Abstract
The effect of B2O3 addition on electrical conductivity of CaO-SiO2-B2O3 slag was investigated using the four-electrode method. The slag structure was determined from Raman and nuclear magnetic resonance spectra. The experimental results show that the electrical conductivity, as the B2O3 content in the slag increased from 0 to 10 pct, monotonously increases from 0.0769 to 0.2642 Ω−1 cm−1 at temperatures ranging from 1783 to 1873 K (1510 °C to 1600 °C). The effects of temperature on electrical conductivity obeyed the Arrhenius law, and the electrical conductivity increased as the temperature increased. The Raman spectra results indicated that addition of B2O3 led to an increase of Q3(Si) at the cost of Q2(Si), which will increase the degree of polymerization of the slag. 11B nuclear magnetic resonance spectra showed that both BO3 trigonal and BO4 tetrahedral structures increased with the increasing B2O3 content. There was a competitive effect between the presence of these structures and an enhanced degree of polymerization of slags in the networks, which resulted in the increased electrical conductivity of the slag. A structure–electrical conductivity model for CaO-SiO2-B2O3 slag was established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The conductivity of slag has an important role in the molten oxide electrolysis process and can directly affect product quality and energy consumption. A large number of metals, including aluminum, magnesium, and titanium, have been produced by the electrolysis of molten slags and salts.[1,2] In recent years, the electrochemical nature of metallurgical reactions is receiving more attention; closely related to this is the electrical conductivity of the slag. Study of the electrical conductivity of the slag can help in understanding the transfer behaviors of oxygen ions and electrons in the slag and the electrochemical nature of reactions.
Electrical conductivity is also an important thermophysical property of a molten slag. This can provide not only a foundation for establishing a model, but also information regarding the structures of a slag. For instance, the earliest evidence for the ionic structure of oxide melts resulted from electrical conductance measurements.[3,4] The transport properties of silicate slags, such as conductivity and viscosity, are predominantly affected by their structure. Knowledge of the structure of silicate melts is an effective way to understand the conductivity properties of molten slags.
There have been numerous attempts in the fields of metallurgy and glass science to measure these macroscopic thermophysical properties as a function of the composition of silicates based on a microscopic view of the structure.[5,6,7,8,9,10,11,12,13,14,15,16,17] Most attempts have studied the relationship between the structure and viscosity of a slag. Research on electrical conductivity relies on the relationship between composition and conductivity. In this study, the relationship between the structure and electrical conductivity of CaO-SiO2-B2O3 slag was investigated.
CaO-SiO2-B2O3 slag has important significance for the smelting and refining processes of silicon metallurgy, so the study of its structure and macroscopic properties is useful. The macroscopic properties of these glasses and melts are primarily determined by their microscopic structures. In this study, a macroscopic property (electrical conductivity) of CaO-SiO2-B2O3 slag with different B2O3 contents was measured, and Raman and nuclear magnetic resonance (NMR) spectroscopy were used to study their microscopic structures. This enabled better evaluation of relationships between the structure and thermophysical properties, such as conductivity, of these melts. A structure–electrical conductivity model for CaO-SiO2-B2O3 slag was also established.
Experimental
Conductivity Measurement
A four-electrode technique was used to measure the electrical conductivity of CaO-SiO2-B2O3 slag. To obtain accurate values, the resistances of lead wires and electrodes should be excluded from the total measured resistance, and the four-electrode method avoids the influence of interface polarization.
A series of CaO-SiO2-B2O3 ternary samples were designed with a fixed CaO/SiO2 mass ratio of 0.67, but with the gradually increasing B2O3 content. The sample compositions are shown in Table I. Slag samples were prepared using reagent grade SiO2, CaCO3, and B2O3 powders (analytically pure, Sinopharm Chemical Reagent Co., Ltd, China), all of which were calcined at 1273 K (1000 °C) for 10 hours in a muffle furnace before use to decompose any contained carbonate and hydroxide present before being used. Each sample mixture was accurately weighed according to the compositions shown in Table I and thoroughly mixed in an agate mortar before use.
