Abstract
Zinc oxide (ZnO) is emerging as a promising n-type thermoelectric material (TE) for power harvesting due to its high melting point and large Seebeck coefficient. However, the TE performance of ZnO is limited by high thermal conductivity and low carrier mobility. Adding or doping a divalent element such as cadmium oxide (CdO) can lower the thermal conductivity and enhance the carrier concentration of ZnO. In this paper, the thermoelectric transport properties of ZnO-CdO nanocrystalline thin films are investigated by varying the Zn/Cd ratio at temperatures ranging from room temperature (RT) to 423 K. The electrical conductivity, carrier concentration and mobility of ZnO were enhanced by increasing the Cd concentration. The maximum power factor of 2.75 × 10−4 W m−1 K−2 was obtained at 423 K for the Zn/Cd = 1:3 sample. The thermal conductivity was dominated by lattice thermal conductivity in which Umklapp scattering occurs between anharmonic phonons. The thermal conductivity of ZnO decreased significantly with increasing Cd concentration. The highest estimated figure of merit (ZT) of 0.59 was found at 413 K for the Zn/Cd = 1:3 sample, which is 223 times greater than for ZnO, indicating that the film is efficient in energy generation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, serious concern has arisen regarding energy consumption and pollution. Thermoelectric (TE) materials have received much attention because they are capable of converting waste heat into energy. Thermoelectric materials can convert thermal energy to electrical energy without moving parts. Therefore, researchers across the world are looking for efficient thermoelectric materials. There are three major obstacles to the wide dissemination of TE harvesters: toxicity and scarcity of elements constituting TE materials, low conversion efficiency, and low stability of thermoelectric materials. Commercial materials for thermoelectric power generation include Pb, Hg, and Se, which are toxic,1,2,3 and Te and Bi which are expensive.4,5 Oxide materials are an environmentally friendly and cost-effective alternative for practical applications of thermoelectric power generation, and the development of improved oxide materials remains the most important issue.
Binary oxide semiconductors have attracted considerable attention due to their unique properties and potential use in energy storage, photodetector, photocatalyst, electrocatalyst and gas sensing applications.6,7 The transport properties of binary nanocomposites such as ZnO-CdO, ZnO-MgO, ZrO2-Y2O3 and SnO2-TiO2 have garnered the attention of the TE community due to their excellent stability at higher temperatures in an oxidizing atmosphere which is useful for fabricating various electronic devices.8,9,10,11 Many research groups have investigated various properties of ZnO-CdO composite films. These films display very high optical conductivity on the order of ~ 1014, a tunable band gap, high photosensitivity and semiconducting behavior.12,13,14 Generally, ZnO and CdO are both n-type semiconductor material.14 In the visible region, ZnO thin film is highly transparent, while the transparency of CdO thin film is low.12,13,14 On the other hand, the electrical conductivity of ZnO thin film is low, while the electrical conductivity of CdO thin film is high. ZnO has a large band gap of 3.10 eV to 3.37 eV15 while CdO has a band gap ranging from 2.20 eV to 2.50 eV.16 Thus, the band gap can be reduced by incorporating an appropriate amount of CdO into ZnO, making the composition suitable for the fabrication of heterojunctions in optoelectronic devices, and therefore the ability to convert thermal energy into electrical energy. As a result, the binary ZnO-CdO system may be considered a promising alternative for the synthesis of high-quality, conductive thermoelectric materials. Recent studies also report the suitability of ZnO-CdO for thermoelectric applications.17,18,19
A variety of physical and chemical deposition techniques have been reported for the preparation of ZnO-CdO composite thin films including screen-printing,20 pulsed laser deposition,21 molecular beam epitaxy,22 sol–gel processing,23 RF-magnetron sputtering24 and spray pyrolysis.25 Among these techniques, spray pyrolysis has the greatest advantages owing to its simplicity and cost-effectiveness. It is also suitable for large-area and nano-structured film deposition and offers ease of handling.
In the present work, the impact of the Zn/Cd ratio on the morphology and thermo-transport properties of ZnO and ZnO-CdO (Zn/Cd = 3:1, Zn/Cd = 1:1 and Zn/Cd = 1:3) thin films prepared by spray pyrolysis is investigated. Electronic transport properties including thermoelectric power, electrical resistivity and carrier concentration are measured experimentally. The total thermal conductivity is combined with the lattice thermal conductivity, estimated theoretically using the Slack model,26,27 and electronic thermal conductivity, calculated from the Wiedemann-Franz law. The results show that the estimated figure of merit ZT increases remarkably for Zn/Cd = 1:3 samples due to higher electrical conductivity.
