Abstract
The electrical conductivity of molten slags is an extremely important factor in the design of electric smelting furnaces. As a fundamental study on properties of the TiO x -bearing slags, the total electrical conductivity and electronic/ionic properties of TiO x -SiO2-CaO slags were measured at different oxygen potentials and temperatures using four-electrode method. The experiment results show that for a specific composition, the temperature dependences of ionic, electronic, and total conductivity obey the Arrhenius law. The stepped potential chronoamperometry method was employed for measuring the electronic transference number, which exhibits a strong dependence on oxygen potential, but is unaffected by temperature. The total electrical, electronic, and ionic conductivities present similar increasing trends with increasing the ratio of CO/CO2, which is resulted from increasing of Ti3+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The electrical conductivity of molten slags plays an important role in understanding the structure of molten slags and operation of the electric smelting furnace. For instance, molten oxide electrolysis (MOE)[1–7] is a carbon–neutral electrochemical technique to decompose metal oxide directly into liquid metal and oxygen gas upon use of an inert anode. Compared to other technologies, its potential environmental advantage of no greenhouse gas emissions is the most important characteristic. For such electrometallurgy process, the electrical conductivity of molten slags is particularly important. The electrical conductivities of TiO2-BaO,[8] FexO-CaO-SiO2,[9–12] FexO-CaO-MgO-SiO2,[13] FexO-CaO-SiO2-Al2O3,[14] NixO-CaO-SiO2,[15] and NixO-CaO-MgO-SiO2[15] slags had been measured and reported in published literatures. Due to the high melting point of slags and the transfer of charge between the slag and the adsorbed gas species, the data regarding the electronic properties of TiO x -bearing slags are very limited. The objective of this work was to study the electrical and electronic conductivity of TiO x -CaO-SiO2 slags at various temperatures and oxygen potentials which was controlled by the ratio of CO/CO2. These fundamental researches will be used in the modeling and design of electric smelting furnaces.
Experiments
The compositions of slag samples are shown in Table I. In each group, the mass ratio of CaO/SiO2 remains constant, but the content of TiO2 gradually increases. Slag samples were prepared using reagent grade TiO2, SiO2, and CaCO3 powders (analytically pure, Sinopharm Chemical Reagent Co., Ltd, China), all of which were calcined at 1273 K (1000 °C) for 10 hours in a muffle furnace to decompose any carbonate and hydroxide before use. Then, about 12 g mixtures were precisely weighed according to the compositions shown in Table I, and mixed in an agate mortar thoroughly.
To accomplish the electrical conductivity measurements, a four-terminal method was employed in this study. The measurement on the electrical conductivity of TiO x -CaO-SiO2 molten slags is extremely difficult because of its high melting point, reactivity, and conductivity. In order to get an accurate electrical conductivity value, it is necessary to exclude the resistances of lead wires and electrodes from the total measured resistance. If the current electrode is used in common for the potential one, the contribution of the interfacial resistance is usually too large to be disregarded. The application of the four-electrode technique can avoid those difficulties. The electrical conductivity of the melts can be obtained by the following equation:
where C is the cell constant and R x is the slag melts resistance. Many factors, such as the applied frequency, the volume of melt, the immersion depths of electrodes in the melt, the positions of electrode, and the temperature could affect the measurement accuracy of the cell constant. Those factors were carefully handled in the present study.
