Abstract
The corrosion and corrosion inhibition effect of carboxymethyl cellulose (CMC) for mild steel in sulphuric acid medium was investigated using chemical (weight loss and hydrogen evolution) techniques at 30–60 °C. The effect of addition of halide ions (Cl−, Br−, and I−) was also studied. It was found that CMC functions as an inhibitor for acid induced corrosion for mild steel. Inhibition efficiency increases with increase in immersion time but decreases with increase in temperature. Addition of halide ions reveals that chloride ions (Cl−) antagonize the inhibition process whereas iodide ions (I−) exert synergistic effect on the corrosion inhibition by CMC. Corrosion inhibitive effect was afforded by adsorption of CMC molecules onto the mild steel surface both in the absence and presence of halide ions which was found to follow Langmuir adsorption isotherm model. The phenomenon of physical adsorption is proposed from decrease in inhibition efficiency with increase in temperature. The inhibition mechanism was further corroborated by the values of thermodynamic and kinetic parameters obtained from the experimental data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mild steel typically the AISI grades 1005 through 1025 is usually used for structural applications. Aggressive acids predominantly sulphuric acid are widely used for industrial and some specific treatments (e.g. chemical cleaning and pickling) of mild steel most especially when intended for hot-dip galvanizing. However, these acids are known to cause severe corrosion problems. In order to minimize the loss of mild steel, acids inhibitors are always added to the treatment bath. The most effective and efficient acid inhibitors are organic compounds containing polar functions such as oxygen, nitrogen, sulphur and phosphorus in their molecular structures (Maayta and Al-Rawashdeh 2004; Bouklah et al. 2005; Abd El Rehim et al. 2001; Umoren et al. 2007) and inorganic compounds such as chromate, dichromate, nitrite, etc. (Fontana 1986). At the same time, the environmental unfriendliness of these products, especially inorganic inhibitors, cost ineffectiveness, and unavailability have come under criticisms. Consequently, research activities in recent times are geared towards finding alternative acid corrosion inhibitors. Polymers, both naturally occurring and synthetic ones have gained wide acceptance in this regard. The inherent stability, presence of multiple adsorption sites, eco-friendliness, availability, and low cost makes polymers to be a better substitute for inorganic and organic acids inhibitors (Yurt et al. 2007; Rajendran et al. 2005; Umoren et al. 2006a).
Chemical structure of CMC revealed that it contains hydroxyl and carboxyl groups hence fulfill an important criterion to function as a corrosion inhibitor. It has been reported as effective corrosion inhibitor for cadmium (Khairou and El-sayed 2003) and mild steel (Bayol et al. 2008) in HCl solution. We have also reported corrosion inhibitive effect of CMC for mild steel in H2SO4 solution (Solomon et al. 2009). Although the value of inhibition efficiency of 72% at CMC concentration of 0.04% obtained for mild steel in HCl by Bayol et al. was relatively high, the value obtained in our previous study for mild steel in H2SO4 was relatively low being 64.8% from weight loss measurements.
To upgrade the performance of organic corrosion inhibitors, extensive studies have been undertaken to identify synergistic effects of other additives. Synergistic corrosion inhibitor formulation has been advocated as it is an effective means of improving the inhibitive force of inhibitor, decreasing the amount of inhibitor usage and diversifying the applications of the inhibitor in acidic media (Li et al. 2008). Some reports indicating synergistic inhibition of synthetic and naturally occurring polymers with some cations and anions can be found in the literature. Sathiyanarayanan et al. (2008) and Jeyaprabha et al. (2006a) found that cerium, zinc and manganese ions greatly enhanced the inhibition efficiency of polyaniline (PANI) when used as inhibitor for iron in sulphuric acid. Corrosion inhibitor formulation consisting of 50 ppm polyacrylamide (PAA) and 50 ppm Zn2+ and also 50 ppm Zn2+ − 300 ppm phenyl phosphonate (PPA) have been reported by Rajendran et al. (1998) to show synergistic effect. Synergistic inhibition effect has also been reported to exist between carboxymethylchitosan (CMCT) and cu2+ for mild steel corrosion in 1 M HCl (Cheng et al. 2007).
For the anions, halide ions are the most widely studied. It is generally accepted that halide ions facilitates the adsorption of organic inhibitors during mild steel corrosion in acidic media. It is thought that halide ions, which become specifically adsorbed on the metal surface, are able to improve the adsorption of organic cations in solution by forming intermediate bridges between the metal surface and the positive end of the organic inhibitor. Corrosion inhibition synergism thus results from increased surface coverage arising from ion—pair interactions between the organic cations and anions. Influence of halide ions on corrosion inhibition of metals using synthetic polymers have been reported (Larabi et al. 2004; Umoren and Ebenso 2007; Jeyaprabha et al. 2005; Umoren et al. 2006b, 2008a) but the effect with naturally occurring polymers have not been investigated although we have reported a few for mild steel in acidic medium in our laboratory (Umoren and Ebenso 2008; Umoren et al. 2008b, c; Umoren and Ekanem 2009).
In our continuous quest of exploring environmentally friendly and cost effective corrosion inhibitors, the present work reports on the effect of CMC in combination with halide ions for mild steel corrosion in acidic environment at temperature range of 30–60 °C using weight loss and hydrogen evolution techniques.
