Abstract
In this work, electrochemical and surface techniques were applied to study the corrosion inhibition effects of benzothiazolone. 10% HCl was used to simulate the oil and gas well-acidizing fluid. Electrochemical frequency modulation and potentiodynamic polarization indicated that this material has excellent inhibiting features in very low concentrations. The influence of DC trend on electrochemical noise data was evaluated by polynomial fitting, and the optimum polynomial order m = 4 was obtained. After detrending, the charge of corrosion reaction obtained by electrochemical noise decreased with increasing inhibitor concentrations. In addition, the inhibition efficiencies from electrochemical noise were in agreement with data obtained by other electrochemical methods. Surface studies illustrated that the corroded surface was smoother in the presence of benzothiazolone. Electrochemical impedance and noise showed that benzothiazolone blocked the electrode surface by adsorption obeying Langmuir isotherm. According to quantum calculation, S atom in benzothiazolone indicated more tendencies for electrophilic attack in adsorption process. The main reason for high inhibition efficiencies in very low concentrations was the planar and simplicity of inhibitor structure which led to increasing the efficiency of adsorption by functional group especially sulfur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acid solutions are extensively applied in industry, including industrial acid cleaning, acid pickling, acid descaling, and oil well acidizing (Ref 1,2,3). Well stimulation or acidizing is a process to improve oil and gas production which leads to contacting the aggressive fluids with metal structure. Acid is forced over the well for reacting with rocks (dolomite, calcite, and limestone) through chemical reactions and dissolving them, opening new flow channels and enlarging bores (Ref 4, 5). The most frequently utilized acid in the acidizing process is HCl and at concentrations up to 15% (Ref 6). Hydrochloric acid is more cost-effective and reacts quickly with the rocks, but is extremely corrosive for metal structure. Therefore, the acid has to be combined with corrosion inhibitors for protecting the materials’ surface against corrosion. Numerous corrosion inhibitors have been introduced in many acid solutions with diverse levels (Ref 7,8,9).
Generally, the organic compounds with heteroatoms such as N, S, O, and P are observed to act as very useful corrosion inhibitors (Ref 10,11,12,13). The performance of these organic compounds is affected by electron density available around the heteroatoms and electronic features (like the Frontier orbital energies and energy gap), amount of available active centers for adsorption and their charge density, dimension of molecular, and the natures of the adsorbed layers on the surface of the metal (Ref 14, 15). Quantum chemical calculations have been used to associate the inhibition performance to the molecular features of materials. For instance, it has been found that molecules with small bandgap energies are extremely reactive and are effective inhibitors (Ref 16).
Electrochemical frequency modulation (EFM) and electrochemical noise (EN) are attractive in corrosion investigations and monitoring (Ref 17,18,19,20,21,22,23,24,25,26,27,28). The electrochemical frequency modulation technique was reviewed by Obot et al. (Ref 17) as a fast nondestructive electrochemical technique for corrosion research especially inhibitors (Ref 17). Ahmed et al. (Ref 22) used EFM method for corrosion inhibition study of organic compounds. They applied causality factors for accepting EFM data and showed that EFM is an effective method in corrosion inhibition studies. Eldesoky et al. (Ref 20) investigated the inhibition effect of quinolinecarboxaldehyde derivatives by electrochemical techniques. They found that corrosion rates obtained from EFM method were comparable with other techniques but EFM was very fast and nondestructive.
Obot et al. (Ref 27) studied the ability of EN to analyze different electrochemical corrosion phenomena, such as corrosion inhibitors, pitting corrosion, coatings on metals, and microbiologically induced corrosion. They indicated that the EN was capable to investigate the kinetics and mechanism of metallic corrosion with high accuracy by analyzing the stochastic and chaotic electrode potential and current fluctuations at EOCP. In recent years, EN has been used to study the inhibition effect of different organic inhibitors such as azoles and imidazoline derivatives, amino acids, and drugs in acid solutions (Ref 24,25,26,27,28). De Faria Neto et al. investigated the inhibition behavior of Paullinia Cupana plant for corrosion of carbon steel (Ref 28). They showed that EN was an effective method in analyzing corrosion charge over time and the type of localized corrosion.
API X100 steel is one of the developed materials for production of gas transportation pipelines and oil and gas well. The aim of the current research is to assess the corrosion behavior of X100 steel in HCl solutions comprising benzothiazolone using electrochemical methods. 10% HCl was employed to simulate the oil well acidizing fluid. Benzothiazolone is a simple molecule with high inhibition behavior in very low concentrations. This is the predominance of thiazole inhibitor in comparison with other complicated inhibitors. Diverse aspects of electrochemical noise are emphasized to study the corrosion inhibitors. Additionally, it is proposed to seek the relationship between EN and also its power spectral density data with the parameters achieved from EIS and polarization curves. Atomic force microscopy, scanning electron microscopy, energy-dispersive x-ray, and x-ray diffraction are utilized to investigate microstructure and analysis of the steel surface. Theoretical calculation is also performed for finding a relation between molecular and electronic structures of the studied inhibitor and its inhibition performance.
Experimental Details
Materials
The steel samples were mechanically cut into 1 × 1 × 0.5 cm3 and introduced into polyester resin leaving 1-cm2 of the surface area exposed to electrolyte. The working electrode surface was mechanically abraded with 1000, 1200, and 2000 grades of emery paper and washed with distilled water prior to all electrochemical tests. The experiments were carried out in 10% HCl solution comprising different levels of inhibitor.
Methods
The electrochemical tests were performed by computer-controlled Autolab potentiostat/galvanostat. Prior to electrochemical test, the samples were submerged in a test solution at open-circuit potential (EOCP) for 20 min to achieve a steady-state condition. To perform polarization, EIS and EFM tests, a three-electrode cell containing the X100 steel sample as a working electrode, a platinum sheet as a counter electrode, and a saturated calomel electrode as a reference was utilized. Polarization curves were provided at a scan rate of 1 mV s−1 from − 700 to − 200 mV versus SCE. Electrochemical frequency modulation (EFM) was performed with two frequencies 2 and 5 Hz, and the base frequency was 1 Hz. The perturbation signal with amplitude of 10 mV was used for both perturbation frequencies of 2 and 5 Hz. EIS measurements were executed at OCP in the frequency range 100 kHz to 0.01 Hz by a sine wave of 10 mV AC amplitude. Experimental impedance data were fitted to the suggested equivalent circuit, and the corrosion parameters were obtained (Ref 29, 30).
The simultaneous measurement of electrochemical current and potential noise was performed in corrosive solution with two similar steel electrodes with the same area and a calomel reference electrode which was located between the two steel electrodes. Electrochemical noise data with resolution 0.1 µV and 1 nA were obtained during 1024 s at 0.2 s interval. This caused a frequency in the range of 1 mHz to 0.5 Hz measured by the expressions fmax = 1/2Δt and fmin = 1/NΔt where Δt was the specimen interval and N was the total number of data recorded.
The morphology of steel surfaces in the absence and presence of inhibitor was investigated by FESEM model MIRA3 TESCAN and atomic force microscopy (AFM) model Nanosurf easyScan2. Moreover, the elemental and structural analysis of the surface was investigated by x-ray diffraction analysis (XRD) model PHILIPS and energy-dispersive x-ray (EDX).
