Abstract
The inhibition effect of New Fuchsin dye on the corrosion of mild steel in 1 M HCl was studied using electrochemical noise measurements (ENM) and the electrochemical impedance spectroscopy (EIS) method. The experimental results indicated that, the New Fuchsin is effective inhibitor even in very low concentration (5 ppm). EIS studies showed that the polarization resistance of mild steel in 1 M HCl increases as the inhibitor concentration increases. Experimental results showed that the DC trend can affect the results obtained from analysis of electrochemical noise data in time domain. DC trend of electrochemical noise data was removed by MAR method. After trend removal, electrochemical noise resistance is confirmed to be strongly correlated to polarization resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Acid solutions are widely used in industry. The most important areas of application are acid pickling, acid cleaning, acid rescaling and oil well cleaning. Corrosion inhibitors are needed to reduce the corrosion rates of metallic materials in these media [1]. Corrosion inhibitors can significantly decrease the corrosion rate when added to a corrosive environment in small concentrations. The inhibition mechanism is carried out through their adsorption on the metallic surfaces [2]. Most of effective corrosion inhibitors are chemical compound with high toxicity and dangerous for environments. Therefore it is desirable to find effective inhibitors with low toxic characteristics [3]. Several authors have been focused on the investigation of organic dyes as inhibitors for steel [4–6].
There are several electrochemical methods to study the corrosion inhibition characteristics of materials. Among these methods, Electrochemical Noise Measurements (ENM) has also been successfully applied to the study of corrosion inhibitor performance [7–10]. ENM has gained popularity in the recent years and has emerged as a promising technique for corrosion analysis. Electrochemical noise describes the low level spontaneous fluctuations of potential and current that occurs during an electrochemical process [11]. The methods based on ENM do not need any externally imposed perturbation to the electrochemical system that could change its specific properties [12]. The conventional measurement technique that is widely used and accepted is based on the measurement of the current noise between two nominally identical working electrodes (using a zero-resistance ammeter) and the potential noise of the coupled working electrode pair [13].
To make electrochemical noise measurements convenient for field applications, the measurement of a quantity generally called noise resistance (Rn) has been proposed. The noise resistance is defined as the ratio of the standard deviation of the EPN (electrochemical potential noise) and ECN (electrochemical current noise) data. It is found that noise resistance can be comparable with polarization resistance (Rp) obtained by other electrochemical methods such as linear polarization and electrochemical impedance spectroscopy (EIS) although controversy is exist between scientists yet [14, 15].
Instability of the test electrode during the measurement period is a problem that can affect many electrochemical techniques [15]. Electrochemical noise data is regarded as random fluctuations around some mean values. For example, in the case of electrochemical potential noise the mean value is corrosion potential. It is often observed that the corrosion potential tends to drift during an experiment. This phenomenon is referred to as DC trend and the process of removing it is called trend removal [16]. It has been shown that DC trend can affect standard deviations of the potential and current fluctuations (σV, σi), and then can change the noise resistance value (Rn = σV/σi). Therefore trend removal process is necessary prior to statistical analysis [15]. Among different trend removal methods, linear trend removal (LTR) and moving average removal (MAR) are two most prominent methods that have been applied to electrochemical noise data [16]. Disagreement exists yet about the ability of mentioned trend removal methods and more experimental assays need to evaluate the real capacity of these methods to correct the ENM results.
The aim of this study is the investigation of inhibition characteristics of New Fuchsin (NF) dye on the corrosion of steel in hydrochloric acid as low toxic and effective inhibitor by electrochemical noise measurements and comparison of obtained results with EIS (electrochemical impedance spectroscopy) results before and after trend removal using MAR method. The inhibition effect of other Triphenylmethane derivatives, namely fuchsin basic (FB) and fuchsin acid (FA), was investigated against copper corrosion in sulphuric acid and citric acid solutions [17, 18] which are not same derivatives as new fuchsin that are used in the present study.
2 Materials and methods
2.1 Materials
The chemical composition (wt. %) of the steel specimen (determined by SPECTROLAB quantometer) is given in Table 1. The steel specimens were polished with emery papers no. 400–1200 grade. They were degreased with acetone, washed with double-distilled water, and finally dried at room temperature before immersion in the acid solution. The acid solutions (1 M HCl) were made from analytical grade 37% HCl using double-distilled water.
New Fuchsin dye with purity of 99.99% provided by Merck was used in this study. The molecular structure of the NF dye is given in Fig. 1. The employed concentration range of NF was 5–200 ppm.