Experimental
ZnO and ZnO-CdO thin films were deposited on glass substrate using spray pyrolysis in an air atmosphere. The spray solutions were prepared by mixing 0.1 M of zinc acetate dihydrate [Zn(COOCH3)2·2H2O] and cadmium acetate dihydrate [Cd(COOCH3)2·2H2O] in ethanol and deionized water in a 1:1 ratio. Then the ratio of Zn/Cd was varied at 1:0, 3:1, 1:1 and 1:3 to obtain ZnO and ZnO-CdO thin films. In general, the spray pyrolysis unit used to deposit thin films consists of an atomized spray solution and a substrate heater. To prepare the ZnO and ZnO-CdO thin films, 100 mL of spray solution was placed in a beaker fitted with a spray nozzle. A clean glass substrate with a suitable mask was put on the subsector of the heater. The distance between the tip of the nozzle and surface of the substrate heater was maintained at 21 cm. The spraying of solutions was initiated after the glass substrate reached the requisite temperature of 360 °C. The temperature was controlled using a Variac transformer and measured using a copper-constantan thermocouple. The spraying time was 20 min, and the solution flow rate and gas pressure were maintained at 1.0 ml/min and 105 N/m2, respectively. All the parameters were maintained to produce ZnO and ZnO-CdO thin films of almost equal thicknesses. The thickness of thin films was 180 ± 20 nm as determined using Newton’s rings. The details of film thickness measurement using Newton’s rings are reported in our previous works.28
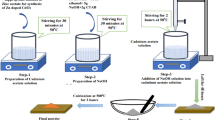
The surface morphology of all samples was studied using a Hitachi S-3400N scanning electron microscope. The temperature-dependent resistivity was determined by the Van der Pauw method with a four-probe configuration in ambient air using a Keithley electrometer. Electrical conductivity was calculated using the resistivity data. The Hall voltage of all samples was measured at a constant magnetic field of 9.815 kilogauss (kG) using the conventional Van der Pauw method. Electromagnets (Newport Instruments Ltd., UK) were used to produce the magnetic field for the Hall effect study. The magnetic pole pieces were placed 2 cm apart. Current and voltage were measured by a digital electrometer and a digital multi-meter, respectively. A voltage stabilizer was used so that a steady supply of current without fluctuation was maintained through the magnet. The circuit arrangement and detailed description of the Hall effect measurement is discussed elsewhere.29 The thermoelectric study of all samples was carried out by the integral method.30,31 The schematic of the thermoelectric measurement using the integral method is shown in Fig. 1. For the thermoelectric power measurement of ZnO-CdO thin films, one end of the sample was placed in an ice bath (273 K) and the other end was heated from room temperature (RT) to 423 K. Samples were covered with a mica sheet during the thermoelectric measurement. The variation in temperature at the hot end is controlled by regulating the heater voltage. The temperature of both hot and cold junctions was monitored using a chromel-alumel thermocouple connected to separate digital multi-meters (KTI, KT 105), with accuracy of ±5°C. The generated thermal emf is measured using another digital multi-meter (KTI, KT 105). The standard deviation of emf is maintained within ±5 μV in the total temperature range. The lattice thermal conductivity of all samples was estimated by a theoretical approach using the Slack model.26,27
Results and Discussion
ZnO-CdO samples are polycrystalline in nature as confirmed by X-ray diffraction (XRD) study and reported elsewhere.12,13,14 It was found that ZnO possess a hexagonal (wurtzite) structure, whereas, Zn/Cd = 3:1, Zn/Cd = 1:1 and Zn/Cd = 1:3 possess a hexagonal-cubic structure. The surface morphology of the ZnO-CdO thin films was characterized by SEM in nanoscale range as shown in Fig. 2a–d.