The geometry of benzothiazolone was optimized without any symmetry constraint using density functional theory (DFT) with at B3LYP level. B3LYP consists of the Beck’s three-parameter exchange functional along with the Lee–Yang–Parr nonlocal correlation functional. DFT is a very useful approach to calculate the structural and electronic properties of different compounds (Ref 31,32,33). Atomic orbitals were described by means of 6-31G (d,p) basis set including polarization functions. Each of these computations was performed in Gaussian software. Fully optimized structures were checked for imaginary frequencies by performing vibrational frequency analysis at the same level of theory.
EHOMO and ELUMO stand for the energy of the highest occupied and lowest unoccupied molecular orbitals, respectively. The important parameters were computed based on (Ref 34) ionization energy I ≃ −EHOMO, electron affinity A ≃ −ELUMO, energy gap ΔEGap = ELUMO − EHOMO, absolute electronegativity χ = (I + A)/2, global hardness η = (I − A)/2, global softness S = 1/η, global electrophilicity index ω = μ2/2η, back donation energy ΔEBackdonation = −η/4, fraction of electron transferred ΔN = (χFe − χinh)/2(ηFe + ηinh), and global nucleophilicity index N = 1/ω (Ref 34). The values of χFe and ηFe were set to 0.2572 and 0 hartree, respectively, for bulk Fe atom based on the Pearson’s electronegativity scale.
Results and Discussion
Potentiodynamic Polarization Measurements
Figure 1 indicates the potentiodynamic polarization curves of carbon steel in 10% HCl solution in the absence and existence of benzothiazolone in the temperatures 25, 45, and 65 °C. The corrosion factors extracted from the polarization curves including corrosion potential (Ecorr), polarization resistance (Rp), cathodic and anodic Tafel slopes (βa and βc), corrosion current density (Icorr), and the degree of surface coverage (θ) are presented in Table 1. The surface coverage degree and the inhibition efficacy for diverse levels of inhibitor are computed by the subsequent equation (Ref 35, 36):
where I and İ refer to the corrosion current densities in the absence and presence of corrosion inhibitor, respectively. According to Fig. 1, both the anodic and cathodic current densities decrease in the existence of thiazole. The key cathodic reaction in acidic media is the reduction of hydrogen ions to generate hydrogen. This outcome offers that adding inhibitor decreases the anodic reaction and retards the hydrogen progress reaction consequently this compound can be categorized as combined cathodic and anodic type corrosion inhibitors, as displacement of electrode potential is less than 85 mV (Ref 35, 36). As seen, Tafel lines of current–potential curves show that the corrosion of the steel is mainly controlled by chemical reaction rate. Diffusion has negligible influence in rate-determining step due to the high concentration of H+ ions. The polarization resistance (Rp) from the Tafel extrapolation technique is computed by the Stern–Geary Equation (Ref 36):
The polarization resistance rises in the existence of inhibitor, demonstrating the inhibitor adsorption on the surface of metal for effective blockage of the active sites and inhibiting corrosion.
Going down the oil and gas well, the difference in temperature per unit well length has been described as geothermal gradient. Difference between down-hole and surface temperatures is an important factor that can influence the effectiveness of the corrosion inhibitor applied for such operations. Generally, in acidic medium, the corrosion rate increases with temperature because the steel dissolution and hydrogen evolution over potential decreases. To examine the ability of benzothiazolone on inhibition of steel corrosion at higher temperatures, polarization was carried out at different temperatures. As seen, enhancing the temperature shows no significant effect on the corrosion potentials; but leads to increasing current densities Icorr. Moreover, benzothiazolone indicates inhibiting properties at all the studied temperatures but the inhibition efficiencies are lower in higher temperature. This implies that benzothiazolone performs effectively at surface conditions but reduces its efficiency some kilometers down the well. The activation energy for the corrosion process is calculated from Arrhenius-type plot (Ref 15):
where R is the universal gas constant, Ea is the apparent activation corrosion energy, T is the absolute temperature and A is the pre-exponential factor. Arrhenius plots for the corrosion current density of steel in the absence and presence of benzothiazolone are given in Fig. 2a. Values of the apparent activation energy of corrosion in 10% HCl are determined from the slope of ln(Icorr) versus T−1 plots and are shown in Table 2. Inspection of the data shows that the activation energy is lower in the absence of inhibitors than in its presence. Based on the concept of activation and collision theory, it can be considered that molecules of the acid must collide with the metal surface before the acid solution corrodes the metal surface. This implies that the acid molecules need extra energy in the inhibited solution for corrosion to occur, therefore corrosion process inhibits. It has been reported that higher Ea in the presence of inhibitor in comparison with blank solution is typically indicating physisorption (Ref 15).
An alternative formulation of Arrhenius is transition state equation (Ref 16):
where h is Planck′s constant, N is Avogadro’s number, ∆Sa is the entropy of activation and ∆Ha is the enthalpy of activation. Figure 2b shows a plot of ln(IcorrT−1) against T−1. ∆Ha and ∆Sa are calculated from the slope and intercept of straight lines and are listed in Table 2. The positive signs of the enthalpies ∆Ha reflect the endothermic nature of the steel dissolution process. Moreover, the endothermic behavior increases in the presence of inhibitor indicating more energy needs for corrosion reaction in the presence of thiazole. The negative values of entropies ∆Sa show that the activated complex in the rate-determining step represents an association rather than a dissociation process, meaning that a decrease in disordering takes place on going from reactants to the activated complex.
Electrochemical Frequency Modulation
The corrosion activity of X100 steel in 10% HCl in the absence and presence of inhibitor are presented in Fig. 3. Each spectrum demonstrates the current response versus frequency. The harmonic and intermodulation bands are obviously visible with values much more than the background noise and are applied to measure the corrosion parameters (Ref 17,18,19,20,21). The electrochemical factors including the Tafel slopes (βa and βc), corrosion current density (Icorr) and the causality factors, CF–2 and CF–3, are calculated from the subsequent equations (Ref 17, 21) and are presented in Table 3.
where I refers to the instantaneous current density at the working electrode determined at frequency ω and U0 is amplitude of the sine wave distortion. Table 3 represents that without using inhibitor, the corrosion current density and corrosion rate is higher representing the vulnerability of X100 steel to corrosion in the strong acid media. Introducing inhibitor into the corrodent decreases corrosion current density indicating that inhibitor reduces the acid-induced corrosion of X100 pipeline steel. The achieved corrosion factors are in accordance with potentiodynamic polarization factors. Furthermore, based on table, the causality factors CF(2) and CF(3) are near to their theoretic values which shows a causal relation between the perturbation signal and so the EFM data are reliable.