2.2 Methods
2.2.1 Electrochemical Impedance Spectroscopy (EIS)
EIS measurements were carried out using AUTOLAB Potentiostat-Galvanostat (PGSTAT30) and the FRA version 4.9. 005Beta software. A sinusoidal voltage signal of 5 mV was applied over a frequency range of 10 KHz–50 mHz. All EIS experiments were performed in open circuit potential using a three–electrode cell configuration consist of a saturated Ag/AgCl electrode as reference, platinum sheet as counter electrode and mild steel sample as working electrode. The working electrode was prepared from steel, mounted in polyester such that the area exposed to solution was 1 cm2. All experiments were performed after 1 hour’s immersion under atmospheric conditions and in 25 °C.
2.2.2 Electrochemical noise (EN)
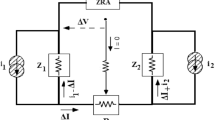
Electrochemical noise data were recorded using an AUTOLAB Potentiostat-Galvanostat (PGSTAT30) and GPES (General Purpose Electrochemical Software) version 4.9 005Beta software. A three–electrode cell configuration (Fig. 2) consists of two identical steel electrodes as dual working electrodes (each other with 1 cm2 surface area) and a saturated Ag/AgCl electrode as a reference electrode was used. The working electrodes were prepared in a similar manner that described for EIS studies. Electrochemical current noise was measured between the two working electrodes and simultaneously, the potential noise was measured between two coupled working electrodes (coupled through the Zero Resistance Ammeter) and the reference electrode. All experiments were carried out in open circuit potential condition. For all the experiments, a sampling interval of 0.5 s was chosen over 512 consecutive data points. The frequency domain corresponding to the sampling conditions was evaluated to be between 1 Hz (f max) and 3.91 mHz (f min), from f max = 1/2Δt, where Δt is the sampling interval and f min = 1/NΔt, where N is the total number of data points [11]. All experiments were performed after 1 hour immersion under atmospheric conditions and in in 25 °C.
2.3 Data analysis
2.3.1 EIS data analysis
Nyquist plots of steel in HCl solution consist of a single semicircle (capacitive like) which can be modeled as an electric equivalent circuit with a parallel combination of double-layer capacitance, Cdl, and polarization resistance, Rp, in series with the solution resistance, Rs, (Fig. 3) as has been reported for steel corrosion in acid media [19, 20].
In evaluation of Nyquist plots the difference in real impedance at lower and higher frequencies is considered as polarization resistance (Rp). To obtain the double layer capacitance (Cdl), the frequency of the maximum imaginary component of the impedance (-Z″max) is found and Cdl values are obtained from the equation [21]:
2.3.2 EN data analysis
The noise resistance, R n, was determined in the time domain, as the ratio of the standard deviation of potential noise, σV, to that of current noise, σI, (R n = σV/σI). As it discussed in introduction section, it is necessary to remove the DC trend before the analysis of noise data in time domain. Among different trend removal methods, we used the MAR method that previously described by Tan et al. [8]. This method is one of the most interesting trend removal methods which remove the average values from the noise record, because the average values should be a good estimation of the DC trend. This method can be briefly explained as follows [8]: consider an experimentally recoded voltage-time series consist of k data point (\( \left\{ {V_n } \right\}{}_n = 1,2,3, \ldots i,i + 1,i + 2, \ldots k \)). Any data point in the series, V i, is regarded as a combination of the real noise component and the DC trend component:
V i,noise is the real noise and is required for noise resistance calculation. V i,DC is the DC trend component which has to be removed. According the above assumption, average value of adjacent data points of V i, can be taken as an estimation of the DC trend and thus the DC trend component of each raw noise data(V i,DC) can be statistically calculate:
where p can be 1, 2, 3 or more. The DC trend in the voltage-time record can therefore be removed and the random fluctuation V i,noise could be deduced as:
3 Results and discussion
3.1 EIS results
Figure 4 shows the typical impedance response of the steel electrode after 1 h immersion in the blank solution (1 M HCl). It corresponds to a single time constant, similarly observed by other authors for steel in hydrochloric solution [1, 19, 20] and [22]. The impedance diagram (Nyquist) contains depressed semicircle with the center under the real axis, with such behavior characteristic for solid electrodes [23]. In such cases, the simple circuit consisting of Rp and Cdl (Fig. 3) is usually a poor approximation especially in the presence of effective inhibitors. Therefore, it is necessary to use a constant phase element, CPE, instead of Cdl to account for non-ideal behavior of double layer. The CPE is modeled by the following equation [24]:
where Z is the impedance, j the square root of −1, ω the frequency, C the capacitance and α is the measure of the non-ideality of the capacitor and has a value in the range 0 < α < 1. The values of polarization resistance (Rp) and CPE for mild steel in blank solution are given in Table 2. Nyquist plots of mild steel in 1 M HCl in the presence of different concentrations of NF dye are shown in Fig. 5 and the corresponding parameters are given in Table 2. It is clear from Fig. 5 that the impedance response of mild steel has significantly changed after the addition of NF at different concentrations in 1 M HCl. It is evident from Table 2 that the value of polarization resistance significantly increases with addition of NF dye. The values of polarization resistance increase when the NF concentration increases. There is a tendency for decreasing of the CPE with the concentration of NF. The decrease in the CPE, which can result from a decrease in local dielectric constant and/or an increase in the thickness of the electrical double layer, suggested that the NF molecule act by adsorption at the metal/solution interface [25].
