Abstract
The corrosion inhibition of mild steel in 0.5 M sulphuric acid by aqueous and acid extracts of leaves of Morinda tinctoria was studied using weight loss, colorimetric and electrochemical techniques. Results obtained indicate that both the extracts are effective in sulphuric acid medium and the efficiency decreases with increase in temperature. Added halide additives improve the efficiency of the inhibitor. From the thermodynamic, spectral, and surface analyses, the nature of adsorption has been found out.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of an inhibitor plays an important role in the corrosion protection of metal in acidic media. The inhibitor may be synthetic or naturally occurring plant products. Both synthetic and naturally occurring inhibitors protect the metal surface against corrosion. The technical, economical, and environmental issues have prompted the researchers to go for green corrosion inhibitors. Green inhibitors are biodegradable, non-toxic, eco-friendly, inexpensive, renewable, and readily available in nature. The corrosion behaviour of metals in different environments has been investigated by many authors. Extracts of various parts of the plant have been used as corrosion inhibitors. For example, the extracts of the leaves of plants like Hibiscus sabdariffa [1], Eucalyptus [2], Murraya koenigii [3], Nauclea latifolia [4], and Azadirachta indica [5] have been used for the corrosion inhibition of various metals in acidic media. There are also reports on the use of fruits [6, 7], flower [8], seeds [5, 9, 10], bark [4, 11, 12], and roots [5, 13] of different plant as corrosion inhibitors for different metals in acidic media. In recent years, most of the work on the corrosion prevention involves the utilization of the extracts of various parts of the plant which contain organic compounds with a lot of functional groups, hetero atoms, and aromatic rings [14–17]. The constituents of the extracts form a protective layer on the metal surface and prevent it from corrosion. In pursuit of an eco-friendly inhibitor, the present work is focused on the use of aqueous and acid extracts of the leaves of Morinda tinctoria for the corrosion inhibition of mild steel (MS) in 0.5 M sulphuric acid medium. M. tinctoria which belongs to the family of Rubiaceae is a weed which grows on its own in the cultivated land. All the parts of M. tinctoria have been studied for its medicinal importance [18], and the leaves of this plant are found to contain many active compounds [19]. Available literature suggests that the extract of any part of M. tinctoria has not been used for corrosion inhibition studies on metals.
Experimental
Specimen preparation
The MS specimen with a dimension of 1 × 5 × 0.2 cm and with an area of 12.3 cm2 was used for the weight loss study, and specimen with an exposed area of 0.95 cm2 was used for electrochemical study. The surface of the specimens were mechanically polished with different grades (600, 800, and 1000) of emery papers, degreased with acetone and stored in a desiccator for their application. The chemical composition (in weight per cent) of MS is: C, 0.14 %; Mn, 0.57 %; Al, 0.05 %; Cr, 0.03 %; Si, 0.02 %; Cu, 0.01 %; and the balance is Fe.
Extract preparation
The aqueous and acid extracts were prepared by refluxing 5 and 0.25 g of dried and powered leaves of M. tinctoria for 3 h at 60 °C in 100 ml of distilled water and 0.5 M H2SO4, respectively. The solutions were allowed to cool at room temperature, filtered, and stored. During the preparation of the extracts, care was taken to maintain the concentration of the acid as constant. The aqueous and sulphuric acid extracts are abbreviated as AE and SE respectively. Distilled water and analar grade H2SO4 were used for preparing the extracts.
Weight loss method
The pre-cleaned and pre-weighed MS specimens in triplicate were suspended in 100 ml test solution with and without inhibitor at different concentrations of AE and SE in 0.5 M H2SO4 for a period of 1 h. After that, the specimens were taken out, washed with distilled water, dried, and weighed. From the weight loss data, percentage inhibition efficiency (% IE) was calculated and the optimum concentration of the inhibitor was identified for both AE and SE. The synergistic influence of halide ions was studied by adding the halide additives (1 % (v/v) of 100 ppm KCl, KBr, and KI) to AE and SE. All these studies were carried out at 30 ± 1 °C. The influence of temperature on the corrosion behaviour of MS in the presence of M. tinctoria extracts was studied in the range of 30–60 °C. The inhibiting power was calculated using the following equation.
where W B and W I are the weight loss of the MS specimens in the absence and in the presence of inhibitor, respectively.