The experimental setup is schematically shown in Figure 1. A detailed description of the device was introduced in our previous study.[18,19] Before measuring the resistance of the slag, the cell was calibrated at temperatures from 293 K to 298 K (20 °C to 25 °C). A standard solution of KCl (1 mol L−1) was used to determine the cell constant. For the conductivity measurements of the slags, each prepared sample was packed into a platinum crucible (Φ26 mm × 26 mm × 0.3 mm), which was first placed inside a corundum crucible (Φ48 mm × 126 mm × 3 mm) and then in the constant-temperature zone of the furnace. Once the target temperature of 1873 K (1600 °C) was reached, it was held there for 2 hours to ensure that a homogeneous slag composition is achieved. The electrodes were slowly lowered until they made contact with the surface of the melt. When the tips of the electrodes contacted the surface of melt, the resistance measured by the electrochemical station rapidly and significantly decreased. The electrodes were then lowered to a depth of 3 mm below the surface. Resistance measurements were carried out at 30 K intervals while cooling from 1873 K (1600 °C). To ensure the equilibrium of the melt, it was held there for 30 minutes at each temperature before the measurement was made. All measurements were carried out at a frequency of 20 kHz.
Structure Determinations
Fifty grams of each sample composition was loaded into a graphite crucible (Φ45 mm × 100 mm × 4 mm) and placed in a muffle furnace. The temperature of the furnace was raised to 1873 K (1600 °C) at a rate of 10 K min−1 and then held for 2 hours under Ar atmosphere to ensure homogeneity the slag composition. The premelting liquid samples were then quickly poured into cold water. The water-quenched samples were dried in an oven at 353 K (80 °C) for 10 hours then crushed and ground to 200 mesh. X-ray diffraction (XRD; SmartLab, Rigaku, Japan) measurements were performed to ensure that the water-quenched samples remained in the glass state. The results, shown in Figure 2, indicated that none of the samples contained precipitated crystals.
The glass samples were characterized using Raman and NMR spectroscopy. A laser confocal Raman spectrometer (LabRAM HR800, Horiba Jobin Yvon Company) was used for this purpose. The power we used was 50 pct and the number of scans is three. The scanning time is 10 seconds. Solid-state 11B MAS-NMR (AVANCE III HD 500 MHz,Avance III 500M, Bruker with a Speed up to 35 KHz high-speed probe) measures were used to identify the structure role of 11B in the glass. The samples were analyzed using an NMR spectrometer employing the 11B one pulse MAS technique equipped with a Bruker AVANCE II 500 operating at 11B frequency of 160.5 MHz. Approximately 50 to 100 mg of samples was placed into a zirconium oxide rotor with a diameter of 4 mm and kel-F caps. For all the measurements, a spinning speed of 7 KHz was applied, and the recycle delays were set to 1 second. About 128 scans were acquired. The power level for pulse was 200 w, and the high–power pulse was 7.5 μs.
Results and Discussion
Conductivity Measurements
The measured electrical conductivity data for different slag compositions at temperatures ranging from 1783 K to 1873 K (1510 °C to 1600 °C) are shown in Figure 3. The experimental results show that as the B2O3 content in the CaO-SiO2-B2O3 slag increased from 0 to 10 pct, the electrical conductivity monotonously increased from 0.0769 to 0.2642 Ω−1 cm−1 in this temperature range. The logarithms of electrical conductivity as a function of reciprocal temperature are shown in Figure 4. There was a linear relationship between these two parameters, and the electrical conductivity increased with the increasing temperature. It was concluded that the temperature dependence of electrical conductivity complied with the Arrhenius equation[20]:
where σ is the electrical conductivity, Ω−1 cm−1; A is the pre-exponential factor, Ω−1 cm−1; E is the activation energy of electrical conductivity, J mol−1; R is the gas constant, 8.314 J mol−1 K−1; and T is the absolute temperature, K. The activation energies were calculated from the Arrhenius equation, as shown in Table II. The values were relatively close to those of CaO-SiO2-FeOx[21] and FexO-SiO2-CaO-Al2O3.[19]
There are two reasons for the increase of electrical conductivity with the increasing temperature. On the one hand, the ionic distance increases with the increasing temperature, the viscous resistance of molten slags decreases, and so the electrical conductivity increases; on the other hand, the spacing between cations and anions increases, and the interionic attraction between cations and anions decreases. Therefore, the binding energy decreases, and the O2− ions can be more easily plundered by the Si4+ ions to break the network, which will decrease the degree of polymerization (DOP) and increase the electrical conductivity of molten slag. Figure 4 shows that conductivity increased with the increasing B2O3 content from 0 to 10 pct.
