitors for copper and copper alloy archaeological artefacts, Proceedings of Metal, National Museum of Australia Canberra ACT 4-8 (2004), ABN 70 592 297 967 (in English)
H. Ashassi-Sorkhabi, B. Shaabani, D. Seifzadeh, Corrosion inhibition of mild steel by some schiff base compounds in hydrochloric acid. Appl. Surf. Sci. 239(2), 154–164 (2005). (in English)
I.B. Obot, N.O. Obi-egbedi, Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation. Corros. Sci. 52(1), 198–204 (2010). (in English)
H. Ashassi-Sorkhabi, Z. Ghasemi, D. Seifzadeh, The inhibition effect of some amino acids towards the corrosion of aluminum in 1 M HCl + 1 M H2SO4 solution. Appl. Surf. Sci. 249(1–4), 408–418 (2005). (in English)
A. Yurt, Y. Mihrican, Quantitative relationships between the structure of some thiol compounds and their inhibition efficiencies. Anti-Corr. Methods Mater. 55(4), 195–203 (2008). (in English)
Jag Mohan, Organic Spectroscopy Principles and Applications, 2nd edn. (Narosa Publishing House, India, 2002), pp. 76–95
Acknowledgment
The authors wish to acknowledge the Post Graduate and Research Department of Chemistry, Sri Ramakrishna Mission Vidyalaya College of Arts and Science, Coimbatore-20 for providing the facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishnaveni, K., Ravichandran, J. A Study on the Inhibition of Copper Corrosion in Sulphuric Acid by Aqueous Extract of Leaves of Morinda tinctoria . J Fail. Anal. and Preven. 15, 711–721 (2015). https://doi.org/10.1007/s11668-015-0002-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-015-0002-0