The corrosion rate (CR) was calculated employing the equation,
where W is the weight loss of the specimen (in milligrams), ρ is the density of the specimen (in grams per cubic centimeter), A is the area of specimen (in square centimeters), and t is the exposure time (in hours).
Colorimetric estimation
In the present study, the amount of iron present in the test solution before and after MS immersion was determined by colorimetric experiment. Ammonium thiocyanate is the colouring agent; the percentage transmittance (% T) of this solution was recorded and used as blank. This was mixed with standard ferric ammonium sulphate solutions of different concentrations and the % T was recorded. A standard graph was plotted between % T and the concentration of iron. MS specimens after the weight loss experiments (1 h immersion) were taken away from the inhibited and uninhibited solutions and the solutions were then tested for dissolved iron by noting the transmittance after mixing with ammonium thiocyanate. From the standard graph the amount of iron was calculated. The % IE was calculated from the following equation.
Electrochemical method
Electrochemical studies were carried out using Electrochemical Analyzer of CH Instruments (Model 608D). The experiments were carried out in a three electrode cell assembly with a platinum wire mesh electrode and a saturated calomel electrode used as auxiliary and reference electrodes respectively. MS specimen was used as the working electrode. AC impendence studies were conducted in the frequency range of 10,000–1 Hz at the rest potential using 0.02 V sine wave as the excitation signal. R ct and C dl values were obtained from the Nyquist plots. The % IE was calculated from,
where R o ct and R i ct are the charge transfer resistance values in the absence and in the presence of inhibitor, respectively. Potentiodynamic polarization studies were carried out in the potential range from −0.750 to −0.250 V at a scan rate of 0.01 V/s. The electrochemical parameters such as corrosion current density (I corr), corrosion potential (E corr), anodic and cathodic slopes (b a and b c) were obtained from Tafel plots and the % IE was determined using the formula,
where I o corr and I i corr are the corrosion current densities in the absence and in the presence of inhibitor respectively.
Surface analysis
Fourier transform infrared (FT-IR) was recorded using Shimadzu spectrophotometer for the liquid extract of SE before and after electrochemical experiments. Ultraviolet–visible (UV–vis) spectrometer (Shimadzu-UV-1700) was used to study the chemical transformation taking place during electrochemical studies. Surface morphology of the MS for inhibited and uninhibited systems were also examined using scanning electron microscope (SEM) of JEOL model (JSM 6390).
Results and discussion
Weight loss method
The results obtained for the corrosion of MS in 0.5 M H2SO4 medium with AE and SE by weight loss studies are given in Table 1, and it is clear from the table that the % IE increases with increasing the concentrations of both AE and SE in 0.5 M H2SO4. The maximum % IE for AE and SE were noticed at the inhibitor concentrations of 22 and 40 % (v/v), respectively. The increase in % IE or decrease in CR reveals the adsorption of various natural products present in the extract on MS. The effect of halide ions on the inhibition efficiency of various organic compounds and natural products was studied by several authors [20–22], and they have observed that the synergistic effect increased in the order Cl− < Br− < I−. According to these authors, the synergistic effect arises from the co-adsorption of halide ions with the inhibitors thereby facilitating the adsorption of organic compounds from the inhibitor solution on the metal surface. The results pertaining to the studies on the influence of halide ions on the performance of the inhibitor are given in Tables 2 and 3. The results show that addition of halide ions considerably improve the inhibition efficiency of the inhibitor and the order of synergistic effect is I− > Br− > Cl−.
Effect of temperature
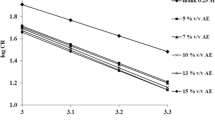
The effect of temperature on corrosion of MS was studied in the presence and in the absence of AE (22 % (v/v)) and SE (40 % (v/v)) to find the nature of the adsorption of inhibitor. The activation energy (E a) for MS corrosion reaction was found out from the slope of the Arrhenius plot (Fig. 1) (log CR vs. 1/T) for which the slope is −E a/2.303R, R is gas constant and T is temperature in absolute scale. Analysis of Table 4 reveals that for both SE and AE, the % IE decreases considerably when the temperature is increased from 303 to 313 K. This might be due to the desorption of the adsorbed inhibitor molecules from the metal surfaces [23]. The decrease in the % IE values at higher temperatures clearly indicates that the inhibitor is efficient around 303 K. The fact that the calculated values of E a (Table 4) for both the inhibited systems are found to be higher than the uninhibited system is an indication of spontaneous adsorption of the inhibitor molecules on MS surface and is attributed to physisorption [24].