Structure Determination
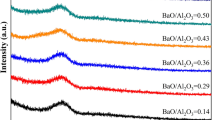
Raman spectra for the various slags are shown in Figure 5. According to the literature.[22,23,24,25] the Raman spectra can comprise three bands attributed to Si-related structures: these are assigned to the O-Si-O bending vibration, Si-O symmetric stretching vibration, and stretching vibration of the SiO4 tetrahedral at 300 to 580, 580 to 770, and 770 to 1150 cm−1, respectively. The B2O3 content had a strong influence on the network structure of the slags. Figure 5 shows that, as the SiO2 content decreased, the relative intensity of the bands associated with O-Si-O bending (300 to 580 cm−1) and Si-O symmetric stretching (580 to 770 cm−1) became less pronounced, and, the peaks at ~ 1080 cm−1, which were assigned to Q3,[26,27,28,29] became more pronounced as the B2O3 content increased. The peaks at about 1350 and 1450 cm−1 were attributed to BO4 tetrahedral and BO3 trigonal structures, respectively.[30,31] These peaks became more pronounced as the B2O3 content increased.
The stretching vibration of the SiO4 tetrahedral (770 to 1150 cm−1) can reflect the DOP of a slag, so essential for understanding the structure of a silicate melt. According to the literature,[32,33,34,35,36,37,38] the bands at about 860, 910, 960, 1080, and 1200 cm−1 can be assigned to Q0, Q1, Q2, Q3, and Q4 respectively. To better understand the structure of the slag, it is necessary to quantitatively calculate the proportions of Q0–Q4. Deconvolution of the Raman spectra was performed in the range of 800 to 1200 cm−1. The results are shown in Figure 6. To reflect changes in the content of Q0–Q4 more intuitively, Eq. [2][39,40,41] was used.
where Ai is the area of the ith peak and Si is the respective Raman scattering coefficient. The values of S0 to S3 are 1, 0.514, 0.242, and 0.009, respectively. The results are shown in Figure 7, from which it can be seen that as the B2O3 content increased, the Q3 content increased significantly, but the Q2 content decreased significantly. In other words, the increase of Q3 occurred at the cost of Q2.
The nonbridging oxygens also can reflect the DOP of a slag. Equation [3][42] was used to reflect the change in the contents of nonbridging oxygens (NBO/T).
The corresponding results are shown in Figure 8. The NBO/T content decreased with the increasing B2O3 content, indicating that the addition of B2O3 increased the DOP of the slag network. In other words, B2O3 acted as a network former in these slags. In the pure B2O3 slag state (B-O-B), all oxygens are assumed to originally exist as bridging oxygens. In a silicate slag, B2O3 will capture the oxygens of Q2 (Si) once it is introduced into the networks; therefore, more Q3 (Si) is formed at the cost of Q2(Si). The reactions can be described in the form of Eqs. [4] and [5]. The network will therefore contain Si-O-Si and Si-O-B bridging oxygens. From Figure 6, it was concluded that the main reaction is the conversion from Q2 to Q3, as shown in Figure 9.
To further study changes of the slag structure, 11B magic-angle spinning (MAS)-NMR spectra results were analyzed, as shown in Figure 10. According to the Raman studies, it was concluded that the regions from − 3.7 to 18 and − 15 to − 3.7 ppm are attributed to the BO3 trigonal and BO4 tetrahedral structures, respectively.[43,44,45] The BO3 trigonal structure was also identified by ring and non-ring BO3 units that are located at ~ 5.5 and ~ 1.5 ppm, respectively. The BO4 tetrahedral was also identified by the BO4 (1B3Si) and BO4 (0B4Si) units located at about − 5.5 and − 10 ppm, respectively.[41,42,43] The 11B MAS-NMR spectra results showed that the intensities of both the BO3 trigonal and BO4 tetrahedral peaks increased with the increasing B2O3 content.