Electrochemical Impedance Spectroscopy Measurements
Figure 4 indicates impedance plots obtained for X100 steel corrosion in 10% HCl solution without and with diverse levels of thiazole attained at Ecorr. The impedance data indicate one capacitive arc and one inductive arc at high-frequency values and at low-frequency values which shows two time constants, respectively. The existence of the inductive arc in low frequencies may be related to the relaxation procedure achieved using adsorption species such as H +ads and Cl −ads on the surface of X100 steel (Ref 37,38,39,40). Moreover, it has been ascribed to the passivated surface redissolution at low-frequency values (Ref 40). In addition, the inductive arc is maybe resulted through stabilizing the layer by the corrosion reaction products on the steel surface like [FeH]ads and [FeOH]ads containing inhibitor molecules (Ref 38). The high-frequency arc results from the time constant corresponding to steel corrosion and thus relates to the electrical double layer and charge transfer resistance.
The inhibited steel impedance enhances by raising the inhibitor’s level and accordingly the inhibition efficacy rises. These curves have the same shape, which is not changed through all examined levels, representing the corrosion mechanism shows no significant change in the presence of inhibitor. The equivalent circuit well matched with the impedance diagram obtained in the absence and existence of inhibitor is represented in Fig. 5. To achieve a suitable impedance simulation for metal corrosion, it is essential to substitute the capacitor, C, with a constant phase element due to the micro- and nano-roughness on the surface (Ref 41, 42). The impedance of the constant phase element is expressed as ZCPE= 1/Q(iw)n, where Q refers to a capacitive element associated with the mean double layer capacitance (Cdl), and n refers to a dimensionless factor associated with the constant phase angle. The electrical circuit elements, Rs, Rct, and CPEdl represent solution resistance, the charge transfer resistance and a CPE relating with double layer capacitance. L and RL represent the inductive elements associated with the relaxation procedure.
Equivalent circuits compatible with the experimental impedance data in Fig. 2 for corrosion of steel electrode at different inhibitor concentrations
The simplest method needs the theoretical transfer function Z(ω) to be expressed by:
ω refers to the frequency in rad/s, ω = 2πf and f represents frequency in Hz. To verify the equivalent circuit, the fitting of empirical data to equivalent circuit is performed and the circuit elements are attained. The inhibition efficiency is calculated by the subsequent equation (Ref 35):
Table 4 shows the equivalent circuit factors for corrosion of X100 steel in 10% HCl solution. The outcomes reveal that this inhibitor has high inhibition efficiencies in very low concentrations. In addition, the presence of thiazole enhances the value of Rct and decreases Qdl representing that the exposed area decreases. Moreover, a reduction in Qdl, which can be resulted from a decrease in local dielectric constant and/or a rise in the electrical double layer thickness, offers the inhibitor adsorption and the creation of a inhibition layer on the surface of electrode (Ref 43). The thickness of this inhibition layer rises with enhance in the amount of inhibitor, as more inhibitor will electrostatically adsorb on the surface of electrode (Ref 44).
Since the Qdl exponent (n) refers to a criterion for the heterogeneity of surface, values of n specify that the surface of steel becomes more and more homogeneous by increasing the amount of inhibitor because of its adsorption on the surface of steel and decreasing corrosion.
Electrochemical Noise Measurements
The noise resistance (Rn) achieves via the standard deviation of potential and the standard deviation of current (Rn = σV/σI), which are calculated using the following equations (Ref 45,46,47).
where σV is the standard deviation of potential noise, σI is the standard deviation of current noise and n refer to total number of measurements. It is observed that the electrochemical noise data are taken into account containing drift or direct current (DC) trend, which might be expressed as an alteration of the average potential or current divided with time, i.e., (Ē2 − Ē1)/(t2 − t1) or (Ī2 − Ī1)/(t2 − t1) (Ref 48). It is believed that this drift could affect the statistical result (Ref 49).
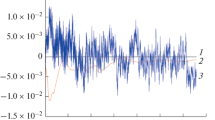
Figure 6 depicts the current time data of the samples immersed in 10% HCl solution and with 0.0002 M of thiazole. The series of current noise time without the inhibitor indicates higher amplitude transients. Moreover, the transients in the existence of the inhibitors are insignificant (Fig. 6b), which can be a superficial sign of efficiency of the inhibitor. Before calculation of noise resistance, a critical step is essential to efficiently eliminate DC trend from experimental noise data, which can largely influence the result of the data analysis (Ref 45). However, there are different ways to eliminate DC trends, such as polynomial fitting and wavelet analysis. Among different DC trend removal methods, polynomial fitting is used in this work which consists of fitting a polynomial of a given order to the current and potential noise time and next, subtracting the calculated curve so as to maintain the residuals. Polynomial detrending of electrochemical noise data with order m should be as subsequent (Ref 49, 50):
where x(t) refers to raw EN time record and fm(t) represents polynomial fitting function. In addition, xm(t) is the residual corresponding to the polynomial fitting with the m order. Figure 7 indicates the detrended current noise time series for X100 steel in different m orders of polynomial fitting in the absence of inhibitor. As can be seen, the detrended EN time fluctuates around zero and their average value is very low, and the overall drifts are removed. A slight dependency on the shape, amplitude, and the width of individual transients on the value of m is obtained from the figure. As can be seen, some relatively trends still exist in m = 1, which still cause unclear transients. Comparing to m = 1, following elimination of drift by m = 4, the transients become more obvious and several long period fluctuations reduce, which is ascribed to the elimination of the low-frequency signals. This type of long period fluctuations is frequently taken into account as the low-frequency trend and should be fully eliminated. In the very high order of drift removal, more useful low-frequency signals are eliminated (Ref 48). Actually, these fluctuations has much shorter period comparing to the measurement period t and thus should not be taken into account as the low-frequency trend. Consequently, the very high order of polynomial fitting is unsuitable for the drift removal and elimination process perhaps just requires removing the general drift in the noise time records (Ref 48).