3.2 EN measurements
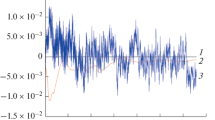
The potential and current noise data were recorded simultaneously as described in the experimental section and the data were analyzed in time domain. Figure 6 shows the raw potential noise data obtained for the steel in 1 M HCl both in the presence and absence of inhibitor at different concentrations and Fig. 7 shows the corresponding raw current noise data.
The noise resistance, R n, was calculated by analysis of raw noise data in the time domain, as the ratio of the standard deviation of potential noise, σV, to that of current noise, σI. The calculated parameters are summarized in Table 3. Analysis of noise data in time domain has the advantages of simplicity and convenience, and this has been demonstrated in several practical applications [8]. It is clear from the Table 3 that the noise resistance of mild steel electrode in 1 M HCl solution significantly increases when the inhibitor adds to corrosive solution in different concentrations. The increase of noise resistance can be directly related to the decrease of σi because of inhibitor film formation. The comparison of obtained results by two different electrochemical methods (EN and EIS) shows that there are high differences between the values of polarization resistance (obtained by EIS) and those of noise resistance (obtained by the analysis of raw noise data in time domain). Tan et al. believe that DC trend can cause inaccuracy in the calculation of noise resistance from time domain analysis, so the DC trend must be removed before the calculation of noise resistance.
Tan’s MAR method [8] was used in this study to remove the DC trend from noise data. Real noise data, used in further statistical analysis, was extracted using this method. MAR was carried out in MATLAB 6.5 with a value of p = 3. Trend removed potential and current noise data are shown in Fig. 6 and Fig. 7 respectively. The analysis of trend removed noise data in time domain was carried out and the calculated noise parameters are given in Table 4. It is clear that the values of noise resistance after trend removal process are quite comparable to Rp values obtained by EIS. However, the values of noise resistance after trend removal are not exactly same as the values of polarization resistance. A possible explanation for this difference could be provided concerning the experimental conditions. In the impedance instrumentation, a fixed potential is imposed, equal to the open circuit potential at the beginning of the experiment, and any differences of local potential on the electrode surfaces are minimal, being corrected through feedback. Under natural conditions, there is, however, no imposed external control, so the local potential is able to vary more and in this condition the resistance of surface can be larger [26]. Fig. 8 shows a comparison of noise resistance before and after of MAR process with the polarization resistance. As it is clear from Fig. 8, good agreement between noise resistance and polarization resistance is obtained only after the trend removal. The results of this study show that the noise resistance is the special form of polarization resistance which can be named statistical polarization resistance. In other words, these results show that the ENM can be used as a powerful method to determine the corrosion rate of metals. Our results also show that the MAR method is a very useful method to remove DC trend from noise data to produce accurate information, as reported by Tan et al. previously [7].
3.2.1 Inhibitor adsorption
Suitable adsorption isotherm can be used to represent the adsorption of inhibitor molecules on the steel surface. Fruendlich isotherm is generally expressed by the following equation [27]:
Where θ is surface coverage, C is the concentration, K and n are coefficients. Surface coverage can be calculated using the following equation [1]:
where R (inh) and R 0 are the corrosion resistance in the presence and absence of inhibitor respectively. The values of surface coverage are listed in Table 4. The fit of the data to the Fruendlich isotherm was very good with a correlation coefficient of 0.9963 (Fig. 9).From Table 2, it is clear that the addition of NF in different concentrations dose not significantly change the corrosion potential of steel in 1 M Hydrochloric solution. This fact shows that studied inhibitor can be classified as mixed type inhibitor which protect metal surface by adsorption. Generally, organic compounds cannot be designed specifically as the anodic or the cathodic inhibitors. They are mixed inhibitors that inhibit both the anodic and cathodic reactions. Small changes in potentials can be a result of the competition of the anodic and the cathodic inhibiting reactions, and of the metal surface condition [28]. Also it is clear that the NF acts as an adsorption inhibitor, but more investigations need to understand the mechanism of adsorption.