Structure–Electrical Conductivity Model for CaO-SiO2-B2O3 slag
The CaO-SiO2-B2O3 slag can be considered to contain ionic conductors, where the Ca2+ cation is assumed to be the main charge carrier when subjected to an electric field. Equation [6] can be used to describe the electrical conductivity:
where σ is the electrical conductivity(S m−1); c is the concentration of Ca2+(mol m−3); z is the charge number of Ca2+; F is Faraday’s constant (C mol−1); and µ is mobility (m2 V−1 s−1). For this slag, the mobility (µ) of the charge carrier is the only unknown factor. Based on the Einstein relation, mobility can be related to a species diffusion coefficient, as shown in Eq. [7]:
where D is the species diffusion coefficient (m2 s−1). Thus, Eq. [6] can be presented as follows:
An electrical conductivity model was developed based on the Nernst–Einstein equation. The species diffusion coefficient D can be represented by the effective diffusivities of the cations,[46] which are described as a function of slag structure. In this slag, the simple oxygen-bonding structures of slag mainly included nonbridging oxygens (bridged, broken, and free oxygen) and B-O bonding (BO3 trigonal and BO4 tetrahedral units). Therefore, the effective diffusivity D is described using Eq. [9]:
where D0 is the pre-exponential factor; E is the activation energy; B is the activation energy when the diffusing species is not being hindered by the medium through which it is diffusing; C is the increase in activation energy due to the increase in polymerization of the slag expressed by the pair fractions of NBO, BO3, and BO4. Equation [8] then can be expressed as follows:
The conductivity of a slag is determined by ionic and electronic conductivities. In silicate melts used in metallurgy, conductance is primarily determined by cation conduction; this was proved by the study of Martin and Derge,[47] which concluded that conductivity in a CaO-SiO2-Al2O3 slag occurs via cation. According to theory, there are simple metal cations and complex polymeric anions in a slag. Since the 1950s, a lot of research has been undertaken on the structure of slags. Most metal oxides release oxygen ions to form metal cations, while some expensive cerium oxides receive oxygen ions from suboxides to form complex anions. Complex anions have a large influence on the electrical conductivity. The electronic conductivity is an important parameter used to measure the transmission speed of electrons in the slag. Under certain conditions, although the electronic conductivity and the number of electron migrations in an ionic slag are small, they will have a significant impact on the electrochemical reaction. Many studies have been undertaken on the electrical conductivity of slags. It has been found that slags that contain FeO, MnO, and TiO exhibit electronic conductivity.[48,49,50,51]
From previous studies, we know the slag that was used in the current study exhibits anion conductivity. The electrical conductivity of the slag is usually determined in two ways: from the ion concentration and from the structure of the slag. For this slag, charge transport is completed by Ca2+ ions. Si4+ and B3+ have large valencies and small ionic radii, and therefore, they have large interactions with nearby ions and form network units, which leads to small contributions of B3+ and Si4+ ions to the charge conductance.[52] Oxygen ions will connect with B3+ or Si4+, forming bridging or nonbridging oxygens, the mobility of which will be weak.[53] Hence, it can be concluded that the main ion that contributes to charge conductance is Ca2+.
For this CaO-SiO2-B2O3 slag, the conduction ion is Ca2+, and the values of z, F, R, and T are constant. The differences between the slags are the concentrations of Ca2+ and the activation energy which is determined by slag structure. The pair fractions of NBO, BO3, and BO4 were calculated from the Raman and NMR data. A structure–electrical conductivity model for the CaO-SiO2-B2O3 slag was established, as follows:
For a specified CaO-SiO2-B2O3 slag, the pair fractions of NBO, BO3, and BO4 can be calculated from the results of Raman and NMR; then, the Eq. [11] can be used to estimate the electrical conductivity of CaO-SiO2-B2O3 slag.
It is known that microscopic structures determine the macroscopic properties of slag melts; therefore, the variation of the electrical conductivity for these slags will be a comprehensive consequence of their microscopic structures. On adding B2O3 to CaO-SiO2-B2O3 slag, the DOP of the melt and the intensities of both the BO3 trigonal and BO4 tetrahedral peaks increased, which affected the diffusivity of the cation: first, an increase in DOP of the slag led to a decrease of electrical conductivity; second, the BO3 trigonal and BO4 tetrahedral units appeared after adding B2O3 to the slag. The BO3 trigonal, as one of the dominant B-related units, is a two-dimensional structure and will greatly reduce the symmetry and the strength of the networks. Furthermore, due to the influence of BO4 group avoidance, the BO4 tetrahedral is preferentially connected to nearby Si atoms and introduced into the silicate networks. This reduces the uniformity of the network structure, and as a result, the strength of the slag structure will decrease. These deformations facilitate Ca2+ to jump from one site to another. In contrast to the effect of the increasing DOP, the increases in BO3 trigonal and BO4 tetrahedral structures could cause the resistance of the charge conductance to decrease observably, resulting in an increase of electrical conductivity. In other words, for these slags, the effect of the increasing electrical conductivity caused by the presence of the BO3 trigonal and BO4 tetrahedral units is stronger than the effect of decreasing electrical conductivity caused by the DOP. This is the reason for the increase of electrical conductivity with the increasing B2O3 content.