Table 5 illustrates the statistical factors of the EN data without and with 0.0002 M thiazole before and after trend removal by different order (m = 1-9) containing standard deviation (σV and σI), the potential and current average value (Ē and Ī), root-mean-square of current (Irms), and noise resistance (Rn). According to the table, with increasing m, Ī and Ē decrease approximately 108-1019 times following drift elimination and become nearly equal to zero, and thus the influences of m on them could be unnoticed in very high m. In this case, according to I 2rms = Ī2+σ 2I , σI should be closely equal to Irms following detrending. σE, σI, and Irms slightly decrease in m ≥ 6 and thus the polynomial fitting looks to be better with moderate m. Therefore, the values of m < 6 would be suggested to prevent large computed errors and great fluctuations at the start and the end of the data. Rn displays very irregular variations following drift elimination with enhancing m. Based on the definition of Rn, its value will be defined using σE and σI, so its alteration following drift elimination would be indefinite. These results demonstrate that one main point of this technique is selecting a proper order. So Rn can be utilized to investigate the influence of order on analysis of the electrochemical noise time records (Ref 31, 51). The effect of order on the Rn at different inhibitor concentrations is shown in Table 6. It is obvious that all inhibited specimens indicate inhibition behavior. In addition, without DC trend elimination, no obvious manner is found for noise resistance versus inhibitor concentrations. These findings prove the necessity for DC trend elimination prior applying additional noise analysis. Also, pretreating time series with m = 1, 2 identify no continuous growing trend for Rn. So, choosing low orders can cause the noise data misinterpretation. When m is equal to 3, 4 and 5, continuous growing trend is obtained with respect to concentrations and suitable trend relationship is found between Rn and charge transfer resistance obtained by EIS measurements. In spite of the trend relation, it is notable that the degree of Rn differs from the relating resistance obtained from other methods. The major reason for this is the difference between experimental conditions of these techniques for measuring corrosion resistance of X100 steel in acid solution. Contrasting DC polarization and EIS, applying external perturbation is not needed in EN measurements. Actually, data are achieved from the natural fluctuations corresponding to potential and current at corrosion potential. This causes the least interference with the system resulting in a more exact data assessment than other electrochemical methods (Ref 52). According to Table 6, enhancing the polynomial order m ≥ 6 causes more trend elimination and no continuous increasing manner for Rn, and thus it is probable that this is accompanied by increasing loss of valuable data (Ref 53). Figure 8 indicates the current noise data resulted from the steel in 10% HCl both in the absence and existence of thiazole in m = 4. As seen, noise current fluctuates around zero in the absence and existence of inhibitor without omission of effective corrosion noise. In addition, the current declines in the existence of thiazole without and with trends removal. Rn is a suitable factor signifying valuable information on the inhibition effects of the materials. According to Table 6, the noise resistance of steel electrode in 10% HCl solution considerably increases with enhancing inhibitor amounts. The increase in Rn can be directly related to the decrease in σI because of the inhibitor film formation. These outcomes again indicate that the electrochemical noise measurement is a very effective technique for monitoring the metals’ corrosion. The inhibition performance values were calculated from EN measurements with the following equation:
where Rn and R on are the noise resistances in the existence and absence of inhibitor, respectively. Table 6 shows alteration of the corrosion inhibition efficacy versus amount of inhibitor. It is clear that the inhibition performances and trend correlation achieved from noise resistance agree with those obtained from EIS. The inhibition performance values achieved from EIS and EN measurements presents that the inhibition is more pronounced with increasing inhibitors concentration. Therefore, more inhibitor molecules are absorbed on the surface of metal, so providing larger surface coverage and the material act as adsorbent. In addition, more accurate data for inhibition efficiencies are obtained by electrochemical noise method due to the absence of external applied potential. The applied sinusoidal potential for EIS and EFM is very low, and therefore, the data obtained by these techniques show more agreement with EN than polarization. The applied cathodic and anodic overpotentials in potentiodynamic polarization are high, and therefore, the obtained inhibition efficiency by this method needs to check its reliability.
Power Spectral Density Analysis
Usual PSD (I) plots of the samples in 10% HCl solutions in the absence and presence of inhibitor are presented in Fig. 9. Without using the inhibitors, the PSD (I) plot either in the frequency-dependent area or in the white noise area is higher comparing with those in the existence of thiazole. As a criterion, for total current energy corresponding to signal, spectral analysis indicates that the current value for corrosion reaction passed throughout the system is higher for uninhibited solution. This means that the presence of inhibitor in the corrosive solution may hinder the steel corrosion due to the inhibitor adsorption on the steel surface (Ref 45).
The charge corresponding to each electrochemical event q can be achieved from the signals of potential and current noise, with the subsequent equation (Ref 54,55,56):
where B is the Stern–Geary coefficient. It is obtained from Eq 14 that the amplitude of PSD(I) and PSD(E) should be considered for getting a suitable characteristic charge.
The corresponding q values in different concentrations of the inhibitors are presented in Table 7. As observed, by enhancing the amount of inhibitor, the q values show a descending trend. The inhibitor reduces rate of metal oxidation in HCl solution causing a decrease in characteristic charge on the surface of metal (Ref 54).
The noise impedance can be derived from PSDE and PSDI according to the equation (Ref 57):
and Zn f→0, the limit of Zn as the frequency tends to zero, is estimated to be comparable with Rp.
Figure 10 shows a comparison between the Bode impedance diagram and noise resistance of steel exposed to HCl in the absence and presence of inhibitor. Moreover, the obtained noise resistance is presented in Table 7. Noise resistance increases with enhancing the amount of inhibitor, which indicates the applicability of thiazole in corrosion inhibition. From the figure, there is a good trend agreement between impedance and noise resistance in the low frequencies. But, there is no complete agreement between the spectral noise plots and Bode plots which is due to the more accurate data obtained by electrochemical noise.
Adsorption Isotherm
Different isotherms of adsorption were fitted to the empirical data and then linear regression coefficient values of (R2) were obtained from the curves. Based on these outcomes, it can be found that the best explanation for the adsorption mechanism of thiazole can be described by Langmuir isotherm. This isotherm can be presented as follows (Ref 58,59,60):
where θ represents the surface coverage, C is the concentration of inhibitor and Kads is the adsorption equilibrium constant relating with the standard adsorption free energy. The linear relationships corresponding to C/θ versus C obtained from EIS and EN data represented in Fig. 11 propose that adsorbing inhibitor on steel surface obeyed the Langmuir isotherm. The interaction of the molecules of inhibitor with the metal surface could be analyzed by the Gibbs free energy value resulting from the subsequent equation (Ref 61):
where 55.5 is the molar concentration of water in the solution described in M (mol l−1). The calculated values obtained by different electrochemical measurements are presented in Table 8.
The high value of Kads for studied thiazole indicates strong adsorption on the steel surface in HCl solution. The ΔGads values obtained from the diverse approaches are in good accordance with each other. The sign of ΔGads values is negative, which suggests the spontaneous adsorption of thiazole on the surface of steel. In general, the −ΔGads values close to or less than 20 kJ mol−1 are related with the electrostatic interaction of the charged surface of metal with the charged inhibitor molecules (physisorption); however, those close to or more than 40 kJ mol−1 specify sharing or transfer of charge from the molecules of inhibitor to the surface of metal for formation of a coordinate bond (chemisorption) (Ref 62). The values of ΔGads are close to −30 kJ mol−1, which specify that thiazole absorption on the surface of X100 steel relates to both chemisorption and physisorption.
In order to predict the inhibition mechanism of thiazole by adsorption, corrosion mechanism of steel should be studied (Ref 63,64,65,66,67). The following mechanism is proposed for steel corrosion in HCl solutions. Anodic dissolution of steel follows the steps:
The cathodic hydrogen evolution follows the steps:
Thiazole molecules are protonated in HCl solution. Inhibition behavior of protonated thiazole can be explained via following adsorption mechanisms (Ref 63,64,65,66,67):
-
(1)
Adsorption can carry out by electrostatic interaction between the positive charge of the protonated thiazole molecules and the negatively charged surface (physical adsorption). The negative charge of surface is provided with a specifically adsorbed anion on metal surface, (FeCl−)ads.
-
(2)
Thiazole molecules in protonated form can adsorb on the cathodic sites of iron in competition with H+ (physical adsorption).
-
(3)
Thiazole molecules can be chemisorbed by interaction of their unshared electron pairs on O, S, and N atoms and/or π orbitals with the steel surface following the deprotonation step of the physically adsorbed protonated thiazole.