Analysis of SEM images reveals that the shape of the grains is transformed from nano-polyhedrons to nano-fiber- or tissue-like, and then to nano-flake-like structures depending on the ratio of Zn/Cd. The larger ionic radius of Cd2+ (0.096 nm) compared to Zn2+ (0.074 nm) and structural phase transition from wurtzite to cubic with the change in Zn/Cd ratio alters the surface morphology of the samples. This structural phase transition is also supported by XRD study reported previously.12,13,14
The temperature-dependent electrical conductivity of ZnO and dual phases of ZnO-CdO (Zn/Cd = 3:1, 1:1 and 1:3) thin films is shown in Fig. 3a. The variation in electrical conductivity is non-linear and increases with increasing Cd content. This can be attributed to the increase of the free carrier concentration. The carrier concentration is directly associated with the conductivity of the material and is in good agreement with the classical Drude theory.32 From the Hall effect measurement, samples are identified as n-type material, with electrons as the predominant charge carriers. The n-type behavior is mainly due to the presence of intrinsic defects such as oxygen vacancies (Vo), Zn interstitials (Zni)33 and Cd interstitials (Cdi)34 in ZnO and ZnO-CdO samples. The carrier concentration and Hall mobility measured at room temperature are shown in Fig. 3b. Both the carrier concentration (left scale) and Hall mobility (right scale) are seen to increase with Cd ratio. However, the charge carrier concentration remains nearly constant when the Zn/Cd ratio changes from 1:0, 3:1 and 1:1, although the mobility shows a near linear rise. As the Zn/Cd ratio changes, the type and concentration of defects can vary. The presence of different types of defects, such as Vo, Zni, and Cdi, can affect the overall carrier concentration. The influence of these defects on the carrier concentration may counterbalance the increase due to Cd doping, leading to the observed nearly constant carrier concentration. On the other hand, the near-linear rise in mobility is likely influenced by changes in crystal quality and scattering mechanisms. The specific behavior depends on the concentration of Cd, growth conditions, and the resulting defect landscape in the thin films.
The thermoelectric power (S) of all samples was calculated using the equation S = \(\pm \frac{\Delta V}{\Delta T}\). The dependence of thermoelectric power (S) on ZnO with the addition of CdO is shown in the thermoelectric power versus temperature graph in Fig. 3c. The thermoelectric power of all samples decreases sharply up to ~ 328 K and decreases slowly or remains steady afterwards. The specific temperature at which S starts to decrease sharply is seen to shift from ~ 313 K to ~ 328 K with the increase of Cd concentration in the films. It is also observed that the value of S increases for Zn-rich films. The decrease of S is due to higher carrier concentration of for Cd-rich films compared to Zn-rich films which is well consistent with the Hall effect study. The increase of Cd concentration enhances the carrier concentration of the Cd-rich film which causes a shift of Fermi level (EF) close to the conduction band (EF ≈ EC). This is because when Cd is added to ZnO as a dopant or alloying element, it introduces additional electrons into the material since CdO is an n-type semiconductor. These extra electrons act as charge carriers, increasing the electron concentration in the conduction band of the ZnO material. As a result, the Fermi level rises to approach the energy level of the conduction band (EF ≈ EC). The shift of Fermi level in ZnO due to doping was reported previously.35,36 Therefore, the behavior of the Cd-rich sample becomes metal-like and S decreases as a consequence.
The power factor (PF) of ZnO-CdO samples is calculated from the formula PF = S2/ρ. The temperature-dependent power factor is shown in Fig. 3d. The power factor of all samples is seen to increase with temperature, whereas the PF of Cd-rich samples increases remarkably due to the higher conductivity of the Cd-rich sample compared to Zn-rich samples. The highest power factor is found to be 2.75 × 10−4 W m−1 K−2 for the Zn/Cd = 1:3 sample.
The thermal conductivity of a material is the material's capacity to conduct heat. A good thermoelectric material must possess high electrical conductivity while maintaining low thermal conductivity. In general, the thermal conductivity is the sum of the lattice thermal conductivity and the electronic thermal conductivity. The lattice thermal conductivity of ZnO and ZnO-CdO is calculated using the Slack equation.
where \(\overline{M }\) is the atomic mass of the atom, θD is the Debye temperature, δ is the volume per atom, the constant A is calculated using the equation A = \(\frac{2.43 \times {10}^{-8 }{\gamma }^{2}}{{\gamma }^{2}-0.514\gamma +0.228}\)37 and n is the number of atoms per unit cell. In our work, the theoretical values of A, θD and γ are taken from first-principles calculations38,39 at zero pressure and room temperature which are tabulated in Table I. The Slack equation is an effective tool for calculating the lattice thermal conductivity of various crystalline solids near the Debye temperature depending on the values of θD and γ.26,27 Theoretical calculations show that the variation in θD and γ is almost constant with temperature ranging from 0 to 900 K.38 Since the lattice thermal conductivity is calculated within the temperature range from RT to 423 K, negligible error is considered as we use constant values (room temperature) of the parameters.