Conclusion
A four-electrode technique was used to investigate electrical conductivity changes of CaO-SiO2-B2O3 slag with the addition of B2O3. It was concluded that conductivity and temperature comply with the Arrhenius equation. As the B2O3 content increased, the electrical conductivity increased. The reason may be that BO3 trigonal and BO4 tetrahedral units were produced with the addition of B2O3. These units decreased the symmetry and uniformity of the structure, and thereby reduced its strength and increased the electrical conductivity of the slag. A structure–electrical conductivity model for CaO-SiO2-B2O3 slag was established
References
R. Winand: Proc. Conf. Extraction Metallurgy, vol. 81, IMM, London, 1981, pp. 20–33.
D.R. Sadoway: U.S. Patent 5, 1993, 185.
3. G.H. Zhang, W.W. Zheng, and S.Q. Jiao: ISIJ Int., 2017, vol. 57, pp. 2091-2096.
4. A.E. Martin and G. Derge: Trans. AIME, 1943, vol. 154, pp. 104.
5. S. Seetharaman, K. Mukai, and D. Sichen: Steel Res. Int., 2005, vol. 76, pp. 267-278.
6. R.E. Aune, M. Hayashi, and S. Sridhar: Ironmaking and Steelmaking, 2005, vol. 32, pp. 141-150.
7. J.H. Park and D.J. Min: J. Non-Cryst. Solids, 2004, vol. 337, pp. 150-156.
8. J.H. Park, D.J. Min and H.S. Song: Metall. Mater. Trans. B, 2004, vol. 35B, pp. 269-275.
9. B.O. Mysen: Eur. J. Mineral., 2003, vol. 15, pp. 781-802.
10. J.H. Park, D.J. Min, and H.S. Song: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 723-29.
11. J.H. Park, D.J. Min, and H.S. Song: ISIJ Int., 2002, vol. 42, pp. 344 -51.
D. Giordano, J.K. Russell, and D.B. Dingwell: Earth Planet. Sci. Lett., 2008, vol. 271, pp. 123–34.
13. J.H. Park, H. Kim, and D.J. Min: Metall. Mater. Trans. B, 2008, vol. 39B pp. 150-53.
14. J.H. Park and D.J. Min: ISIJ Int., 2007, vol. 47, pp. 1368–69.
15. D.R. Neuvill: Chem. Geol., 2006, vol. 229, pp. 28-41.
16. H. Kim, W.H. Kim, J.H. Park, and D.J. Min: Steel Res. Int., 2010, vol. 81, pp. 17–24.
17. P. Richet: J. Non-Cryst. Solids, 2009, vol. 355, pp. 628-35.
18. J.H. Liu, G.H. Zhang, Y.D. Wu, and K.C. Chou: Metall. Mater. Trans. B, 2016, vol. 47(1), pp. 798-803;
19. J.H. Liu, G.H. Zhang, and K.C. Chou: ISIJ Int., 2015, vol. 55, pp. 2325-31.
20. J.H. Liu, G.H. Zhang, and K.C. Chou: Can. Metall. Q., 2015, vol. 54, pp. 170-77.
21. M. Barati and K.S. Coley: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 41-9.
22. P. McMillan: Am. Mineral., 1984, vol. 69, pp. 622-44.
23. J.D. Frantz and B.O. Mysen: Chem. Geol., 1995, vol. 121, pp. 155-76.
24. B.O. Mysen and D.R. Neuville: Geochim. Cosmochim. Acta, 1995, vol. 59, pp. 325-42.
25. D.R. Neuville, D.D. Ligny, and G.S. Henderson: Rev. Mineral. Geochem., 2014, vol. 78, pp. 509-41.
26. Y. Tsunawaki, N. Iwamoto, T. Hattori, and A. Mitsuishi: J. Non-Cryst. Solids, 1981, vol. 44, pp. 369-78.
27. Y.Q. Sun and Z.T. Zhang: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 1549-54.
