Abstract
The electrical conductivity of molten slag has many important and practical effects in modeling and operating the electric smelting furnace. In the present study, the electrical conductivities (total and electronic/ionic properties) of MnO-CaO-SiO2 slags were measured by a four-electrode method at different oxygen potentials and temperatures. Experimental results show that the effects of temperature on the total, electronic, and ionic conductivities obey the Arrhenius law, and all conductivities increase when increasing the temperature. The stepped potential chronoamperometry method was used to measure the electronic transference number, which is affected strongly by oxygen potential but is unaffected by temperature. The total electrical, electronic, and ionic conductivities present similar increasing trends when increasing the CO/CO2 ratio, which resulted from increasing Mn2+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As one of the most basic physical-chemical properties, the electrical conductivity of molten slag has many important and practical effects. On one hand, the electrical conductivity of molten slag plays a prominent role in modeling and operating the electric smelting furnace and optimizing the metallurgical process[1,2,3,4,5,6]; on the other hand, it is important for understanding the structure of molten slags.[7,8,9,10,11,12] For instance, a large number of metals, including titanium, aluminum, and magnesium, have been produced by the electrolysis of molten slags and salts, and the earliest evidence of the ionic structure of oxide melts resulted from electrical conductance measurements. For the preceding aspects, the study of electrical properties of metallurgical melts is particularly necessary and important. Molten oxide electrolysis[13,14,15,16] is a carbon-neutral electrochemical technique, which can decompose metal oxide directly into liquid metal and oxygen gas. Compared to other technologies, its greatest advantage is no greenhouse gas emissions. As an efficient electrolysis, the melt must be predominantly an ionic conductor. Knowledge of the electrical conductivity of slag can help in the design and selection of the proper slag for the electrolysis process. So far, the electrical conductivities of abundant different slags have been reported.[10,17,18,19,20,21,22] High melting point and the charge transfer between the slag and gas make it hard for the conductivity measurement of MnO-bearing slags, which leads to a lack of data of electrical conductivity of MnO-bearing slags. The objective of the present work was to experimentally study the electrical conductivity of MnO-CaO-SiO2 slags at different oxygen potentials controlled by CO-CO2 gas mixtures in the temperature range of 1723 K to 1823 K (1450 °C to 1550 °C).
Experiments
The initial slag compositions are provided in Table I. In each group, the molar ratio of CaO and SiO2 remains constant (CaO:SiO2 = 1:1), but the content of MnO gradually increases. Slag samples were prepared using reagent grade SiO2 and CaCO3 powders (analytically pure, Sinopharm Chemical Reagent Co., Ltd., Beijing, China), both of which were calcined at 1273 K (1000 °C) for 10 hours in a muffle furnace to decompose any carbonate and hydroxide before being used. Then, about 12 g mixtures were precisely weighted according to the compositions shown in Table I and mixed in an agate mortar thoroughly to ensure the components were uniform. In the present study, a four-terminal method was employed to accomplish the electrical conductivity measurement, and this method had already been successfully used to measure the electrical conductivity of molten slags.[17,22] In this way, the effects of polarization at the current-carrying electrodes can be largely eliminated and the effect of alternative paths is reduced. Due to the necessity of calibration, determinations of the cell constant C and resistance R x are obligatory to the measurement accuracy. The relation among the total electrical conductivity σ t , constant C, and resistance R x can be expressed by Eq. [1]:
MnO-bearing slags can be regarded as mixed conductors, since they exhibit both ionic and electronic conductivity (σ i and σ e). The electronic transference numbers (the ratio of electronic conductivity to the total conductivity) were measured by the stepped potential chronoamperometry method.[17] The electronic transference number is defined as
or
where i e and i i are the currents carried by the electronic and ionic charge carriers, respectively. Therefore, the electronic conductivity and ionic conductivity can be calculated by total electrical conductivity and electronic transference number.