Surface Analysis
More studies on the corrosion inhibition ability of thiazole were performed using AFM for characterizing the surface microstructure. Figure 12 and 13 depicts three-dimensional AFM images corresponding to the surface of steel following 8 h exposure in uninhibited and inhibited system at 25 °C. In an uninhibited system, the steel surface is very rough and damaged because of dissolving in corrosive media (Fig. 12a). In the existence of inhibitor (Fig. 13a), the surface appears smoother which is due to the creation of a compact protective film of inhibitor on the surface of metal, thereby inhibiting the corrosion of steel. Average roughness factor (Ra) was obtained from the respective images for quantifying the roughness of surface. The values of Ra show that the roughness of the metal surface submerged in 10% HCl is 1.38 μm. However, addition of inhibitor decreases the Ra to 591 nm.
These outcomes are further supported with height profile graphs (Fig. 12b and 13b). The high heterogeneity is found for the sample submerged in 10% HCl without inhibitor (Fig. 12b). Comparing to the sample in HCl, the height profile graph is smoother in Fig. 13(b) in the presence of inhibitor. This is in good accordance with the outcome achieved from the EIS analysis that the exponent n of the double layer capacitance enhances in the existence of inhibitor in solution.
To assess the exact circumstance of the steel surfaces in contact with HCl solution, FESEM was performed. Surface was examined after 30 h of immersing in 10% HCl in the absence and existence of inhibitor at temperature of 25 °C. FESEM image of the surface with and without inhibitor is shown in Fig. 14. As it is observed, the coupon surface immersed in the free acid solution (Fig. 14a) is strongly damaged because of corrosive attack causing pitting and undulation. However, the coupon surface immersed in the solution including inhibitor (Fig. 14b) is rather smoother and more uniform comparing to that one immersed in the free acid solution. This shows that adding inhibitor decreases corrosive pitting which happens in the free acid solution.
EDX data are collected over the whole surface of corroded sample and presented in Fig. 15 and Table 9. The elements Fe and Mn are attributed to X100 steel substrate (Ref 68). As seen, in the absence of inhibitor (Fig. 15a), the existence of oxygen is due to the corrosion products especially iron oxides and in addition, Cl is related to corrosion in HCl solution or trapped Cl− ions in pits. According to Fig. 15(b) and Table 9, the weight percent of oxygen decreases in the presence of inhibitor which is the result of lower corrosion products and corrosion rate. In addition, the peak between 0.2 and 0.4 eV might be related to Kα transmission of carbon element which is obtained from inhibitor adsorption on steel surface. The EDX is not an effective method for thin adsorbed layer analysis, especially for low-weight elements. Therefore, the carbon peak in this diagram is not absolutely related to organic inhibitor.
Figure 16 illustrates the normal XRD pattern of corroded steel surface after 30 h of immersing in 10% HCl with and without inhibitor in the range 10° < 2θ < 90°. According to studies, the recorded sharp peaks at 2θ of 44.7°, 64.9° and 82.3° are related to body-centered cubic (BCC) structure oriented in (110), (200) and (211) directions of iron (Ref 69,70,71). In the absence and presence of inhibitor (Fig. 16a, b), the XRD diagrams and peak positions are the same and only an increase in the intensity of peaks is obtained in the presence of inhibitor. This is due to the low corrosion products on steel surface which leads to enhancing iron peaks. The analysis of oxide layer of corroded steel is obtained by magnification of baseline and indicated in Fig. 16c, d. It is observed that FeO and Fe2O3 can be detected on steel surface but these peaks are decreased in the presence of inhibitor. This is in agreement with data obtained by energy-dispersive x-ray method.
Computational Investigation
Quantum chemical computation using DFT/B3LYP/6-31G(d,p) technique is employed to study the inhibition behavior of Benzothiazolone molecule. Optimized structure and frontier molecule orbital density distributions of the inhibitor: HOMO and LUMO are depicted in Fig. 17. As it is observed from Fig. 17, the electron density corresponding to HOMO and LUMO distributed over the benzene rings, sulfur, nitrogen and oxygen atoms. Table 10 shows the detailed data of quantum chemical computations. EHOMO of the inhibitor represents its ability for donating electrons to suitable acceptor molecule of low empty molecular orbital energy, i.e., it reflects oxidation. Also, ELUMO stands for the lowest unoccupied molecular orbital which is defined as a tendency of the molecule to receive electrons from the metal d-orbitals. The calculated fraction of electron transferred (ΔN) in the present study is 0.64. Agreeing with Lukovits’s research (Ref 72) if ΔN < 3.6, the inhibition performance enhances with increasing electron-donating ability at the surface of metal. In addition, absolute hardness, η, and softness, σ, are important properties to measure the molecular stability and reactivity. A hard molecule has a large energy gap, and a soft molecule has a small energy gap. Soft molecules are more reactive than hard ones because they could easily offer electrons to an acceptor. According to Table, the Global softness for this inhibitor is obtained 0.35 eV−1 which indicates that this inhibitor has a high reactivity. For the simplest transfer of electrons, adsorption could occur at the part of the molecule where σ has the highest value and η the lowest value. The electrophilicity index, ω, which measures the electrophilic power of a molecule was calculated for neutral form of benzothiazolone. It has been reported that the higher the value of ω, the higher the capacity of the molecule to accept electrons. In this study, the inhibitor has very high value of ω and by extension the highest capacity to accept electrons from the metal. This process increases the adsorption capacity of the inhibitor on the steel surface. The lower value of ionization potential (I) and higher electron affinity (A) enhance the inhibition efficiency.
In addition, Fukui functions are assessed by Mulliken population analysis of atoms in the inhibitor (Eq 24 and 25) based on the electron transfer direction.
where qk refers to the gross charge of atom k in the molecule. N, (N + 1), and (N−1) correspond to the number of electrons, an anion with an electron entered to the LUMO, and cation with an electron exited from the HOMO corresponding to the neutral molecule, respectively (Ref 73). These parameters can be used to represent reactive regions (the areas in a molecule vulnerable for electrophilic and nucleophilic attack) in a molecule. The calculated net charges by Mulliken population method and natural population analysis (NPA), and the Fukui functions over the heteroatoms, to simplify the discussion, are summarized in Table 11. In these calculations, f + k and f – k are applied to show the site for nucleophilic and electrophilic attack, respectively. For the mentioned inhibitor, it can be deduced that the more effective site for electrophilic attack is S atom. Moreover, the planar and simplicity of inhibitor structure lead to enhancing the efficiency of inhibitor adsorption on steel surface by functional groups especially sulfur and this is the main reason for high inhibition efficiencies in very low concentrations. This result is also depicted in Fig. 18.
Conclusion
The Benzothiazolone was studied as a corrosion inhibitor for steel electrode in 10% HCl solution with different concentrations by EIS, EFM, potentiodynamic polarization and electrochemical noise methods. The subsequent points can be highlighted:
-
Electrochemical frequency modulation and potentiodynamic polarization showed that thiazole compound had an effective inhibition in very low concentrations for the steel corrosion in 10% HCl solution.
-
Noise resistance and inhibition performance obtained from EN measurement were in good trend accordance with other approaches.