The electronic thermal conductivity was calculated using the Wiedemann-Franz law:
where L = 2.44 × 10−8 WΩK−2 and is known as the Lorenz number.
Figure 4a–c shows the variation in thermal conductivity with temperature for the ZnO-CdO samples. From Fig. 4a, it is observed that κlat decreases from 0.42 W m−1 K−1 to 0.15 W m−1 K−1 with the increase in temperature and Cd alloying indicating that the thermal transport is dominated by Umklapp scattering between anharmonic phonons.40,41 The difference in ionic radius between Zn and Cd may introduce some lattice point defects, suppressing the heat transfer of the short wavelength phonons and enhancing the scattering process. Moreover, the increase in grain boundaries may effectively enhance the scattering for long-wavelength phonons. The values of κlat are found to be lower for Cd-rich samples. The increase in atomic mass with the higher Debye temperature and lattice strain fluctuations due to the addition of Cd concentration impede the propagation of phonons, which leads to a decrease in the lattice thermal conductivity for Cd-rich samples.42 On the other hand, κel increases from 1.64 × 10−5 W m−1 K−1 to 0.039 W m−1 K−1 with the increase in Cd concentration as shown in Fig. 4b. The values of κel are highly dependent on the electrical conductivity of the samples. The total thermal conductivity (κ) of ZnO and ZnO-CdO is shown in Fig. 4c. The total thermal conductivity of all samples is dominated by lattice thermal conductivity and decreases from 0.40 W m−1 K−1 to 0.168 W m−1 K−1 with the increase in Cd concentration. It is seen that κ is lower for Cd-rich samples. The figure of merit of a thermoelectric device is a measure of its thermoelectric performance. It is defined as ZT = S2/ρκ, where S is the Seebeck coefficient, ρ is the resistivity and κ is the thermal conductivity. The variations in ZT values with temperature are shown in Fig. 4d. The estimated ZT values increase with increasing Cd content, whereas ZT increases remarkably for Cd-rich samples. The highest value of ZT is estimated at 0.59 at 413 K for the Zn/Cd = 1:3 sample, which is 223 times higher than for ZnO. A comparison of the thermoelectric data values with reported ZnO, doped ZnO and ZnO-CdO is shown in Table II.
Conclusions
ZnO-CdO nano-crystalline thin films with different ratios of Zn/Cd = 1:0, 3:1, 1:1 and 1:3 were deposited on glass substrates by spray pyrolysis under optimized deposition conditions. The surface morphology and thermoelectric transport properties were investigated with the variation in the Zn/Cd ratio. Scanning electron microscopy (SEM) showed that the presence of nano-grains depend on the Zn/Cd ratio. The electrical conductivity, carrier concentration and mobility of the ZnO-CdO samples were increased by increasing the Cd concentration. Both the Hall effect and thermoelectric power measurement confirmed that ZnO-CdO is an n-type material. The thermoelectric power varied from −270 to −650 µV K−1 depending on the temperature and the amount of Cd. The maximum power factor of 2.75 × 10−4 W m−1 K−2 was obtained for the Zn/Cd = 1:3 sample, mainly due to the low resistivity of the sample. The total thermal conductivity decreased with the addition of Cd and was dominated by lattice thermal conductivity. The highest estimated ZT value was 0.59 at 413 K for the Zn/Cd = 1:3 sample, which is 223 times greater than that for ZnO. Considering the above features, ZnO-CdO thin films have potential as novel thermoelectric materials for thermoelectric applications.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
O. Caballero-Calero, J.R. Ares, and M. Martín-González, Environmentally friendly thermoelectric materials: high performance from inorganic components with low toxicity and abundance in the earth. Adv. Sustain. Sys. 5, 2100095 (2021).
S.M.K. Nazrul Islam, M.B. Cortie, and X. Wang, Grape juice: an effective liquid additive for significant enhancement of thermoelectric performance of Cu2Se. J. Mater. Chem. A 8, 16913 (2020).
S.M.K. Nazrul Islam, M. Li, U. Aydemir, X. Shi, L. Chen, G.J. Snyder, and X. Wang, Giant enhancement of the figure-of-merit over a broad temperature range in nano-boron incorporated Cu2Se. J. Mater. Chem. A 6, 18409 (2018).