Adsorption isotherm
Adsorption process is determined by using the surface coverage data which plays an important role in the construction of the adsorption isotherm and provides information about the nature of interaction between the metal surface and inhibitor. The free energy of adsorption (∆G ads) at different temperatures was calculated using the following equation.
where, K = θ/C inh (1–θ), θ is surface coverage, C inh is concentration of inhibitor and the constant value of 55.5 represents the concentration of water in solution. In general, a value of ∆G ads less negative than 20 kJ mol−1 signifies physisorption and a value more negative than about 40 kJ mol−1 indicates chemisorption [25]. Analysis of Table 4 shows that the values of ∆G ads are less negative than 20 kJ mol−1 indicating that the process of inhibition is through physisorption. Furthermore, the negative values of ∆G ads point out the stability of the adsorbed layer and the spontaneity of adsorption. Table 4 also indicates that for both SE and AE, ∆H ads values are negative and ∆S ads are positive. The negative value of ∆H ads and the positive value of ∆S ads indicate the feasibility of the reaction. Furthermore, the ∆G ads, ∆H ads, and ∆S ads values indicate that the process of adsorption of both AE and SE are spontaneous and exothermic in nature. Among all the adsorption isotherms tested, Langmuir and Freundlich isotherms were found to fit well with the experimental data for AE and SE, respectively. The equations are stated as follows.
Langmuir isotherm
Freundlich isotherm
where K ads is adsorption equilibrium constant and is related to the free energy of adsorption. The Langmuir and Freundlich isotherms were obtained by plotting log (θ/1–θ) vs. log C and log θ vs. log C, respectively and are shown in Fig. 2a, b, respectively.
Colorimetric estimation
The results obtained from colorimetric estimation give the amount of iron in the inhibited and uninhibited solution which can be used to calculate % IE. The % IE values were calculated by applying the relation described in “Colorimetric estimation” and the results are given in Tables 1 and 3. Analysis of Table 1 reveals that with the increase in the concentration of inhibitor, the iron content of the solution decreases suggesting the decrease in CR which in turn indicates the increase in % IE. Moreover, the addition of halide salts improves the efficiency of the inhibitors. Careful analysis of Table 3 also shows that addition of halide ions brings about noticeable improvement in the performance of the inhibitor, at lower concentration of the inhibitors. However at higher concentration of the inhibitors, the increase in the % IE is minimal. The % IE values calculated from the colorimetric data are in good agreement with the data obtained from weight loss studies.
Electrochemical method
Impedance studies were carried out by varying the concentrations of inhibitor (SE and AE) and the added halide ions. The Randles equivalent circuit used for impedance studies is given in Fig. 3a, where R s is solution resistance, C dl is the double layer capacitance and R ct is the charge transfer resistance. The impedance data obtained from Nyquist plots are given in Tables 5, 6, and 7. The representative Nyquist and Bode’s plots for various concentrations of inhibitor SE and AE are given in Figs. 3 and 4, respectively. The values of R ct increased with increase in concentration of the inhibitors AE and SE as well as with the addition of halide additives. The impedance diagrams have an approximately semicircular appearance and the diameter of the semicircles varied upon increasing the concentration of inhibitor suggesting that the corrosion of MS is controlled by a charge transfer process. In general, the values of C dl decrease with the increase in the concentration of the organic compounds which undergo adsorption on metal surface. This is due to the increase in the thickness of the double layer. However, in the present study, it has been noticed that there is a general tendency for an increase in the C dl value with increase in the inhibitor concentration. This abnormal behaviour of the C dl component can be attributed to the phenomenon of specific adsorption of the compounds in the plant extract on the metal surface. According to this phenomenon of specific reactant adsorption noticed by Senda and Delahay [26], Delahay [27], Delahay [28], Baticle and Perdue [29], and Timmer et al. [30], if the species undergoing reaction on metal surface (depolarizers) involves itself in specific adsorption, the C dl values increase.