-
It is observed that the polynomial detrending was significantly affected by choosing order. Taking high order of m may lead to over filtering. Also, for low m, the drift could be observed.
-
In the case of m = 4, a good trend relation was found between Rn and the resistances achieved from EIS.
-
Power spectral density analysis indicated that the noise impedance increased and the charge of corrosion procedure decreased in the existence of inhibitor and the noise impedance was in agreement with electrochemical impedance.
-
Outcomes achieved from EIS and EN measurements showed that the adsorption of thiazole on steel in 10% HCl followed the Langmuir isotherm.
-
The values of ΔGads showed that the adsorption of inhibitor on the surface of steel occurs via both physical and chemical adsorptions.
-
Quantum chemical study at the B3LYP/6-31G (d p) basis set level was done to find out the correlation between molecular structure and inhibition performance. Theoretical calculations suggest that the HOMO densities are located around the benzene ring.
-
The planar and simplicity of inhibitor structure cause to enhancing the efficiency of adsorption by functional groups especially sulfur and this is the main reason for high inhibition efficiencies in very low concentrations.
References
R.C. Nascimento, L.B. Furtado, M.J.O.C. Guimarães, P.R. Seidl, J.C. Rocha, J.A.C. Ponciano, and M.T.M. Cruz, Synergistic Effect of Propargyl Alcohol, Octadecylamine, and 1,3-Dibutyl Thiourea for API, P110 Alloys in Acetic and Formic Acidic Solutions used in Oil Well Acidizing, J. Mol. Liq., 2018, 256, p 548–557
K. Haruna, I.B. Obot, N.K. Ankah, A.A. Sorour, and T.A. Saleh, Gelatin: A Green Corrosion Inhibitor for Carbon Steel in Oil Well Acidizing Environment, J. Mol. Liq., 2018, 264, p 515–525
E. Ituen, O. Akaranta, and A. James, Electrochemical and Anticorrosion Properties of 5-Hydroxytryptophan on Mild Steel in a Simulated Well-Acidizing Fluid, J. Taibah Univ. Sci., 2017, 11, p 788–800
M.A. Hegazy, A.Y. El-Etre, M. El-Shafaie, and K.M. Berry, Novel Cationic Surfactants for Corrosion Inhibition of Carbon Steel Pipelines in Oil and Gas Wells Applications, J. Mol. Liq., 2016, 214, p 347–356
M.A. Deyab, K. Eddahaoui, R. Essehli, T. Rhadfi, S. Benmokhtar, and G. Mele, Experimental Evaluation of New Inorganic Phosphites as Corrosion Inhibitors for Carbon Steel in Saline Water from Oil Source Wells, Desalination, 2016, 383, p 38–45
E. Ituen, O. Akaranta, and A. James, Green Anticorrosive Oilfield Chemicals from 5-hydroxytryptophan and Synergistic Additives for X80 Steel Surface Protection in Acidic Well Treatment Fluids, J. Mol. Liq., 2016, 224, p 408–419
E.B. Ituen, A.O. James, and O. Akaranta, Fluvoxamine-Based Corrosion Inhibitors for J55 Steel in Aggressive Oil and Gas Well Treatment Fluids, Egypt. J. Pet., 2017, 26, p 745–756
G.R. Argade, S. Sanders, G. Mohandass, A. Alsaleh, F. D’Souza, T.D. Golden, and R.S. Mishra, Corrosion Inhibition Study of Mg-Nd-Y High Strength Magnesium Alloy using Organic Inhibitor, J. Mater. Eng. Perform., 2019, 28, p 852–862
M. Moradi, Z. Song, and T. Xiao, Exopolysaccharide Produced by Vibrio Neocaledonicus sp. as a Green Corrosion Inhibitor: Production and Structural Characterization, J. Mater. Sci. Technol., 2018, 34, p 2447–2457
M. Li, C. Dai, B. Yang, Y. Qiao, and Z. Zhu, New and Green Multi-component Scaling and Corrosion Inhibitor for the Cooling Water of Central Air Conditioners, J. Mater. Eng. Perform., 2017, 26, p 764–772
M.B. Radovanovic and M.M. Antonijevic, Inhibition of Brass Corrosion by 2-Mercapto-1-Methylimidazole in Weakly Alkaline Solution, J. Mater. Eng. Perform., 2016, 25, p 921–937
G.R. Argade, S. Sanders, G. Mohandass, A. Alsaleh, F. D’Souza, T.D. Golden, and R.S. Mishra, Corrosion Inhibition Study of Mg-Nd-Y High Strength Magnesium Alloy Using Organic Inhibitor, J. Mater. Eng. Perform., 2019, 28, p 852–862
M.A. Domínguez-Aguilar, M. Díaz-Cruz, A. Cervantes-Tobón, and B. Castro-Domínguez, The Effect of Jet Flow Impingement on the Corrosion Products Formed on a Pipeline Steel in Naturally Aerated Sour Brine, J. Mater. Eng. Perform., 2019, 28, p 431–447
P. Mohan and G.P. Kalaignan, 1, 4-Bis (2-nitrobenzylidene) Thiosemicarbazide as Effective Corrosion Inhibitor for Mild Steel, J. Mater. Sci. Technol., 2013, 29, p 1096–1100
K. Hachama, A. Khadraoui, M. Zouikri, M. Khodja, A. Khelifa, K. Echiker, and B. Hammouti, Synthesis, Characterization and Study of Methyl 3-(2-oxo-2H-1,4-benzoxazin-3-yl) Propanoate as New Corrosion Inhibitor for Carbon Steel in 1 M H2SO4 Solution, Res. Chem. Intermed., 2016, 42, p 987–996
A. Khadraoui, A. Khelifa, H. Boutoumi, Y. Karzazi, B. Hammouti, and S.S. Al-Deyab, The Oil from Mentha Rotundifolia as Green Inhibitor of Carbon Steel Corrosion in Hydrochloric Acid, Chem. Eng. Commun., 2016, 203, p 270–277
I.B. Obot and I.B. Onyeachu, Electrochemical Frequency Modulation (EFM) Technique: Theory and Recent Practical Applications in Corrosion Research, J. Mol. Liq., 2018, 249, p 83–96
I.B. Obot, N.K. Ankah, A.A. Sorour, Z.M. Gasem, and K. Haruna, 8-Hydroxyquinoline as an Alternative Green and Sustainable Acidizing Oilfield Corrosion Inhibitor, Sustain. Mater. Technol., 2017, 14, p 1–10
H.J. Habeeb, H.M. Luaibi, R.M. Dakhil, A.A.H. Kadhum, A.A. Al-Amiery, and T.S. Gaaz, Development of New Corrosion Inhibitor Tested on Mild Steel Supported by Electrochemical Study, Results Phys., 2018, 8, p 1260–1267