Experimental
Materials
A flat sheet of mild steel 0.09 cm in thickness with the following composition: C = 0.13%; Si = 0.18%; Mn = 0.39%; P = 0.40%; S = 0.04%; Cu = 0.025%; and the balance Fe was used in the study. The mild steel was mechanically press-cut into coupons of 5 cm × 4 cm (surface area = 20 cm2) dimension, for weight loss study and 3 cm × 3 cm dimension for hydrogen evolution measurements. These coupons were used without further polishing to ensure reproducible surface. However, they were degreased in absolute ethanol, dried in acetone and stored in a moisture- free desiccator prior to use in corrosion testing. The inhibitor, carboxymethyl cellulose (CMC) used is a product of Sigma–Aldrich, Inc. Germany and was used as obtained. The concentration of CMC used in the study ranged between 0.1 and 0.5 g/L. Analytical grade potassium halide salts (KCl, KBr and KI) (Sigma–Aldrich) was used to study the effect of the halide additives by adding a fixed concentration of 5 mM to different concentrations of CMC. The corrodent concentration used was 2 M H2SO4 (Sigma–Aldrich) prepared from 98% reagent grade. All reagents used for the study were Analar grade, and were used as procured without further purification. Deionised water was used for the preparation of all solutions.
Weight loss measurements
In weight loss experiments the pre-cleaned mild steel coupons were suspended in 250 mL beakers containing 200 mL of test solutions maintained at 30, 40, 50, and 60 °C in a thermostated bath with the aid of glass rods and hooks. The coupons were retrieved at 2 h intervals progressively for 10 h, washed thoroughly in 20% NaOH solution containing 200 g/L of zinc dust (Jones 1996) with bristle brush, rinsed severally in deionised water, cleaned, dried in acetone, and re-weighed. The weight loss, in grammes, was taken as the difference in the weight of the mild steel coupons before and after immersion in different test solutions.
The corrosion rate (g cm−2 h−1) in the absence and presence of CMC and CMC-halide mixtures were determined using Eq. 1 (Umoren 2008):
where ΔW is the weight loss of the mild steel coupon after 10 h of immersion in grammes, A is the sectional area of the mild steel coupon in cm2 and t is the exposure time in hours.The inhibition efficiency of CMC and CMC-halide mixtures were evaluated from Eq. 2:
where CR blank and CR inh are the corrosion rates of the mild steel coupons in the absence and presence of additives, respectively, in 2 M H2SO4 at the same temperature.
Hydrogen evolution measurements
The hydrogen evolution measurement was carried out using gasometric assembly. The assembly is given in Fig. 1 and detailed description is reported elsewhere (Umoren and Ekanem 2009). In this method, 100 mL each of 2 M H2SO4, 0.1–0.5 g/L CMC and the different concentrations of CMC in combination with 5 mM KCl, KBr, and KI was introduced into a reaction vessel which was connected to a burette through a delivery tube. The initial volume of air in the burette was recorded. Mild steel coupons of dimension 3 cm × 3 cm were carefully dropped into the different test solutions placed in the reaction vessel. The volume of hydrogen gas evolved from the reaction was monitored by the depression (in cm3) in the level of paraffin oil contained in the burette at fixed time intervals (Umoren and Ebenso 2007; Ebenso et al. 2006). This was conducted at the temperatures of 30, 40, 50, and 60 °C maintained with the help of a thermostated water bath.
The corrosion rates from hydrogen evolution measurements were computed from Eq. 3 and inhibition efficiency from Eq. 2.
where V t and V i are the volumes of hydrogen evolved at time t t and t i , respectively.
Results and discussion
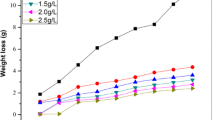
Corrosion inhibition performance of organic compounds as corrosion inhibitors can be evaluated using electrochemical and chemical techniques. For the chemical methods, weight loss measurement is ideally suited for long term immersion test. Corroborative results between weight loss and other techniques have been reported in the literature (Aytac et al. 2005; Moussa et al. 1998; Ebenso and Oguzie 2005). Earlier studies (Solomon et al. 2009) have shown that CMC inhibited acid induced corrosion of mild steel and the highest inhibition efficiency of 64.8% was obtained at 30 °C with CMC concentration of 0.5 g/L. This concentration was therefore chosen to evaluate the effect of halide ions additives. Figure 2 shows the weight loss of mild steel in 2 M H2SO4 in the absence and presence of CMC (0.5 g/L) and CMC in combination with 5 mM KCl, KBr and KI at (a) 30, (b) 40, (c) 50 and (d) 60 °C, respectively. Inspection of the figure reveals that the weight loss of mild steel was reduced in the presence of CMC compared to the free acid solution; an indication of the inhibiting effect of acid corrosion of mild steel. Further reduction in weight loss was observed on addition of halide ions to CMC solution especially for Br− and I− ions with most profound effect noticed with I− ions at all the temperatures studied. However, a different behaviour for weight loss of mild steel was noted with CMC − Cl− mixtures particularly at longer immersion period where the weight loss was observed to be lower compared to that of CMC alone. Further inspection of the figures also show that weight loss of mild steel in 2 M H2SO4 increases with increase in temperature both in the absence and presence of the additives.