Figure 5a, b depicts the representative Tafel plots of the corrosion inhibition studies on MS with varying the concentration of the inhibitors of AE, SE, and added additives ions. The polarisation data obtained are given in Tables 5, 6, and 7. Results show that the additions of inhibitor and halide ions alter both b a and b c values suggesting that the inhibitor reduces both anodic dissolution and cathodic hydrogen evolution and thereby indicating the mixed nature of the inhibitor [31]. On increasing the concentration of inhibitors, the I corr values decrease which is due to the increase in the surface coverage by the inhibitor. Comparison of the results in Tables 5, 6, and 7 shows that the % IE calculated from I corr values of polarisation studies are less than that obtained from impedance studies. This is due to the fact that the real polarisation resistance or the charge transfer resistance is lower than polarisation resistance obtained from EIS method. This deviation is a result of the resistance of the diffuse layer [32]. Tables 1, 2, 3, 5, 6, and 7 clearly shows that the % IE values obtained from weight loss data are higher than that obtained from electrochemical data. This could be due to the fact that during the electrochemical process the adsorbed inhibitor molecule may undergo electrochemical redox reaction and get converted into new compounds which are not as efficient as that of the original inhibitor. It is also possible that the molecules of the original inhibitor and their conversion products may also present on the electrode surface when chemical changes occur [33]. Electrochemical studies were repeated to confirm this large deviation in the results noticed.
To find out whether the nature of working electrode has any influence on the results, the experiments were carried out using the same working electrode with the extract of another inhibitor namely Albizia amara (Family: Fabaceae). To our surprise, A. amara inhibitor gave normal results, i.e. the % IE values obtained from electrochemical data were comparable with that the obtained gravimetric data. Similarly, when the experiments are carried with other working electrodes, i.e.(MS specimens of different composition) both AE and SE exhibited a similar trend suggesting that the decrease in efficiency noticed during electrochemical studies is not due to the nature of working material but due to the behaviour of the inhibitors AE and SE during electrochemical studies.
The review of literature predicts that the plant extract contains natural products with functional groups like NH2, OH, COOH, NH, etc. To confirm the chemical transformations which could have taken place during electrochemical studies, the inhibitor sample (before and after electrochemical studies) was extracted with CHCl3 and the CHCl3 layer so obtained was tested for functional groups. The solution before electrochemical studies gave brisk effervescence with sodium bicarbonate indicating the presence of carboxylic acid. However, when the CHCl3 extract obtained from the test solution after electrochemical studies was tested for carboxylic acid group, it has been found to be negative. Similarly, the test for aldehyde on the CHCl3 extracts before and after electrochemical studies clearly indicate the presence of aldehydic group in the extract after electrochemical studies while the CHCl3 extract obtained before electrochemical studies does not contain aldehydic group.
To authenticate the above results, the experimental solution after electrochemical study was treated with phenyl hydrazine hydrochloride and sodium acetate and the mixture was boiled for 15 min and cooled. A yellow colour precipitate was obtained conforming the formation of phenyl hydrazone (>C=N−) representing the presence of >C=O group. This was further confirmed by subjecting the precipitate thus obtained to FT-IR and UV–vis spectral analyses. The above results reasonably conclude that the % IE values obtained from electrochemical studies are lower due to the chemical transformation that occurs at the surface of the electrode.