A.M. Eldesoky and S.G. Nozha, The Adsorption and Corrosion Inhibition of 8-Hydroxy-7-Quinolinecarboxaldehyde Derivatives on C-Steel Surface in Hydrochloric Acid, Chin. J. Chem. Eng., 2017, 25, p 1256–1265
R.W. Bosch, J. Hubrecht, W.F. Bogaerts, and B.C. Syrett, Electrochemical Frequency Modulation: a New Electrochemical Technique for Online Corrosion Monitoring, Corrosion, 2001, 57, p 60–70
M.H. Othman Ahmed, A.A. Al-Amiery, Y.K. Al-Majedy, A.A.H. Kadhum, and T.S. Gaaz, Synthesis and Characterization of a Novel Organic Corrosion Inhibitor for Mild Steel in 1 M Hydrochloric Acid, Results Phys., 2018, 8, p 728–733
H.J. Habeeb, H.M. Luaibi, T.A. Abdullah, R.M. Dakhil, and A.A. Al-Amiery, Case Study on Thermal Impact of Novel Corrosion Inhibitor on Mild Steel, Case Stud. Therm. Eng., 2018, 12, p 64–68
C. Wang, L. Wu, F. Xue, R. Ma, and W. Ke, Electrochemical Noise Analysis on the Pit Corrosion Susceptibility of Biodegradable AZ31 Magnesium Alloy in Four Types of Simulated Body Solutions, J. Mater. Sci. Technol., 2018, 34, p 1876–1884
N. Palaniappan, L. Raju Chowhan, S. Jothi, I.G. Bosco, and I.S. Cole, Corrosion Inhibition on Mild Steel by Phosphonium Salts in 1M HNO3 Aqueous Medium, Surf. Interf., 2017, 6, p 237–246
G. Golestani, M. Shahidi, and D. Ghazanfari, Electrochemical Evaluation of Antibacterial Drugs as Environment-Friendly Inhibitors for Corrosion of Carbon Steel in HCl Solution, Appl. Surf. Sci., 2014, 308, p 347–362
I.B. Obot, I.B. Onyeachu, A. Zeino, and S.A. Umoren, Electrochemical Noise (EN) Technique: Review of Recent Practical Applications to Corrosion Electrochemistry Research, J. Adhes. Sci. Technol., 2019, 33, p 1453–1496
A.D.R. De Faria Neto, A.P.N. De Souza, R.R. Passos, M.D.S. Pereira, L.A. Pocrifka, R.F.B. De Souza, and E.D.C. Rios, Evaluation of Paullinia Cupana as a Green Corrosion Inhibitor for Carbon Steel Utilizing Gravimetric and Electrochemical Noise Techniques, Mater. Res. Express., 2019, 6, p 076522
J.R. Macdonald, Note on the Parameterization of the Constantphase Admittance Element, Solid State Ion., 1984, 13, p 147–149
I. Danaee and S. Noori, Kinetics of the Hydrogen Evolution Reaction on NiMn Graphite Modified Electrode, Int. J. Hydrog. Energy, 2011, 36, p 12102–12111
A. Becke, Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior, Phys. Rev., 1988, 38, p 3098–3100
D. Becke, Density-functional thermochemistry III. The role of exact exchange, J. Chem. Phys., 1993, 98, p 5648–5652
C. Lee, W. Yang, and R. Parr, Development of the ColleSalvetti Correlation-Energy Formula into a Functional of the Electron Density, Phys. Rev., 1988, 37, p 785–789
J.H. Al-Fahemi, M. Abdallah, E.A.M. Gad, and B.A.A.L. Jahdaly, Experimental and Theoretical Approach Studies for Melatonin Drug as Safely Corrosion Inhibitors for Carbon Steel using DFT, J. Mol. Liq., 2016, 222, p 1157–1163
A. Khadraoui, A. Khelifa, M. Hadjmeliani, R. Mehdaoui, and A. Zarrouk, Extraction, Characterization and Anti-corrosion Activity of Mentha Pulegium Oil: Weight Loss, Electrochemical, Thermodynamic and Surface Studies, J. Mol. Liq., 2016, 216, p 724–731
A.R. Hoseinzadeh, I. Danaee, and M.H. Maddahy, Thermodynamic and Adsorption Behaviour of Medicinal Nitramine as a Corrosion Inhibitor for AISI, Steel Alloy in HCl Solution, J. Mater. Sci. Technol., 2013, 29, p 884–892
M.A. Amin, S.S. Abd El-Rehim, E.E.F. El-Sherbini, R.S. Bayyomi, The Inhibition of Low Carbon Steel Corrosion in Hydrochloric Acid Solutions by Succinic Acid: Part I. Weight Loss, Polarization, EIS, PZC, EDX and SEM studies, Electrochim. Acta, 2007, 52, p 3588–3600.
N. Labjar, M. Lebrini, F. Bentiss, N. Chihib, S. El Hajjaji, and C. Jama, Corrosion Inhibition of Carbon Steel and Antibacterial Properties of Aminotris-(Methylenephosphonic) Acid, Mater. Chem. Phys., 2010, 119, p 330–336
A.R. Hosein Zadeh, I. Danaee, and M.H. Maddahy, Thermodynamic and Adsorption Behaviour of Medicinal Nitramine as a Corrosion Inhibitor for AISI, Steel Alloy in HCl Solution, J. Mater. Sci. Technol., 2013, 29, p 884–892
A.R. Hosein Zadeh, I. Danaee, M.H. Maddahy, and M. RashvandAvei, Taurine as a Green Corrosion Inhibitor for AISI, 4130 Steel Alloy in Hydrochloric Acid Solution, Chem. Eng. Commun., 2014, 201, p 380–402
I. Danaee, N. Bahramipanah, S. Moradi, and S. Nikmanesh, Impedance Spectroscopy Studies on Corrosion Inhibition Behavior of Synthesized N, N’-bis(2,4-dihydroxyhydroxybenzaldehyde)- 1,3-Propandiimine for API-5L-X65 steel in HCl Solution, J. Electrochem. Sci. Technol., 2016, 7, p 153–160
A. Moteshakker and I. Danaee, Microstructure and Corrosion Resistance of Dissimilar Weld-Joints between Duplex Stainless Steel 2205 and Austenitic Stainless Steel 316L, J. Mater. Sci. Technol., 2016, 32, p 282–290
R. Mehdaoui, A. Khelifa, A. Khadraoui, O. Aaboubi, A. Hadj Ziane, F. Bentiss, and A. Zarrouk, Corrosion Inhibition of Carbon Steel in Hydrochloric Acid Solution by Some Synthesized Surfactants from Petroleum Fractions, Res. Chem. Intermed., 2016, 42, p 5509–5526
H. Gerengi and H.I. Sahin, Schinopsis Lorentzii Extract as a Green Corrosion Inhibitor for Low Carbon Steel in 1M HCl Solution, Ind. Eng. Chem. Res., 2012, 51, p 780–787
B.P. Markhali, R. Naderi, M. Mahdavian, M. Sayebani, and S.Y. Arman, Electrochemical Impedance Spectroscopy and Electrochemical Noise Measurements as Tools to Evaluate Corrosion Inhibition of Azole Compounds on Stainless Steel in Acidic Media, Corros. Sci., 2013, 75, p 269–279
E. Garc´ıa-Ochoa and J. Genescan, Understanding the Inhibiting Properties of 3-Amino-1,2,4- Triazole from Fractal Analysis, Surf. Coat. Technol., 2004, 184, p 322–330