The computed values of corrosion rate and inhibition efficiency for the different systems at all the temperatures studied are listed in Table 1. From the table, it is clearly seen that the corrosion rate of mild steel in 2 M H2SO4 is lower in the presence of the additives compared to the blank solution. Again corrosion rate is observed to be in the order CMC + Cl− > CMC > CMC + Br− > CMC + I−. Increase in corrosion rate of mild steel on addition of Cl− ions to CMC in comparison to CMC alone could mean that Cl− ions stimulated the corrosion of mild. On the other hand, lower corrosion rates were recorded on addition of Br− and I− ions to CMC compared to the presence of CMC alone which indicates that both Br− and I− ions addition to CMC enhanced the corrosion inhibition effect of CMC with greater effect obtained with I− than Br− ions. Results in Table 1 also show that corrosion rate increases with increase in temperature with the highest values obtained at 60 °C for all the systems investigated. Inhibition efficiency values also presented in Table 1 show a decreasing trend with increasing experimental temperatures for all the systems studied. Also the trend of inhibition efficiency with respect to the systems was found to follow the order CMC + I− > CMC + Br− > CMC > CMC + Cl−. For instance at 30 °C, the inhibition efficiency obtained for CMC alone was 65%. On addition of 5 mM KCl, inhibition efficiency was downgraded to 51% and upgraded to 67 and 89% on addition of the same concentration of 5 mM KBr and KI, respectively.
Inhibition of mild steel corrosion in 2 M H2SO4 by CMC alone may be attributed to the adsorption of CMC onto the mild steel surface leading to corrosion inhibition phenomenon. Corrosion inhibition is initiated by the displacement of adsorbed water molecules by the inhibitor species leading to specific adsorption of the inhibitor on the metal surface (Umoren and Ebenso 2007). Decrease in inhibition efficiency with temperature indicates that the molecules of CMC were physically adsorbed on the mild steel surface. The physisorption mechanism can be rationalized by considering the structure of CMC which shows that it contains carboxyl functional group (–COOH) in addition to the hydroxyl functional group (–OH) in its molecule. In acid solution, the carbonyl oxygen (C = O) may be protonated and the molecule exists as a polycation. However, in H2SO4 solution, steel is assumed to be positively charged and having hydrated ions of sulphate (SO4 2−) being weakly adsorbed on the surface thereby given rise to negatively charged steel surface (Abd El-Maksoud 2008). The formation of positively charged protonated species facilitates adsorption of the compound on the metal surface through electrostatic interaction between the CMC molecule and the mild steel surface (physisorption). Furthermore, it has been reported that, the substitution process in CMC is slightly cooperative rather than random, giving slightly higher than expected unsubstituted and trisubstituted areas (Batdorf and Rossman 1973). The molecules are therefore most extended (rod-like) at low temperatures but at higher temperatures, the molecules overlap and coil up and then, entangle to become a thermoreversible gel. This behaviour at higher temperatures may be responsible for the low inhibition efficiency of CMC observed as the thermoreversible gel nature may not appreciably cover the mild steel surface.
Mechanism of inhibition and effect of halide ions additives requires some knowledge of interaction between the protective compound and the metal surface. According to the mechanism for the dissolution of iron in acidic sulphate solution initially proposed by Bockris et al. (1961), iron electro dissolution in acidic sulphate solution depends primarily on the adsorbed intermediates as follows:
The cathodic hydrogen evolution follows the steps:
In the presence of halide ions (X−), the mechanism of the anodic dissolution is given as (Chin and Nobe 1972; MacFarlane and Smedley 1986):
The inhibitor (CMC) molecules may then combine with the adsorbed intermediates to form metal-inhibitor complex. The resulting complexes could either catalysed or inhibit further metal dissolution depending on its solubility (Okafor and Zheng 2009). From the results obtained in this study, it follows that on addition of Cl− ions to CMC a readily soluble complex formation occurred, thus increasing the metal’s corrosion rate and lowering inhibition efficiency. On the other hand addition of Br− and I− ions to CMC results in the formation of insoluble complex leading to reduction in corrosion rate and raised inhibition efficiency particularly with respect to iodide ions. Synergistic inhibition effect between iodide ions in combination with arginine against copper corrosion (Zhang et al. 2009), aliphatic amines (Fouda et al. 2005), methionine (Oguzie et al. 2007), propargyl alcohol (Feng et al. 1999) for mild steel corrosion; diphenylamine (Jeyaprabha et al. 2006b) for iron corrosion in acidic media have been reported. The highest synergistic effect of iodide ions has been attributed to chemisorption with metal surface due to its larger size, ease of polarizability, high hydrophobicity and low electronegativity compared to the other halide ions (Jeyaprabha et al. 2006c). The strong chemisorption of iodide ions on the metal surface is responsible for the synergistic effect of iodide ions in combination with CMC. The CMC polycation is then adsorbed by coulombic attraction at the metal surface, where iodide ions are already chemisorbed. Stabilization of the adsorbed iodide ions by means of electrostatic interaction with CMC polycation leads to greater surface coverage and thereby greater inhibition efficiency (Azim et al. 1995).