U. Syafiq, E. Isotta, N. Ataollahi, K. Lohani, S. Luong, V. Trifiletti, O. Fenwick, and P. Scardi, Facile and low-cost fabrication of Cu/Zn/Sn-based ternary and quaternary chalcogenides thermoelectric generators. ACS Appl. Ener. Mater. 5, 5909 (2022).
P. Fan, Z.-H. Zheng, Y.-Z. Li, Q.-Y. Lin, J.-T. Luo, G.-X. Liang, X.-M. Cai, D.-P. Zhang, and F. Ye, Low-cost flexible thin film thermoelectric generator on zinc based thermoelectric materials. Appl. Phy. Lett. 106, 073901 (2015).
Y. Zhang, L. Li, H. Su, W. Huang, and X. Dong, Binary metal oxide: advanced energy storage materials in supercapacitors. J. Mater. Chem. A 3, 43 (2015).
M. Yang, Z. Ye, M.A. Iqbal, H. Liang, and Y.-J. Zeng, Progress on two-dimensional binary oxide materials. Nanoscale 14, 9576 (2022).
G. Hu and Y. Zhang, Quantum piezotronic devices based on ZnO/CdO quantum well topological insulator. Nano Ener. 77, 105154 (2020).
K. Aziz and C. Ekuma, Electronic and vibrational spectroscopy of miscible MgO-ZnO ternary alloys. J. Appl. Phy. 127, 075706 (2020).
T.S. Hofer, F.M. Kilchert, and B.A. Tanjung, An effective partial charge model for bulk and surface properties of cubic ZrO2, Y2O3 and yttrium-stabilised zirconia. Phys. Chem. Chem. Phys. 21, 25635 (2019).
M. Diantoro, M.B. Zaini, T. Suprayogi, N. Mufti, S. Zulaikah, and A. Hidayat, Effect of (SnO2:TiO2) nanoparticles on charging performance of integrated dye-sensitized solar cell-supercapacitor. AIP Confer. Proceed. 2231, 040007 (2020).
A.M.M.T. Karim, M.K.R. Khan, and M.M. Rahman, Effect of Zn/Cd ratio on the optical constants and photoconductive gain of ZnO-CdO crystalline thin films. Mater. Sci. Semicond. Process. 41, 184 (2016).
A.M.M.T. Karim, M.M. Rahman, M. Shahjahan, and M.K.R. Khan, Study of the morphology, photoluminescence and photoconductivity of ZnO-CdO nanocrystals. Mater. Res. Expr. 2, 036402 (2015).
A.M.M.T. Karim, M.K.R. Khan, and M.M. Rahman, Structural and opto-electrical properties of pyrolized ZnO-CdO crystalline thin films. J. Semicond. 36, 053001 (2015).
K. Davis, R. Yarbrough, M. Froeschle, J. White, and H. Rathnayake, Band gap engineered zinc oxide nanostructures via a sol-gel synthesis of solvent driven shape-controlled crystal growth. RSC adv. 9, 14638 (2019).
E. Przeździecka, P. Strąk, A. Wierzbicka, A. Adhikari, A. Lysak, P. Sybilski, J. Sajkowski, A. Seweryn, and A. Kozanecki, The Band-Gap Studies of Short-Period CdO/MgO Superlattices. Nanoscale Res. Lett. 16, 1 (2021).
C. Gayner, Improved electrical conductivity and thermoelectric performance of ZnO by doping with NaCl and CdO. Chem. Eng. J. 413, 128149 (2021).
D.V.C.L. Han, A. Bhowmik, S.B. Simonsen, L.T. Hung, E. Abdellahi, Y.Z. Chen, N.V. Nong, S. Linderoth, and N. Pryds, Scandium-doped zinc cadmium oxide as a new stable n-type oxide thermoelectric material. J. Mater. Chem. A. 4, 12221 (2016).
X. Zha, L. Gao, H. Bai, N. Fu, J. Wang, G. Fu, and S. Wang, Towards a high thermoelectric performance in CdO ceramics by introducing multiscale scattering centers via ZnCO3 doping. Mater. Res. Expr. 5, 035908 (2018).
R.A. Zargar, Fabrication and improved response of ZnO-CdO composite films under different laser irradiation dose. Sci. Rep. 12, 1 (2022).
A.M. Mostafa and A. Menazea, Laser-assisted for preparation ZnO/CdO thin film prepared by pulsed laser deposition for catalytic degradation. Rad. Phy. Chem. 176, 109020 (2020).