The electronic transference numbers were measured using the stepped potential chronoamperometry (SPC) method.[16,17] Basically, the current response upon applying a constant voltage (stepped potential) is monitored as a function of time in this technique. The electronic transference number can be calculated using the following equation:
where \( i_{t \to 0} \) is the initial value of the current and \( i_{t \to \infty } \) is the long-time value of the current. Figure 1 shows a typical current vs time curve. The experimental arrangement is drawn schematically in Figure 2, and the descriptions of experiment device have already mentioned in our previous study.[18] The electrodes were made from Pt-Rh (30 wt pct), and each electrode consisted of a tip which is 25-mm long and 0.8-mm in diameter welded to a thinner extension wire which consisted of the same material, but 0.3-mm in diameter and 800-mm long. The electrodes were sheathed into two twin-bore alumina tubes (The distance between two holes is 3 mm) so that the tips were extended about 20 mm outside the tubes. The two twin-born alumina tubes were fixed with a short alumina tube of the same diameter and these tubes were then passed down two support tubes which were stabilized rigidly, so that these two central electrodes separate for 6 mm. The two twin-born alumina tubes could be moved up and down along the two support tubes. Before each measurement, the cell calibration for determination of C was performed at low temperatures [293 K to 298 K (20 °C to 25 °C)], using standard aqueous KCl solutions (1.0 mol/L), whose preparation and specific conductance were well documented. The resistance was measured at different immersion depths of the electrodes to determine C by Eq. [1].
After obtaining the cell constant, the prepared slag samples were packed into a platinum crucible (The diameter and height of the crucible are 26 mm and 25 mm, respectively. The depth of the melt is about 12 mm.) and then placed at the constant temperature zone inside the furnace, where the temperature variations measured by a type B (Pt-6 pct Rh/Pt-30 pct Rh) thermocouple could be negligible. During the heating process, the tips of the electrodes were located at about 2 cm above the slag surface. After the target temperature 1843 K (1570 °C) was reached and held for 2 hours, the electrodes were lowered slowly until touched the surface of the melt. During this process, the resistance was monitored. When the tips of the electrodes contacted with the surface of melt, the resistance will significantly decrease, and this position is considered as the zero point. And then the electrodes were lowered further to reach the desired depth. In all experiments, the immersion depth of the electrodes was 3 mm, and the electrodes were in the intermediate position of the crucible during the whole experimental process.
During the whole heating process and the first 2 hours holding at the target temperature, the slag was exposed to CO2, and the gas rate was controlled by a flowmeter. The input gas composition varied from pure CO2 to CO/CO2 mixed gas to control oxygen partial pressure. The total flow rate of CO2 and CO was fixed at 200 mL/min. The slag was kept for 2 hours in each atmosphere for the purpose of equilibrium and uniformity of slag. Once the slag and gas reached equilibrium, the electrical measurements were carried out at every 30 K interval on cooling from 1843 K (1570 °C).
The slag resistance was measured using a Model CHI 660a Electrochemical Workstation, and the electronic transference number was measured by the SPC method, using a direct current signal. The resistance was found to be independent of the frequency, over the range 0.5 to 100 kHz. All of the measurements were carried out at 20 kHz.
Results and Discussion
Total Electrical Conductivity
Influence of oxygen potential on total electrical conductivity
The total electrical conductivity for different compositions at 1843 K (1570 °C) as a function of CO/CO2 ratio is shown in Figure 3, from which it can be seen that for slags with various TiO2 contents, the total electrical conductivities increase as increasing the ratio of CO/CO2, and the higher the content of TiO2 is, the higher the total electrical conductivities will be in fixed CO/CO2 ratio.
Influence of temperature on total electrical conductivity
It is widely accepted that the temperature dependence of electrical conductivity can be expressed by the Arrhenius law as
Or
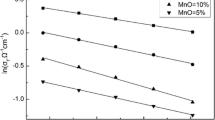
where σ is electrical conductivity, Ω−1 cm−1; A is pre-exponent factor; E is activation energy, J/(mol K); R is the gas constant, 8.314 J/(mol K); and T is the absolute temperature, K. Figure 4 shows the change of total electrical conductivity as a function of temperature for different slags, at CO/CO2 = 30. It can be seen from Figure 4, electrical conductivity increases as increasing the temperature, furthermore, the temperature dependence of electrical conductivity obeys the Arrhenius law very well, with the activation energies shown in Table II. At the higher temperature, ion mobility will be stronger, which resulted in the increase of electrical conductivity. In addition, temperature will affect the ratio of Ti3+/Ti4+, which also influences the electrical conductivity of slags.