The descriptions of the device and experimental principle have been described already in detail in our previous study.[23–25] In the experimental process, the oxygen partial pressure was controlled by the ratio of CO to CO2, whose gas rate was controlled by a flowmeter. The total flow rate of CO2 and CO was fixed at 200 mL min−1. The slag was kept for 2 to 3 hours in each atmosphere for the purpose of equilibrium and uniformity of slag. Once the slag and gas reached equilibrium, the electrical measurements were carried out at every 25 K interval on cooling from 1823 K (1550 °C). All the measurements were completed using a CHI 660a electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd., Shanghai, China).
Results and Discussion
Influence of Temperature
The Arrhenius law can be used to express the effect of temperature on electrical conductivity:
or
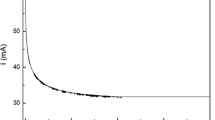
where σ is the electrical conductivity, Ω−1 cm−1; A is the pre-exponent term; E is the activation energy, J (mol K)−1; R is the gas constant, 8.314 J (mol K)−1; and T is the absolute temperature, in Kelvin. The changes of total electrical conductivity as functions of temperature for different slags at CO/CO2 = 1 are shown in Figure 1. As seen in this figure, the temperature dependence of total electrical conductivity obeys the Arrhenius law well; furthermore, electrical conductivity increases when increasing the temperature, since the charge transport rate is faster at the higher temperature.
The electronic transference number was measured under conditions identical to those of the electrical conductivity measurements. The results are provided in Figure 2, from which it can be seen that under the present experimental conditions, the electronic transference number is essentially independent of the temperature. The negligible effect of temperature on the transference number has also been reported by other authors.[18,26] Furthermore, it is obvious from Figure 2 that when increasing the MnO content, there is an increase of the electronic transference number.
The effect of temperature on the ionic conductivity and electronic conductivity is shown in Figures 3 and 4, respectively. Similar to the case of total electrical conductivity, the ionic conductivity and electronic conductivity increase when increasing the temperature, and the relationships between temperature and both ionic and electronic conductivities also follow the Arrhenius law. The positive effect of temperature on ionic conductivity can be related to the increased mobility of cations at higher temperature, due both to a greater diffusion coefficient and to more depolymerization of the silicate structure.
Influence of Oxygen Potential
The partial pressure of oxygen \( P_{{{\text{O}}_{2} }} \) can be controlled by CO-CO2 gas mixtures according to Eq. [6]:
Figure 5 shows the total electrical conductivity for different compositions at 1823 K (1550 °C) as a function of CO/CO2 ratio. Figure 5 shows that for all experimental slags, the total electrical conductivities increase when increasing the CO/CO2 ratio in fixed MnO content, and in the case of fixed CO/CO2 ratio, the higher the MnO content is, the higher the total electrical conductivities will be.
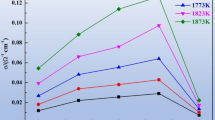
The change of the electronic transference number as different CO/CO2 ratios is shown in Figure 6. From Figure 6, under the current experimental conditions, the electronic transference numbers vary from about 21 to 50 pct and always increase when increasing the MnO content and CO/CO2 ratio.
The change of the ionic conductivity at 1823 K (1550 °C) as different CO/CO2 ratios is shown in Figure 7. From Figure 7, for all experimental slags, the ionic conductivity increases when increasing the CO/CO2 ratio. There are several ions in the present MnO-CaO-SiO2 slags, but Mn2+ and Ca2+ ions are the most important charge carriers. The reaction between manganese ions and gas is shown as follows:
The existence form of Mn4+ is a much weaker mobility compared with Mn2+ ion. On the basis of Eq. [7], more and more Mn2+ will replace Mn4+ when increasing the CO/CO2 ratio, which will lead to an increase of the ionic conductivity.