Surface analysis
Comparison of the SEM images of uninhibited and inhibited surfaces in Fig. 6 clearly reveals that the surface of the MS is covered with inhibitor molecules of AE and SE. This is further confirmed by the FT-IR spectra of the extract of SE taken before and after electrochemical studies and are represented in Fig. 7a, b, respectively. The peak at 3,361 cm−1 in Fig. 7a is due to the O–H stretching of carboxylic acid. This disappears and a new peak is obtained at 2,978 cm−1 in Fig. 7b which is due to C–H stretching of aldehydic group. Besides, the peak at 1,641 cm−1(Fig. 7a) is due to C=O stretching of carboxylic acid and this is shifted to 1,753 cm−1 (Fig. 7b) which is due to the C=O stretching of aldehydic group. The formation of an aldehyde is further confirmed by the sharp peak at 1,639 cm−1 (Fig.7c) for >C=N− stretching in the FT-IR spectrum of the phenyl hydrazone obtained by the reaction of phenyl hydrazine with C=O group [34]. Furthermore, the band at 436 nm for the phenyl hydrazone on the visible region of the UV–vis spectrum (Fig. 8) is due to the π → π* transition of >C=N−, and this chromophore is responsible for the yellow colour. These results clearly indicate that during the electrochemical studies, the −COOH group after adsorption on the MS surface undergoes electrochemical transformation to aldehyde leading to the decrease in the inhibition efficiency of the inhibitor.
Conclusions
Inhibition efficiency increases with increase in the concentration of inhibitor and decreased with increasing the temperature. The additions of halide ions improve the performance of both AE and SE. The negative ∆G ads values predict that the inhibition processes are spontaneous. The values of ∆H ads and ∆S ads suggest that the process of adsorption of the inhibitors is through physisorption. The calculated values of E a are found to be higher for inhibited system than that for uninhibited system advocating the weak Van der Waals force of attraction/weak electrostatic force of attraction of the inhibitor molecules on metal surface. The adsorption of SE and AE on MS follow Freundlich and Langmuir isotherms respectively. AC impedance studies favour charge transfer mechanism and the potentiodynamic polarisation studies indicate the mixed nature of the inhibitor. Surface analysis techniques (SEM and FT-IR) also confirm the adsorption of inhibitor molecule on the metal surface. The results from weight loss method are in good agreement with the colorimetric method but deviate from that obtained from electrochemical studies.
References
Noor EA (2009) Potential of aqueous extract of Hibiscus sabdariffa leaves for inhibiting the corrosion of aluminium in alkaline solutions. J Appl Electrochem 39(9):1465–1475
Minhaj A, Saini PA, Quraishi MA, Farooqi IH (1999) A study of natural compounds as corrosion inhibitors for industrial cooling systems. Corros Prev Contr 46(2):32–38
Quraishi MA, Singh A, Singh VK, Yadav DK, Singh AK (2010) Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Mater Chem Phys 122(1):114–122
Uwah IE, Okafor PC, Ebiekpe VE (2013) Inhibitive action of ethanol extracts from Nauclea latifolia on the corrosion of mild steel in H2SO4 solutions and their adsorption characteristics. Arab J Chem (in press)
Okafor PC, Ebenso EE, Ekbe UJ (2010) Azadirachta indica extracts as corrosion inhibitor for mild steel in acidic medium. Int J Electrochem Sci 5(7):978–993
Sayed MEI, Mansour OY, Selim IZ, Ibrahim MM (2001) Identification and utilization of banana plant juice and its liquor as anti-corrosive materials. J Scienti Industr Res 60(9):738–747
da Rocha JC, da Cunha Ponciano Gomes JA, D’Elia E (2010) Corrosion inhibition of carbon steel in hydrochloric acid solution by fruit peel aqueous extract. Corros Sci 52(7):2341–2348
Rajenderan S, Jeyasundari J, Usha P, Selvi JA, Narayanasamy B, Regis APP, Renga P (2009) Corrosion behavior of aluminium in the presence of an aqueous extract of Hibiscus rosa sinensis. Portug Electrochim Acta 27(2):153–164
Okafor PC, Ikpi ME, Uwah IE, Ebenso EE, Ekpe UJ, Umoren SA (2008) Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros Sci 50(8):2310–2317
Okafor PC, Ebenso EE (2007) Inhibitive action of Carica papaya extracts on the corrosion of mild steel in acidic media and their adsorption characteristics. Pigm Resin Technol 36(3):134–140