28. F.A. Seifert, B.O. Mysen, and D. Virgo: Am. Miner., 1982, vol. 67, pp. 696-717.
29. J. Tan, S.R. Zhao, W.F. Wang, G. Davies, and X.X. Mo: Mater. Sci. Eng. B, 2004, vol. 106, pp. 295-99.
30. G. Padmaja and P. Kistaiah: J. Phys. Chem. A, 2009, vol. 113, pp. 2397-2402
31. B.N. Meera and J. Ramakrishna: J. Non-Cryst. Solids, 1993, vol. 159, pp. 1-21
32. P. McMillan: Am. Miner., 1984, vol. 69, pp. 622-644
33. D.W. Matson, S.K. Sharma and J.A. Philpotts: J. Non-Cryst. Solids, 1983, vol. 58, pp. 323-352.
34. K. Fukumi, J. Hayakawa and T. Komiyama: J. Non-Cryst. Solids, 1990, vol. 119, pp. 297-302.
35. B.O. Mysen and J.D. Frantz: Am. Miner., 1993, vol. 78, pp. 699-709.
36. J.L. You, G.C. Jiang and K.D. Xu: J. Non-Cryst. Solids, 2001, vol. 282, pp. 125-131.
37. B.O. Mysen and J.D. Frantz: Contrib. Miner. Petrol., 1994, vol. 117, pp. 1-14.
38. B.O. Mysen, L.W. Finger, D. Virgo and F.A. Seifert: Am. Miner., 1982, vol. 67, pp. 686-695.
39. J.D. Frantz and B.O. Mysen: Chem. Geol., 1995, vol. 121, pp. 155-76.
40. B.O. Mysen and J.D. Frantz: Am. Mineral., 1993, vol. 78, pp. 699-709.
41. Y.Q. Wu, G.C. Jiang, J.L. You, H.Y. Hou, and H. Chen: Acta Phys. Sin., 2005, vol. 54, pp. 961-66.
42. J. Kline, M. Tangstad, and G. Tranell: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 62-73.
43. L.S. Du and J.F. Stebbins: J. Non-Cryst. Solids, 2003, vol. 315, pp. 239-55.
44. S.K. Lee, H.N. Kim, B.H. Lee, H.I. Kim, and E.J. Kim: J. Phys. Chem. B, 2010, vol. 114, pp. 412-20.
45. L.S. Du and J.F. Stebbins: J. Phys. Chem. B, 2003, vol. 107, pp. 10063-76.
46. E. Thibodeau and I. Jung: Metall. Mater. Trans. B, 2016, vol. 47B, pp. 355-83.
47. A.E. Martin and G. Gerge: Trans. Inst. Min. Mrtall, 1943, vol. 154, pp. 104-115.
48. M.T. Simnad and G. Derge: J. Chem. Phys, 1953, vol. 21, pp. 933-34.
49. M.T. Simnad, G. Derge and I. George, Trans. Inst. Min. Mrtall, 1954, vol. 200, pp. 1386-90.
50. D.A. Dukelow and G. Derge: Trans. Inst. Min. Mrtall, 1960, vol. 218, pp.136-139.
51. E.A. Dancy and G. Derge: Trans. Inst. Min. Mrtall, 1966, vol. 236, pp. 1642-48.
52. G.H. Zhang and K.C. Chou: J. Iron Steel Res. Int., 2011, vol. 18, pp. 13-16.
53. F. Fincham and F.D. Richardson: Proc. R. Soc. Lond. A, 1954, vol. 223, pp. 40-62.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (51704271 and U1702251) and National Key R&D Program of China (2018YFC1901801). The authors thank Kathryn Sole, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a previous draft of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Manuscript submitted July 8, 2018.
Rights and permissions
About this article
Cite this article
Zhang, P., Liu, J., Wang, Z. et al. Effect of B2O3 Addition on Electrical Conductivity and Structural Roles of CaO-SiO2-B2O3 Slag. Metall Mater Trans B 50, 304–311 (2019). https://doi.org/10.1007/s11663-018-1472-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-018-1472-8