The seeming negative effect of Cl− ions with CMC as observed in this present study is not surprising given the fact that halide ions have been known to stimulate and inhibit corrosion of metals. The negative effects of addition of halide ions to naturally occurring materials including plant extracts used as corrosion inhibitor for mild steel in acidic media has been reported in the literature. Oguzie (2006) reported antagonistic effect with Cl− and synergistic effect with Br− and I− ions on addition to 10% extract of Occimum viridis in 2 M HCl. Eddy et al. (2009) observed that addition of Br− and I− ions to Lasianthera africana antagonized inhibition of mild steel in 0.1 M H2SO4 while Cl− ions produced synergistic effect. Other antagonistic behaviour between mixture formulations as corrosion inhibitors can also be found in the literature. Rajendran et al. (1998) found that corrosion inhibitor formulation consisting of 50 ppm polyacrylamide and 300 ppm phenyl phosphonate showed antagonistic effect on the inhibition of corrosion of mild steel in neutral aqueous environment. Similarly, antagonistic effect between amino trimethylene phosphonic acid as well as 1-hydroxyethane-1,1-diphosphonic acid and polyacrylamide has been reported (Rajendran et al. 1996, 1997). In all cases, the observed antagonistic effects have been attributed to formation of soluble adsorption intermediates which exhibit fast dissolution rates especially at higher temperatures.
To gain insight into the possible cause of negative effect of Cl− ions on corrosion inhibition by CMC, experiments were undertaken to asses the effect of immersion time on the inhibition efficiency of CMC alone and on addition of halide ions. Figure 3 shows the plot of immersion time against inhibition efficiency for the different systems studied. From the figure, it is seen that inhibition efficiency increased with immersion time for CMC, CMC + Br− and CMC + I− which could be attributed to increase in surface coverage due to increased inhibitor adsorption as time increases and stability of the adsorbed layer on the mild steel surface. However, in the presence of Cl− ions an initial increase in inhibition efficiency with time is observed which could be attributed to the initial spontaneous adsorption of the inhibitor on the metal surface. A precipitous decrease in inhibition efficiency was also observed with increase immersion time which could be likened to the onset of the dissolution of the adsorbed film and a decrease in surface coverage. The result is in agreement with that of the weight loss measurements.
Hydrogen evolution measurements
This technique provides a rapid and reliable means of assessing the inhibitive performance of corrosion inhibitors on mild steel corrosion in acidic media at short term immersion period. The relative speed, effectiveness and suitability for monitoring in situ, any perturbation by an inhibitor with respect to gas evolution in the metal/solution interface have been well established in the literature (Ebenso and Oguzie 2005; Solmaz et al. 2008; Umoren and Obot 2008). The volume of H2 gas evolved in the uninhibited (2 M H2SO4) solution and solutions containing CMC and CMC-halide mixtures were monitored as a function of exposure time at fixed time interval. Figure 4 shows the plot of volume of H2 gas evolved against time for mild steel corrosion in 2 M H2SO4 in the absence and presence of CMC and CMC in combination with KCl, KBr and KI at 30, 40, 50, and 60 °C represented by panels a, b, c, and d, respectively. It is seen from the figure that addition of CMC to the corrosive medium causes a considerable reduction in the volume of H2 gas evolved, suggesting that CMC molecules adsorbed onto the metal surface and blocked the electrochemical reaction efficiently by decreasing the available surface area. Further inspection of the figures reveal that further reduction in the volume of H2 gas evolved was observed on addition of 5 mM KBr and KI onto CMC while the volume of H2 gas evolved increases on addition of 5 mM KCl compared to the volume of H2 gas evolved in the presence of CMC alone. Also the volume of H2 gas evolved increases with increase in temperature for all the systems studied.
Table 2 shows the computed values of hydrogen evolution rate which can be correlated to the corrosion rate of mild steel coupons in the absence and presence of CMC and CMC—halide mixtures at different temperatures. Results in the table indicate that hydrogen evolution rate followed the order CMC + KI < CMC + KBr < CMC < CMC + KCl which is in agreement with results obtained from weight loss measurements. The increase in hydrogen evolution rate when Cl− ions was combined with CMC compared to CMC alone also points to the decreasing inhibitive effect of CMC in the presence of the chloride ions while the decrease in hydrogen evolution rate observed for CMC − I− combination indicates enhancement of inhibitory action of CMC in the presence of iodide ions. The hydrogen evolution rate was also found to increase with increase in temperature with the highest values obtained at 60 °C for all the systems investigated. The calculated values of inhibition efficiency are also listed in Table 2 and can be seen to follow the trend reported for weight loss measurements. It increases in the order CMC + KI > CMC + KBr > CMC > CMC + KCl but decreases with increase in temperature.
Comparison of the values of inhibition efficiency from the two independent methods as presented in Tables 1, and 2 for weight loss and hydrogen evolution techniques, respectively, it is observed that the values obtained from the hydrogen evolution technique are lower for all the systems. This can be attributed to differences in immersion time needed for the inhibiting species to get adsorbed and form a protective film on the mild steel surface thereby isolating the metal from attack of the aggressive anions present in solution. In addition, it has been argued that corrosion rate values from hydrogen evolution method represent instantaneous values while those one from weight loss method represent average values.