Figure 8 shows the effect of the CO/CO2 ratio on electronic conductivity at 1823 K (1550 °C). From Figure 8, for slags with various MnO contents, the electronic conductivity increases when increasing the CO/CO2 ratio, and in the case of the fixed CO/CO2 ratio, the electronic conductivity increases when increasing the MnO content. Barati and Coley proposed a diffusion-assisted charge transfer model that has already successfully explained the experimental phenomenon of electronic conductivity for CaO-SiO2-FeO x slags.[27]
The variation phenomenon of the present electronic conductivity can be explained by the diffusion-assisted charge transfer model. This model requires that the electron hopping between Mn2+ and Mn4+ ions takes place when they are at appropriate distance. That is to say, the ions that are far apart from each other first travel to reach separation distances sufficiently short, and then the activated charge hopping between Mn2+ and Mn4+ ions can take place. The schematic diagram of the diffusion-assisted charge transfer model is shown in Figure 9. According to the diffusion-assisted charge transfer model, when the slag composition, temperature, and oxygen partial pressure are constant, the electronic conductivity is related to the concentration of Mn2+ and Mn4+; therefore, the expression of electronic conductivity can be simplified and shown as \( \sigma_{\text{e}} = kc_{{\left( {{\text{Mn}}^{2 + } } \right)}} c_{{\left( {{\text{Mn}}^{4 + } } \right)}} \); in other words, the electronic conductivity is proportional to the product of the concentration of Mn2+ and Mn4+. When the content of manganese oxide is fixed, the amounts of Mn2+ and Mn4+ are constant, that is, \( \frac{{c_{{\left( {{\text{Mn}}^{2 + } } \right)}} }}{{c_{{\left( {\text{MnO}} \right)}} }} + \frac{{c_{{\left( {{\text{Mn}}^{4 + } } \right)}} }}{{c_{{\left( {\text{MnO}} \right)}} }} = 1 \), and the product of the concentration of Mn2+ and Mn4+ will be maximal when \( c_{{\left( {{\text{Mn}}^{2 + } } \right)}} = c_{{({\text{Mn}}^{4 + } )}} \). According to Eqs. [6] and [7], when increasing the CO/CO2 ratio, the concentration of Mn2+ will increase and the concentration of (MnO4)4– will decrease, which leads to the value of the electronic conductivity increasing first and then decreasing; there should be a maximum value when \( c_{{\left( {{\text{Mn}}^{2 + } } \right)}} = c_{{\left( {{\text{Mn}}^{4 + } } \right)}} \). However, the present experimental results show that the electronic conductivity increases monotonously when increasing the CO/CO2 ratio, which may result from the concentration of Mn2+ always being less than that of Mn4+ in current experimental conditions, even in the largest CO/CO2 ratio, just like Figure 8. In addition, it also can be seen from Figure 8 that the higher the MnO content, the higher the electronic conductivity will be. When increasing the MnO content, the concentration of Mn2+ and Mn4+ will increase, which results in larger contact probability between Mn2+ and Mn4+, eventually leading to the increase of the electronic conductivity.
Conclusions
-
1.
The effects of temperature on the electrical conductivity of MnO-SiO2-CaO slags obey the Arrhenius law; when increasing the temperature, all the electrical conductivities increase.
-
2.
The electronic transference number is affected strongly by oxygen potential but is unaffected by temperature.
-
3.
When increasing the CO/CO2 ratio, the ionic conductivity exhibits the increasing trend because of a higher concentration of Mn2+.
-
4.
In the current experimental conditions, the electronic conductivity increases when increasing the ratio of CO/CO2. The effect of CO/CO2 on electronic conductivity is suggestive of a small polaron-hopping mechanism between Mn2+ and Mn4+ ions.
References
G. Gruener, D. De Sousa Meneses, P. Odier, and J.P. Loup: J. Non-Cryst. Solids, 2001, vol. 281, pp. 117–24.
M.T. Simnad, G. Derge, and I. George: Trans. AIME, 1954, vol. 6, pp. 1386–90.