4 Conclusion
The following main conclusions are drawn from the present study:
-
1.
New Fuchsin acts as an effective corrosion inhibitor for mils steel in 1 M hydrochloric acid even in very low concentration.
-
2.
As the inhibitor concentration increases the values of polarization resistance increase and the values of double layer capacitance decrease. These results can be explained by inhibitor adsorption on the metal surface.
-
3.
Analysis of noise data in time domain and comparison of calculated noise resistance with the polarization resistance obtained by EIS reveal that the DC trend can strongly influence noise parameters accuracy.
-
4.
It is found that the MAR trend removal method is very useful so that analysis of trend removed noise data in time domain produced some data that was in very good agreement with EIS results.
-
5.
Adsorption of NF on the metal surface obey Fruendlich isotherm. NF acts as mixed type inhibitor.
References
Ashasi-Sorkhabi H, Shaabani B, Seifzadeh D (2005) Electrochim Acta 50:3446
Garcıa-Ochoa E, Genesca J (2004) Surf Coat Technol 184:322
Ashasi-Sorkhabi H, Seifzadeh D (2006) 1:92
Oguzie EE, Onuoha GN, Onuchukwu AI (2005) Mater Chem Phys 89:305
Ebensoa EE, Oguzie EE (2005) Mater Lett 59:2163
Oguzie EE, Unaegbu C, Ogukwea CN, Okolue BN, Onuchukwu AI (2004) Mater Chem Phys 84:363
Dong ZH, Guo XP, Zheng JX, Xu LM (2002) J Appl Electrochem 32:395
Tan YJ, Bailey S, Kinsella B (1996) Corros Sci 38:1681
Tan YJ, Bailey S, Kinsella B (1998) Corros Sci 40:513
Aballe A, Bethencourt M, Botana J, Marcos M, Osuna RM (2002) Electrochim Acta 47:1415
Girija S, Kamachi Mudali U, Raju VR, Dayal RK, Khatak HS, Raj B (2005) Mater Sci Eng A 407:188
Smulko J, Darowicki K (2003) J Electroanal Chem 545:59
Cottis RA (2006) Russ J Electrochem 42:497
Eden DA, Hladky K, John DG, Dawson JL (1998) Electrochemical noise. Simultaneous monitoring of potential and current noise signals from corroding electrodes, corrosion 86, paper 274. NACE, Houston, TX
Mansfeld F, Sun Z, Hsu CH, Nagiub A (2001) Corros Sci 43:341
Lowe AM (2002) Estimation of electrochemical noise impedance and corrosion rates from electrochemical noise measurements. PhD Thesis, Curtin University of Technology, Curtin, Australia
Bastidas JM, Pinilla P, Cano E, Polo JL, Miguel S (2003) Corros Sci 45:427
Cano E, Pinilla P, Polo JL, Bastidas JM (2003) Mater Corros 54:222
Bentiss F, Lagrenee M, Traisnel M, Hornez JC (1999) Corros Sci 41:789
Elkadi L, Mernari B, Traisnel M, Bentiss F, Lagrenee M (2000) Corros Sci 42:703
Ozcan M, Dehri I, Erbil M (2004) Appl Surf Sci 236:155
Ashasi-Sorkhabi H, Shaabani B, Seifzadeh D (2005) Appl Surf Sci 239:154
Chauhan LR, Gunasekaran G (2007) Corros Sci 49:1143
Roeper DF, Chidambaram D, Clayton CR, Halada GP (2008) Electrochim Acta 53:2130
Benabdellah M, Touzani R, Aouniti A, Dafali A, El Kadiri S, Hammoutia B, Benkaddour M (2007) Mater Chem Phys 105:373
Gouveia-Caridade C, Isabel M, Pereira S, Brett C (2004) Electrochim Acta 49:785
Raghuvanshi SP, Singh R, Kaushik CP (2004) 2:35
Stupnisek-Lisac E, Gazivoda A, Madzarac M (2002) Electrochim Acta 47:4189
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashassi-Sorkhabi, H., Seifzadeh, D. Analysis of raw and trend removed EN data in time domain to evaluate corrosion inhibition effects of New Fuchsin dye on steel corrosion and comparison of results with EIS. J Appl Electrochem 38, 1545–1552 (2008). https://doi.org/10.1007/s10800-008-9602-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9602-7