Adsorption considerations
Some authors (Solmaz et al. 2008; Vracar and Drazic 2002) have pointed out that adsorption on corroding surfaces never reaches the real equilibrium and tends to an adsorption steady state. However, when the corrosion rate is sufficiently small, the adsorption steady state has a tendency to become quasi-equilibrium state. Therefore, it is reasonable to consider the quasi-equilibrium adsorption in a thermodynamic manner using the appropriate equilibrium isotherms. Adsorption isotherms provide information about the interaction among adsorbed molecules themselves as well as their interactions with the metal surface. Values of the degree of surface coverage (θ) for the different systems studied at 30 °C after 10 h of immersion were used to determine which isotherm best described the adsorption process. Surface coverage values were evaluated from the weight loss measurements assuming direct relationship between inhibition efficiency and surface coverage as follows: η(%) = θ × 100. The surface coverage values were fitted to different adsorption isotherm models and best result judged by the correlation coefficient (R 2) was obtained with Langmuir adsorption isotherm. Langmuir isotherm is given by the expression:
where θ is the surface coverage, C is the concentration, K ads is the equilibrium constant of adsorption process. K ads is related to the free energy of adsorption \( \Updelta G_{\rm ads}^{o} \) by the equation (Noor 2009):
where \( C_{{{\text{H}}_{2} {\text{O}}}} \) is the concentration of water expressed in g L−1 (the same as that of inhibitor concentration), R is the molar gas constant (kJ mol−1 K−1) and T is the absolute temperature (K). The plot of C/θ as a function of C is shown in Fig. 5. Linear plots were obtained with very good correlation coefficient which seems to suggest that adsorption of CMC alone and on addition of halide ions follow Langmuir adsorption isotherm. Adsorption parameters obtained from this isotherm are listed in Table 3. From the table, it is seen that K ads values are in the order CMC + KI > CMC + KBr > CMC + KCl > CMC. Large values of K ads imply more efficient adsorption hence better inhibition efficiency (Refay et al. 2004). The large value of K ads obtained for CMC + KI system accord with the high inhibition efficiency obtained. Though the linearity of the Langmuir plot may be interpreted to mean that the adsorption of CMC alone and in the presence of the halide ions additives follow Langmuir isotherm, the considerable deviation of the slope from unity shows that the isotherm can not be strictly applied. This deviation may be explained on the basis of the interaction among adsorbed species on the surface of the metal. It has been postulated in the derivation of Langmuir isotherm equation that adsorbed molecules do not interact with one another, but as we pointed out in our earlier report (Solomon et al. 2009) this is not true in the case of large organic molecules such as CMC having polar atoms or groups which can adsorb on the cathodic and anodic sites of the metal surface. Such adsorbed species interact by mutual repulsion or attraction. It is therefore pertinent to say that the adsorption behaviour of CMC alone and with halide ions combination can be more appropriately represented by a modified Langmuir equation suggested by Villamil et al. (1999) taking into consideration the interactions between adsorbate species as well as changes in heat of adsorption with changing surface coverage as follows:
The suitability of modified Langmuir adsorption isotherm in this study corroborates the findings of Cheng et al. (2007) in their study of carboxymethyl chitosan (a naturally occurring polymer) as an ecofriendly inhibitor for mild steel in HCl solution. Generally, corrosion inhibitors are found to protect steel corrosion in acid solutions by adsorbing themselves on steel surface. Adsorption is a separation process involving two phases between which certain components can become differentially distributed. Adsorption can be described by two main types of interaction (Adamson 1990; Noor and Al-Moubaraki 2008) namely physisorption and chemisorption. Physisorption involves electrostatic forces between ionic charges or dipoles on the adsorbed species and the electric charge at the metal/solution interface while chemisorption involves charge sharing or charge transfer from the inhibitor molecules to the metal surface to form a coordinate type of bond. Thermodynamic adsorption parameters are a useful tool for clarifying the adsorption behaviour of an inhibitor. The free energy of adsorption \( \Updelta G_{\text{ads}}^{o} \) was computed using Eq. 14 and the values are listed in Table 3 for the different systems studied. From the table, it is seen that the values of \( \Updelta G_{\text{ads}}^{o} \) in all cases are negative. The negative values of \( \Updelta G_{\text{ads}}^{o} \) indicate the spontaneous adsorption of the additives on the mild steel surface. Survey of literature reveals that negative values of \( \Updelta G_{\text{ads}}^{o} \) around 20 kJ mol−1 or lower are consistent with the electrostatic interaction between charged molecules and the charged metal (physisorption) while those around 40 kJ mol−1 or higher involve charge sharing or transfer from organic molecules to the metal surface to form coordinate type of bond (chemisorption) (Obot et al. 2009). Results presented in the table indicate that the values of \( \Updelta G_{\text{ads}}^{o} \) for all the systems studied lies between −21.7 and −16.8 kJ mol−1 signifying spontaneous adsorption of the additives via physisorption mechanism.