H.C. Jang, K. Saito, Q. Guo, K.M. Yu, W. Walukiewicz, and T. Tanaka, Realization of rocksalt Zn1-xCdxO thin films with an optical band gap above 3.0 eV by molecular beam epitaxy. Cryst. Eng. Comm. 22, 2781 (2020).
T.K. Pathak, J.K. Rajput, V. Kumar, L. Purohit, H. Swart, and R. Kroon, Transparent conducting ZnO-CdO mixed oxide thin films grown by the sol-gel method. J. Coll. Inter. Sci. 487, 378 (2017).
S. Cheemadan and M. Kumar, Optoelectronic properties of transparent conducting CdO:ZnO composite thin films by RF-magnetron sputtering. J. Mater. Sci. Mater. Electron. 33, 15638 (2022).
O. Aguilar, S. de Castro, M.P. Godoy, and M.R.S. Dias, Optoelectronic characterization of Zn1-xCdxO thin films as an alternative to photonic crystals in organic solar cells. Opt. Mater. Expr. 9, 3638 (2019).
G.A. Slack, Nonmetallic crystals with high thermal conductivity. J. Phy. Chem. Sol. 34, 321 (1973).
D.T. Morelli and G.A. Slack, High thermal conductivity materials (New York, NY: Springer, 2006).
A.M.M.T. Karim, M.S. Hossain, M.K.R. Khan, M. Kamruzzaman, M.A. Rahman, and M.M. Rahman, Solution-processed mixed halide CH3NH3PbI3−xClx thin films prepared by repeated dip coating. J. Mater. Sci. 54, 11818 (2019).
A.M.M.T. Karim, M.K.R. Khan, and M.S. Hossain, Temperature dependency of excitonic effective mass and charge carrier conduction mechanism in CH3NH3PbI3−xClx thin films. Sci. Rep. 11, 10772 (2021).
V.D. Das and J.C. Mohanty, Size and temperature effects on thermoelectric power of β-tin thin films. J. Appl. Phy. 54, 977 (1983).
A.M.M.T. Karim, M.K.R. Khan, and M.S. Hossain, Evaluation of the thermoelectric properties and thermal conductivity of CH3NH3PbI3−xClx thin films prepared by chemical routes. ACS Omega 6, 16775 (2021).
F. Wooten, Optical properties of solids. Am. J. Phy. 41, 939 (1973).
J. Kaupužs, A. Medvids, P. Onufrijevs, and H. Mimura, Origin of n-type conductivity in ZnO crystal and formation of Zn and ZnO nanoparticles by laser radiation. Opt. Las. Technol. 111, 121 (2019).
M. Burbano, D.O. Scanlon, and G.W. Watson, Sources of conductivity and doping limits in CdO from hybrid density functional theory. J. Am. Chem. Soc. 133, 15065 (2011).
J.-Q. Wen, J.-M. Zhang, Z.-G. Qiu, X. Yang, and Z.-Q. Li, The investigation of Ce doped ZnO crystal: the electronic, optical and magnetic properties. Physica B: Conden. Mat. 534, 44 (2018).
J. Jia, A. Takasaki, N. Oka, and Y. Shigesato, Experimental observation on the Fermi level shift in polycrystalline Al doped ZnO films. J. Appl. Phy. 112, 013718 (2012).
C.L. Julian, Theory of heat conduction in rare-gas crystals. Phys. Rev. 137, A128 (1965).
N. Aarifeen, A. Ahmad, H.B. Munir, and A. Bakar, First principles study of thermodynamic properties of CdxZn1−xO (0≤ x≤ 1) ternary alloys. Zeit. für Naturfor. A 74, 1115 (2019).
N. Aarifeen and A. Afaq, Lattice thermal conductivity of CdxZn1−xX (X=O, S, Se, Te) from first principles. Mater. Chem. Phy. 251, 123099 (2020).
L. Gao, S. Zhai, R. Liu, N. Fu, J. Wang, G. Fu, and S. Wang, Enhanced thermoelectric performance of CdO ceramics via Ba2+doping. J. Am. Ceram. Soc. 98, 3285 (2015).
G. Fu, L. Gao, R. Liu, X. Zha, J. Wang, and S. Wang, Synergistically tuning the electrical and thermal transport properties of CdO: Cu thermoelectric ceramics. Mater. Res. Exp. 4, 075502 (2017).