J.M. Sanches-Amaya, R.A. Cottis, and F.G. Botana, Shot Noise and Statistical Parameters of the Estimation of Corrosion Mechanism, Corros. Sci., 2005, 47, p 3280–3299
J.Y. Huang, Y.B. Qiu, and X.P. Guo, Comparison of Polynomial Fitting and Wavelet Transform to Remove Drift in Electrochemical Noise Analysis, Corros. Eng. Sci. Technol., 2010, 45, p 288–294
D. Seifzadeh, H. Basharnavaz, and A. Bezaatpour, A Schiff Base Compound as Effective Corrosion Inhibitor for Magnesium in Acidic Media, Mater. Chem. Phys., 2013, 138, p 794–802
U. Bertocci, F. Huet, R.P. Nogueira, and P. Rousseau, Drift Removal Procedures in the Analysis of Electrochemical Noise, Corrosion, 2002, 58, p 337–348
M. Hernandez and J. Genesca, Correlation between Electrochemical Impedance and Noise Measurements of Waterborne Coatings, Corros. Sci., 2009, 51, p 499–510
H. Ashassi-Sorkhabi and D. Seifzadeh, Analysis of Raw and Trend Removed EN Data in Time Domain to Evaluate Corrosion Inhibition Effects of New Fuchsin Dye on Steel Corrosion and Comparison of Results with EIS, J. Appl. Electrochem., 2008, 38, p 1545–1552
A.M. Homborg, T. Ting, X. Zhang, E.P.M. Van Westing, P.J. Oonincx, J.H.W. De Wite, and J.M.C. Mole, Time–Frequency Methods for Trend Removal in Electrochemical Noise Data, Electrochim. Acta, 2012, 70, p 199–209
Ł. Lentka and J. Smulko, Methods of Trend Removal in Electrochemical Noise Data—Overview, Measurement, 2019, 131, p 569–581
M.A. Rubio, K. Bethune, A. Urquia, and J. St-Pierre, Proton Exchange Membrane Fuel Cell Failure Mode Early Diagnosis with Wavelet Analysis of Electrochemical Noise, Int. J. Hydrog. Energy, 2016, 41, p 14991–15001
D. Seifzadeh, A. Bezaatpour, and R. Asadpour Joghani, Shot Noise Analysis to Investigate the Corrosion Inhibition of AZ91D Magnesium Alloy in Sulfuric Acid Solution, Prot. Met. Phys. Chem. Surf., 2016, 52, p 329–338
J.J. Perdomo and P.M. Singh, Electrochemical Noise (ECN) Measurements as a Corrosion Monitoring Tool: A Review, Corros. Rev., 2002, 20, p 359–378
A. Khadraoui, A. Khelifa, K. Hachama, H. Boutoumi, and B. Hammouti, Synergistic Effect of Potassium Iodide in Controlling the Corrosion of Steel in Acid Medium by Mentha Pulegium Extract, Res. Chem. Intermed., 2015, 41, p 7973–7980
A. Khadraoui, A. Khelifa, K. Hachama, and R. Mehdaoui, Thymus Algeriensis Extract as a New Eco-friendly Corrosion Inhibitor for 2024 Aluminium Alloy in 1M HCl Medium, J. Mol. Liq., 2016, 214, p 293–297
H.G. Hosseini, S.F.L. Mertens, and M.R. Arshadi, Synergism and Antagonism in Mild Steel Corrosion Inhibition by Sodium Dodecylbenzenesulphonate and Hexamethylenetetramine, Corros. Sci., 2003, 45, p 1473–1489
J. Flis and T. Zakroczymski, Impedance Characterization of the Activation of Iron Surface for Hydrogen Entry from Alkaline Solution, Electrochim. Acta, 1996, 41, p 1245–1250
S. Deng, X. Li, and H. Fu, Two Pyrazine Derivatives as Inhibitors of the Cold Rolled Steel Corrosion in Hydrochloric Acid Solution, Corros. Sci., 2011, 53, p 822–828
A. Yurt, B. Duran, and H. Dal, An Experimental and Theoretical Investigation on Adsorption Properties of Some Diphenolic Schiff Bases as Corrosion Inhibitors at Acidic Solution/Mild Steel Interface, Arab. J. Chem., 2014, 7, p 732–740
P. Kannan, T.S. Rao, and N. Rajendran, Improvement in the Corrosion Resistance of Carbon Steel in Acidic Condition using Naphthalen-2-ylnaphthalene-2-Carboxammide Inhibitor, J. Colloid Interf. Sci., 2018, 512, p 618–628
K.R. Ansari, M.A. Quraishi, and A. Singh, Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Some Pyridine Derivatives: An Experimental and Quantum Chemical Study, J. Ind. Eng. Chem., 2015, 25, p 89–98
Y. El Bakri, L. Guoc, E.H. Anouar, and E.M. Essassi, Electrochemical, DFT and MD Simulation of Newly Synthesized Triazolotriazepine Derivatives as Corrosion Inhibitors for Carbon Steel in 1M HCl, J. Mol. Liq., 2019, 274, p 759–769
A.O. Yüce, E. Telli, B.D. Mert, G. Kardaş, and B. Yazıcı, Experimental and Quantum Chemical Studies on Corrosion Inhibition Effect of 5,5 Diphenyl 2-thiohydantoin on Mild Steel in HCl Solution, J. Mol. Liq., 2016, 218, p 384–392
Y. Li, J. Liu, Y. Deng, X. Han, W. Hu, and C. Zhong, Ex situ Characterization of Metallurgical Inclusions in X100 Pipeline Steel Before and After Immersion in a Neutral pH Bicarbonate Solution, J. Alloy Compd., 2016, 673, p 28–37
C. Wang, Z. Farhat, G. Jarjoura, M.K. Hassan, and A.M. Abdullah, Indentation and Erosion Behavior of Electroless Ni-P Coating on Pipeline Steel, Wear, 2017, 376–377, p 1630–1639
J. Zhao, D. Xiong, Y. Gu, Q. Zeng, and B. Tian, A Comparative Study on the Corrosion Behaviors of X100 Steel in Simulated Oilfield Brines under the Static and Dynamic Conditions, J. Pet. Sci. Eng., 2019, 173, p 1109–1120
H.D. Omar, To Investigation the Structure and Morphology of Iron Metallic by Difference Techniques, J. Nano. Adv. Mat., 2015, 3, p 57–61
I. Lukovits, E. Kalman, and F. Zucchi, Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency, Corrosion, 2001, 57, p 3–8
P. Udhayakala, Density Functional Theory Calculations on Corrosion Inhibitory Action of Five Azlactones on Mild Steel, J. Chem. Pharm. Res., 2014, 6, p 117–127
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Danaee, I., Nikparsa, P. Electrochemical Frequency Modulation, Electrochemical Noise, and Atomic Force Microscopy Studies on Corrosion Inhibition Behavior of Benzothiazolone for Steel API X100 in 10% HCl Solution. J. of Materi Eng and Perform 28, 5088–5103 (2019). https://doi.org/10.1007/s11665-019-04272-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-019-04272-z