Effect of temperature
In this study the effect of temperature on the corrosion of mild steel in 2 M H2SO4 and its inhibition by CMC alone and on addition of halide ions was evaluated using weight loss method at the temperature range 30–60 °C. The results of effect of temperature on corrosion rate and inhibition efficiency as indicated in Table 1 show that corrosion rate increases with increase in temperature both in the uninhibited and inhibited solutions while inhibition efficiency decreases with temperature rise for the different systems investigated. The dependence of corrosion rate on temperature can be expressed by the Arrhenius equation:
where CR is the corrosion rate, E a is the apparent activation energy, R is the molar gas constant, T is the absolute temperature, and A is the frequency factor. Arrhenius plots obtained for the corrosion of mild steel in the uninhibited and inhibited acid solutions are shown in Fig. 6. Linear plots were obtained and activation energy E a was evaluated from the slope (−E a /2.303R) of the linear plots and listed in Table 4. E a values in the table are higher for inhibited solutions (for the different systems) than the uninhibited one, indicating a strong inhibitive action of the additives by increasing energy barrier for the corrosion process, emphasizing the electrostatic character of the inhibitor’s adsorption on the mild steel surface (physisorption). Experimental corrosion rate values obtained from weight loss measurements for mild steel in 2 M H2SO4 in the absence and presence of CMC and CMC-halide ions combination was used to further gain insight on the change of enthalpy (ΔH *) and entropy (ΔS *) of activation for the formation of the activation complex in the transition state using transition state equation (Noor and Al-Moubaraki 2008):
where CR is the corrosion rate, h is the Planck’s constant, N is the Avogadro’s number, R is the universal gas constant and T is the absolute temperature. Figure 7 shows the plot of log CR/T versus 1/T for mild steel corrosion in 2 M H2SO4 for the different systems studied. Straight lines were obtained with slope of (−ΔH */2.303R) and an intercept of [log (R/Nh) + (ΔS */2.303R)] from which the values of ΔH * and ΔS *, respectively, were calculated and listed in Table 4. The positive values of ΔH * both in the absence and presence additives reflect the endothermic nature of the steel dissolution process. Results in Table 4 further indicate that the activation enthalpies vary in the same manner as the activation energies, supporting the proposed inhibition mechanism. Large and negative values of ΔS * in the uninhibited and inhibited systems implies that the activation complex in the rate determining steps represent association rather than dissociation step, meaning that a decrease in disordering takes place on going from reactants to the activated complex. Similar observations have been reported in the literature for mild steel dissolution in the absence and presence of inhibitors in sulphuric acid solution (Noor and Al-Moubaraki 2008; Tao et al. 2009; Oguzie et al. 2008; Badr 2009].
Conclusions
The influence of halide ions on the corrosion inhibition of carboxymethyl cellulose (CMC) for mild steel in 2 M H2SO4 has been investigated using weight loss and hydrogen evolution methods at 30–60 °C. It was found that CMC inhibited the acid induced corrosion of mild steel by virtue of adsorption of CMC molecules on the steel surface via physical adsorption mechanism both in the absence and presence of halide ions additives. Addition of halide ions to CMC exhibited both antagonistic and synergistic behaviour. Antagonistic effect was observed with addition of Cl− ions while synergistic effect was noted with I− ions at all the temperatures studied. Adsorption of CMC alone and on addition of halide ions followed Langmuir adsorption isotherm and was spontaneous in nature. Experimental data was further corroborated by the kinetic and thermodynamic parameters obtained.
References
Abd El-Maksoud SA (2008) The effect of organic compounds on the electrochemical behaviour of steel in acidic media—a review. Int J Electrochem Sci 3:528–555
Abd El Rehim SS, Mam I, Khaled KF (2001) 4-Amino antipyrine as an inhibitor of mild steel corrosion in HCl solution. Mater Chem Phys 70:268–273
Adamson AW (1990) Physical chemistry of surfaces. Wiley, New York
Aytac A, Ozmen U, Kabasakaloglu M (2005) Investigation of some Schiff-bases as acidic corrosion of alloy AA3102. Mater Chem Phys 89:176–181
Azim SS, Muralidharan S, Iyer V (1995) Studies on the influence of iodide ions on the synergistic inhibition of the corrosion of mild steel in acidic solution. J Appl Electrochem 25:495–500