J.O.M. Bockris, I.A. Kitchener, and S.A. Ignatowicz: Disc. Faraday Soc., 1948, vol. 4, pp. 265–81.
J.O.M. Bockris, I.A. Kitchener, and S.A. Ignatowicz: Trans. Faraday Soc., 1952, vol. 48, pp. 75–91.
L. Segers, A. Fontana, and R. Winand: Can. Metall. Q., 1983, vol. 22, pp. 429–35.
A. Fontana, L. Segers, and R. Winand: Can. Metall. Q., 1980, vol. 20, pp. 209–14.
J. W. Tomlinson and H. Inouye: J. Chem. Phys., 1952, vol. 20, p. 193.
M.T. Simnad and G. Derge: J. Chem. Phys., 1953, vol. 21, pp. 933–34.
H. Inouye, J.W. Tomlinson, and J. Chipman: Trans. Faraday Soc., 1953, vol. 49, pp. 796–801.
N.A. Fried, K.G. Rhoads, and D.R. Sadoway: Electrochim. Acta, 2001, vol. 46, pp. 3351–58.
G.M. Haarberg, K.S. Osen, R.J. Heus, and J.J. Egan: J. Electrochem. Soc., 1990, vol. 137, pp. 2777–81.
J.H. Park: ISIJ Int., 2012, vol. 52, pp. 1627–36.
D. Wang, A.J. Gmitter, and D.R. Sadoway: J. Electrochem. Soc., 2011, vol. 158, pp. E51–E54.
D.R. Sadoway: J. Mater. Res., 1995, vol. 10, pp. 487–92.
S.L. Schiefelbein, N.A. Fried, K.G. Rhoads, and D.R. Sadoway: Rev. Sci. Instrum., 1998, vol. 69, pp. 3308–13.
J.H. Liu, G.H. Zhang, and K.C. Chou: J. Electrochem. Soc., 2015, vol. 162, pp. E314–E318.
K. Narita, T. Onoye, T. Ishll, and K. Uemura: ISIJ Int., 1975, vol. 61, pp. 2943–51.
M. Barati and K.S. Coley: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 41–49.
L. Bobok, L. Bodnar, and J. Schmiedl: Hutnicke Listy, 1982, vol. 37, pp. 419–24.
S.N. Shin, S.A. Lyamkin, R.I. Gulyaeva, and V.M. Chumarev: Rasplavy, 1998, vol. 5, pp. 20–24.
J.H. Liu, G.H. Zhang, Y.D. Wu, and K.C. Chou: Can. Metall. Q., 2016, vol. 55, pp. 221–25.
M. Kawahara, K.J. Morinaga, and T. Yanagase: Can. Metall. Q., 1983, vol. 22, pp. 143–47.
J.H. Liu, G.H. Zhang, Y.D. Wu, and K.C. Chou: Metall. Mater. Trans. B, 2015, vol. 47B, pp. 798–803.
J.H. Liu, G.H. Zhang, and K.C. Chou: Can. Metall. Q., 2015, vol. 54, pp. 170–76.
J.H. Liu, G.H. Zhang, and K.C. Chou: ISIJ Int., 2015, vol. 55, pp. 2325–31.
W.R. Dickson and E.B. Dismukes: Trans. AIME, 1962, vol. 224, pp. 505–11.
M. Barati and K.S. Coley: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 51–60.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (Grant No. 51422405).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 28, 2017.
Rights and permissions
About this article
Cite this article
Liu, JH., Zhang, GH. & Wang, Z. Experimental Study on Electrical Conductivity of MnO-CaO-SiO2 Slags at 1723 K to 1823 K (1450 °C to 1550 °C) and Various Oxygen Potentials. Metall Mater Trans B 48, 3359–3363 (2017). https://doi.org/10.1007/s11663-017-1072-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-017-1072-z