S.M.K. Nazrul Islam, P. Mayank, Y. Ouyang, J. Chen, A.K. Sagotra, M. Li, M.B. Cortie, R. Mole, C. Cazorla, D. Yu, X. Wang, R.A. Robinson, and D.L. Cortie, Copper diffusion rates and hopping pathways in superionic Cu2Se. Acta Mater. 215, 117026 (2021).
K. Kim, J. Park, S. Hong, S.H. Park, S.G. Jeon, C. Ahn, and J.Y. Song, S, Jeon, Anomalous thermoelectricity of pure ZnO from 3D continuous ultrathin nanoshell structures. Nanoscale 10, 3046 (2018).
G. Li, S. Liu, Y. Piao, B. Jia, Y. Yuan, and Q. Wang, Joint improvement of conductivity and Seebeck coefficient in the ZnO: Al thermoelectric films by tuning the diffusion of Au layer. Mater. Des. 154, 41 (2018).
A.I. Abutaha, S.R.S. Kumar, and H.N. Alshareef, Crystal orientation dependent thermoelectric properties of highly oriented aluminum-doped zinc oxide thin films. Appl. Phys. Lett. 102, 053507 (2013).
N.H.T. Nguyen, T.H. Nguyen, M. Y-r Liu, A.T.T. Aminzare, S. Pham, D.P. Cho, K.-H. Wong, T. Seetawan, N.K. Pham, H.K.T. Ta, V.C. Tran, and T.B. Phan, Thermoelectric properties of indium and gallium dually doped ZnO thin films. ACS Appl. Mater. Inter. 8, 33916 (2016).
V. Sethi, K. Sun, D. Newbrook, D. Runacres, T. Zhang, V. Greenacre, C. H. de Groot, R. Huang, Ultralow Thermal Conductivity and Improved Thermoelectric Properties of Al-Doped ZnO by In Situ O2 Plasma Treatment. Small Struct. 2300140 (2023)
S. Liu, G. Li, M. Lan, M. Zhu, K. Miyazaki, and Q. Wang, Role of intrinsic defects on thermoelectric properties of ZnO: Al films. Ceram. Int. 47, 17760 (2021).
A.T.T. Pham, H.K.T. Ta, M. Y-r Liu, D.P. Aminzare, T.H. Wong, N.K. Nguyen, T.B.N. Le Pham, T. Seetawan, H. Ju, S. Cho, K.-H. Chen, V.C. Tran, and T.B. Phan, Effect of annealing temperature on thermoelectric properties of Ga and In dually doped-ZnO thin films. J. Alloy. Compd. 747, 156 (2018).
P.M.S. Saini, H. Honda, T. Suzuki, K. Matsumoto, K. Miyazaki, A. Ichinose, L. Molina Luna, R. Carlini, and A. Tiwari, Effect of self-grown seed layer on thermoelectric properties of ZnO thin films. Thin Sol. Film. 605, 289 (2016).
Acknowledgments
The authors would like to acknowledge the support of the Department of Advanced Materials Science & Technology University of Yamanashi, Japan, for providing SEM study.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
AMMTK: Conceptualization, Formal analysis, Investigation, Methodology, Writing–original draft, review & editing. MRI: Investigation, Formal analysis, Writing–original draft. HK: Formal analysis, Writing–original draft, review & editing. MKRK: Conceptualization, Supervision, Writing–review & editing. MMR: Supervision, Writing–review & editing. MS: Supervision, Writing–review & editing. MFH: Formal analysis, Writing–review & editing. MAH: Formal analysis, Writing–review & editing. SMKNI: Supervision, Formal analysis, Writing–review & editing.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
I, Al Momin Md Tanveer Karim consciously assure that for the manuscript “Ultrahigh thermoelectric performance of ZnO-CdO thin films” the following is fulfilled: This material is the authors' own original work, which has not been previously published elsewhere. The paper is not currently being considered for publication elsewhere. The paper reflects the authors' own research and analysis in a truthful and complete manner. The paper properly credits the meaningful contributions of co-authors and co-researchers. The results are appropriately placed in the context of prior and existing research. All authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karim, A.M.M.T., Rumana Islam, M., Khatun, H. et al. Ultrahigh Thermoelectric Performance of ZnO-CdO Thin Films. J. Electron. Mater. 53, 188–195 (2024). https://doi.org/10.1007/s11664-023-10761-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10761-8