Badr GE (2009) The role of some thiosemicarbazide derivatives as corrosion inhibitors for C-steel in acidic media. Corros Sci 51:2529–2536
Batdorf JB, Rossman JM (1973) Sodium carboxymethyl cellulose. In: Whistler L (ed) Industrial gums. Academic Press, New York, pp 695–729
Bayol E, Gurten AA, Dursun M, Kayakirilmaz K (2008) Adsorption behaviour and inhibition corrosion effect of sodium carboxylmethyl cellulose on mild steel in acidic medium. Acta Physco-Chim Sinica 24:2236–2242
Bockris JOM, Drazic D, Despic AR (1961) The electrode kinetics of the deposition and dissolution of iron. Electrochim Acta 4:325–361
Bouklah M, Hammouti B, Benkaddour M, Benhadda T (2005) Thiophene derivatives as effective inhibitors for the corrosion of mild steel in 0.5 M H2SO4. J Appl Electrochem 35:1095–1101
Cheng S, Chen S, Liu T, Chang X, Yin Y (2007) Carboxymethyl chitosan + Cu2+ mixture as an inhibitor used for mild steel in 1.0 M HCl. Electrochim Acta 52:5932–5938
Chin RJ, Nobe K (1972) Electrodissolution kinetics of iron in chlorides solution. III. Acidic solutions. J Electrochem Soc 119:1457–1461
Ebenso EE, Oguzie EE (2005) Corrosion inhibition of mild steel in acidic media by some organic dyes. Mater Lett 59:2163–2165
Ebenso EE, Ekpe UJ, Umoren SA, Jackson E, Abiola OK, Oforka NC (2006) Synergistic effect of halide ions on the corrosion inhibition of aluminium in acidic medium by some polymers. J Appl Polym Sci 100:2889–2894
Eddy NO, Odoemelam SA, Odiongenyi AO (2009) Joint effect of halides and ethanol extract of Lasianthera africana on inhibition of corrosion of mild steel in H2SO4. J Appl Electrochem 39:849–857
Feng Y, Siow KS, Teo WK, Hsieh AK (1999) The synergistic effects pf propargyl alcohol and potassium iodide on the inhibition of mild steel in 0.5 M sulfuric acid solution. Corros Sci 41:829–852
Fontana MG (1986) Corrosion engineering. McGraw Hill, New York, p 275
Fouda AS, Mostafa HA, El-Taib F, Elewady GY (2005) Synergistic influence of iodide ions of C-steel in sulphuric acid by some aliphatic amines. Corros Sci 47:1988–2004
Jeyaprabha C, Sathiyanarayanan S, Venkatachari G (2005) Co-adsorption effect polyaniline and of halide ions on the corrosion of on iron in 0.5 M H2SO4 solutions. J Electroanal Chem 583:232–240
Jeyaprabha C, Sathiyanarayanan S, Venkatachari G (2006a) Effect of cerium ions on corrosion inhibition of PANI for iron in 0.5 M H2SO4. Appl Surf Sci 253:432–438
Jeyaprabha C, Sathiyanarayanan S, Venkatachari G (2006b) Influence of halide ions on the adsorption of diphenylamine on iron in 0.5 M H2SO4 solutions. Electrochim Acta 51:4080–4088
Jeyaprabha C, Sathiyanarayanan S, Muralidharan S, Venkatachari G (2006c) Corrosion inhibition of iron in 0.5 mol L−1 H2SO4 by halide ions. J Braz Chem Soc 17:61–67
Jones AD (1996) Principles of corrosion control and prevention, 2nd edn. Printice-Hall, Saddle River, p 32
Khairou KS, El-sayed A (2003) Inhibition effect of some polymers on the corrosion of cadmium in hydrochloric acid solution. J Appl Polym Sci 88:866–871
Larabi L, Harek Y, Traisnel M, Mansri A (2004) Synergistic influence of poly (4-vinylpyridine) and potassium iodide on inhibition of corrosion of mild steel in 1 M HCl. J Appl Electrochem 34:833–839
Li X, Deng S, Fu H, Mu G (2008) Synergism between rare earth cerium (IV) ion and vanillin on the corrosion of cold rolled steel in 1 M HCl solution. Corros Sci 50:3599–3609
Maayta AK, Al-Rawashdeh NA (2004) Inhibition of acidic corrosion of pure aluminum by some organic compounds. Corros Sci 46:1129–1140
MacFarlane DR, Smedley SI (1986) The dissolution mechanism of iron in chloride solutions. J Electrochem Soc 133:2240–2244
Moussa MN, Foula AS, Taha AI, Elnenaa A (1998) Some thiosemicarbazide derivatives as corrosion inhibitors for aluminium in sodium hydrogen solution. Bull Korea Chem Soc 9:192–195
Noor EA (2009) Potential of aqueous extract of Hibiscus sabdariffa leaves for inhibiting the corrosion of aluminium in alkaline solutions. J Appl Electrochem 39:1465–1475
Noor EA, Al-Moubaraki AH (2008) Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4’ (-X)-styrlpyridinium iodides/hydrochloric acid systems. Mater Chem Phys 110:145–154
Obot IB, Obi-Egbedi NO, Umoren SA (2009) Antifungal drugs as corrosion inhibitors for aluminium in 0.1 M HCl. Corros Sci 51:1868–1875
Oguzie EE (2006) Studies on the inhibitive effect of Occimum viridis extract on the acid corrosion of mild steel. Mater Chem Phys 99:441–446
Oguzie EE, Li Y, Wang FH (2007) Corrosion inhibition and adsorption behaviour of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J Colloid Interf Sci 310:90–98
Oguzie EE, Njoku VO, Enenebeaku CK, Akalezi CO, Obi C (2008) Effect of hexamethylpararosaniline chloride (crystal violet) on mild steel corrosion in acidic media. Corros Sci 50:3480–3486