Loto CA (2001) The effect of mango bark and leaf extract solution additives on the corrosion inhibition of mild steel in dilute sulphuric acid—part 2. Corros Prev contr 48(2):59–64
Loto CA (2001) The effect of mango bark and leaf extract solution additives on the corrosion inhibition of mild steel in dilute sulphuric acid—part 1. Corros Prev contr 48(1):38–41
Obot IB, Obi-Egbedi NO (2009) Ginseng root: a new efficient and effective eco-friendly corrosion inhibitor for aluminium alloy of type AA 1060 in hydrochloric acid solution. Int J Electrochem Sci 4(9):1277–1288
Umoren SA, Obot IB, Ebenso EE, Obi-Egbedi NO (2009) The Inhibition of aluminium corrosion in hydrochloric acid solution by exudate gum from Raphia hookeri. Desali 247:561–572
Li X, Deng S (2012) Inhibition effect of Dendrocalamus brandisii leaves extract on aluminum in HCl, H3PO4 solutions. Corros Sci 65:299–308
Oguzie EE (2007) Corrosion inhibition of aluminium in acidic and alkaline media by Sansevieria trifasciata extract. Corros Sci 49:1527–1539
Raja PB, Sethuraman MG (2008) Inhibitive effect of black pepper extract on the sulphuric acid corrosion of mild steel. Mater Lett 62:2977–2979
Nisha K et al (2011) Phytochemical screening of plant Morinda tinctoria (Family Rubiaceae) using different solvents. J Pharmacogn Herb Formulations 1:6
Shanthi G, Saridha D, Mariappan V (2012) Pharmacognostical studies on Morinda tinctoria. Roxb Int J Pharm Pharm Sci 4(2):636–638
Gomma GK (1998) Corrosion of low-carbon steel in sulphuric acid solution in presence of pyrazole-halides mixture. Mater Chem Phys 55:241–246
Oguzie EE (2004) Influence of halide ions on the inhibitive effect of Congo red dye on the corrosion of mild steel in sulphuric acid solution. Mater Chem Phys 87:212–217
Oguzie EE (2008) Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corros Sci 50:2993–2998
Stanly JK, Parameswaran G (2010) Corrosion inhibition of mild steel in hydrochloric acid solution by Schiff base furoin thiosemicarbazone. Corros Sci 52:224–228
Ashassi-Sorkhabi H, Shaabani B, Seifzadeh D (2005) Corrosion inhibition of mild steel by some schiff base compounds in hydrochloric acid. Appl Surf Sci 239:154–164
Obot IB, Obi-Egbedi NO (2010) Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation. Corros Sci 52:198–204
Mitsugi S, Paul D (1961) Electrode processes with specific or non-specific adsorption: faradaic impedance and rectification. J Phys Chem 65(9):1580–1588
Paul D (1966) Charge separation and recombination without external current at nonideal polarized electrodes. J Phys Chem 70(6):2067–2068
Paul D (1966) Electrode processes without a priori separation of double-layer charging. J Phys Chem 70(7):2373–2379
Baticle AM, Perdu F (1966) Adsorption et impedanck faradique: I Etudk theorique. J Electroanal Chem 12:15–26
Timmer B, Sluyters-Rehbach M, Sluyters JH (1967) On the impedance of galvanic cells: XXII. Determination of the parameters of the double-layer impedance from the electrode impedance and the electrocapillary curve in the case of specific. J Electroanal Chem Interfac 15:343–357
Abdel-Gaber AM, Abd-El-Nabey BA, Sidahmed IM, El-Zayady AM, Saadawy M (2006) Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros Sci 48:2765–2779
Yurt A, Mihrican Y (2008) Quantitative relationship between the structure of some thiol compounds and their inhibition efficiencies. Anti-Corros Method M 55(4):195–203
Damaskin Boris B, Petrii Oleg A, Valerii Batrakov V (1971) Adsorption of organic compounds on electrodes. Plenum Press, New York, pp 294–295
Jag Mohan (2002) Organic spectroscopy principles and applications. Second edn. Narosa Publishing House, New Delhi, pp.76–95
Acknowledgements
The authors wish to acknowledge the PG and Research Department of Chemistry, Sri Ramakrishna Mission Vidyalaya College of Arts and Science, Coimbatore-20 for providing the facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishnaveni, K., Ravichandran, J. & Selvaraj, A. Inhibition of mild steel corrosion by Morinda tinctoria leaves extract in sulphuric acid medium. Ionics 20, 115–126 (2014). https://doi.org/10.1007/s11581-013-0954-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0954-6