Okafor PC, Zheng Y (2009) Synergistic inhibition behaviour of methylbenzyl quaternary imidazoline derivative and iodide ions on mild steel in H2SO4 solutions. Corros Sci 51:850–859
Rajendran S, Apparao BV, Palaniswamy N (1996) EUROCORR’96, Paper II. P1 Nice, Acropolis, Sep 24–26
Rajendran S, Apparao BV, Palaniswamy N (1997) Synergistic, antagonistic and biocidal effects of amino (trimethylene phosohonic acid), polyacrylamide and zinc on the inhibition of corrosion of mild steel in neutral aqueous environment. Anticorros Methods Mater 44:308–313
Rajendran S, Apparao BV, Palaniswamy N (1998) Synergistic and antagonistic effects existing among polyacrylamide, phenyl phosphonate and Zn2+ on the inhibition of corrosion of mild steel in neutral aqueous environment. Electrochim Acta 44:533–537
Rajendran S, Srideri SP, Anthony N, Andraj AJ, Sundaravadivedi M (2005) Corrosion behaviour of carbon steel in polyvinyl alcohol. Anti-corros Methods Mater 52:102–107
Refay SA, Taha F, Abd El-Malak AM (2004) Inhibition of stainless steel corrosion in acidic medium by 2-mercaptobenzoxazole. Appl Surf Sci 236:175–185
Sathiyanarayanan S, Jeyaprabha C, Venkatachari G (2008) Influence of metal cations on the inhibitive effect of polyaniline for iron in 0.5 M H2SO4. Mater Chem Phys 107:350–355
Solmaz R, Mert ME, Kardas YaziciB, Erbil M (2008) Adsorption and corrosion inhibition effect of 1, 1′-thiocarbonyldiimidazole on mild steel in H2SO4 solution and synergistic effect of iodide ion. Acta Physco-Chim Sinica 24:1185–1191
Solomon MM, Umoren SA, Udosoro II, Udoh AP (2009) Inhibitive and adsorption behaviour of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corros Sci. doi:10.1016/j.corsci.2009.11.041
Tao Z, Zhang S, Li W, Hou B (2009) Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros Sci 51:2588–2595
Umoren SA (2008) Inhibition of aluminium and mild Steel corrosion in acidic medium using Gum Arabic. Cellulose 15:751–761
Umoren SA, Ebenso EE (2007) The synergistic effect of polyacrylamide and iodide ions on the corrosion inhibition of mild steel in H2SO4. Mater Chem Phys 106:387–393
Umoren SA, Ebenso EE (2008) Studies of the anti-corrosive effect of Raphia hookeri exudates gum-halide mixtures for aluminium corrosion in acidic medium. Pigm Resin Technol 37:173–182
Umoren SA, Ekanem UF (2009) Inhibition of mild steel corrosion in H2SO4 using exudate gum from Pachylobus edulis and synergistic potassium halides additives. Chem Eng Comm (in Press)
Umoren SA, Obot IB (2008) Polyvinylpyrollidone and polyacrylamide as corrosion inhibitors for mild steel in acidic medium. Surf Review Lett 15:277–286
Umoren SA, Ebenso EE, Okafor PC, Ogbobe O (2006a) Water soluble polymers as corrosion inhibitors of mild steel in acidic medium. Pigm Resin Technol 35:346–352
Umoren SA, Ogbobe O, Ebenso EE, Ekpe UJ (2006b) Effect of halide ions on the corrosion inhibition of mild steel in acidic medium using polyvinyl alcohol. Pigm Resin Technol 35(5):284–292
Umoren SA, Ogbobe O, Ebenso EE, Okafor PC (2007) Polyethylene glycol and polyvinyl alcohol as corrosion inhibitors for aluminium in acidic medium. J Appl Polym Sci 105:3363–3370
Umoren SA, Eduok UM, Oguzie EE (2008a) Synergistic inhibition of mild steel corrosion in 1 M H2SO4 by polyvinyl pyrrolidone and iodide ions. Portug Electrochim Acta 26:533–546
Umoren SA, Ogbobe O, Igwe IO, Ebenso EE (2008b) Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros Sci 50:1998–2006
Umoren SA, Obot IB, Ebenso EE, Obi-Egbedi NO (2008c) Synergistic inhibition between naturally occurring exudate gum and halide ions on the corrosion of mild steel in acidic medium. Int J Electrochem Sci 3:1029–1043
Villamil RFV, Corio P, Rubin JC, Agostinho SML (1999) Effect of sodium dodecylsulfate on copper corrosion in sulfuric acid media in the absence and presence of benzotriazole. J Electroanal Chem 472:112–119
Vracar LJM, Drazic DM (2002) Adsorption and corrosion inhibitive properties of some organic molecules on iron electrode in sulfuric acid. Corros Sci 44:1669–1680
Yurt A, Butun V, Duran B (2007) Effect of the molecular weight and structure of some novel water soluble triblock copolymers on the electrochemical behaviour of mild steel. Mater Chem Phys 105:114–121
Zhang DQ, He XM, Cai QR (2009) Arginine self-assembled monolayers against copper corrosion and synergistic effect of iodide ions. J Appl Electrochem 39:1193–1198
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umoren, S.A., Solomon, M.M., Udosoro, I.I. et al. Synergistic and antagonistic effects between halide ions and carboxymethyl cellulose for the corrosion inhibition of mild steel in sulphuric acid solution. Cellulose 17, 635–648 (2010). https://doi.org/10.1007/s10570-010-9409-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-010-9409-7