Electronic Transference Number
Influence of equilibrium oxygen potential on electronic transference number
The CO/CO2 dependence of electronic transference number at 1843 K (1570 °C) is shown in Figure 5. It can be seen that the electronic transference numbers vary from about 42 to 52 pct under the experimental conditions. From Figure 5, the electronic transference numbers always increase as increasing of the CO/CO2 ratio.
Influence of temperature on electronic transference number
The effect of temperature on the electronic transference number is shown in Figure 6, from which it can be seen that the electronic transference number increases with increasing TiO2 content at CO/CO2 = 30. It also obviously shows that the electronic transference number is essentially independent of the temperature under the experimental conditions. Similar trends have been reported by other authors.[19,20]
Ionic Conductivity
Influence of oxygen potential on ionic conductivity
The ionic conductivity at 1843 K (1570 °C) as a function of CO/CO2 ratio is shown in Figure 7. It is evident from Figure 7 that the ionic conductivity of all slags increases with increasing the ratio of CO/CO2. Tranell et al.[21] studied the Ti4+-Ti3+ equilibria in CaO-SiO2-TiO x system. The total amounts of reduced titanium (Ti3+ and Ti2+) in the slags were determined by a modified version of an indirect potentiometric redox titration method.[22] This scheme involved dissolution of the slag in HF-HCl-H2O solution with excess Fe3+ under an argon atmosphere. The reduced titanium is oxidized by the ferric ion through the following reaction:
The quantity of titanium in the Ti4+ valency state was calculated as the difference between total and reduced titanium. Since the concentration of Ti2+ is low in comparison with the Ti3+ under the present experimental conditions, all reduced titanium was approximately considered as Ti3+. The reaction among Ti4+, Ti3+, and gas is shown as follows:
From Eq. [6], it can be known that more and more Ti3+ will replace Ti4+ with increasing the CO/CO2 ratio. The tendency of the Ti4+ toward covalent binding with oxygen is strong enough to stimulate the formation of highly covalent anions (TiO4−), which will lead to greatly reduced mobility compared with Ti3+. Therefore, the ionic conductivity of all slags increases with increasing the ratio of CO/CO2. Figure 8 shows the ionic conductivities plotted as a function of Ti3+/Ti4+ ratio, values of which were taken from the work by Tranell et al.[21] And the data of Figure 8 have been shown in Table III. It is seen that an increase in the fraction of Ti3+ increases slightly the ionic conductivity for all slags. These results can be justified by the weaker mobile ability of Ti4+ ion relative to that of Ti3+ ion, because of its larger electrostatic field.
Influence of temperature on ionic conductivity
Figure 9 shows the effect of temperature on the ionic conductivity. It can be seen that the Arrhenius law is always obeyed, and as temperature increases, ionic electrical conductivity increases, because of the decrease of viscosity and the increase of ion mobility. In addition, according to the study of Tranell et al.,[21] temperature increase results in stabilization of the lower oxidation state of titanium (Ti3+ more stable than Ti4+), which will lead to the increase of ionic conductivity.
Electronic Conductivity
Influence of oxygen potential on electronic conductivity
Figure 10 shows the effect of the CO/CO2 ratio on electronic conductivity at 1843 K (1570 °C). The electronic conductivity increases with increasing the ratio of CO/CO2. Barati and Coley[23] proposed a diffusion-assisted charge transfer model to successfully explain the electronic conductivity change of CaO-FeO-SiO2 system. Based on this model, charge transfer can be regarded as a bimolecular reaction between Ti3+ and Ti4+ ions. In the first step, ions should travel to reach separation distances sufficiently short for electron hopping. In the next step, the electron hopping can take place. In other words, this model requires neighboring Ti3+ and Ti4+ ions to interact. As increasing the ratio of CO/CO2 in a certain range, the percentage of Ti3+ ion will increase while that of Ti4+ ion will decrease. According to the diffusion-assisted charge transfer model, the electronic conductivity is proportional to the product of concentrations of Ti3+ and Ti4+ ions. Therefore, the electronic conductivity will increase with increasing the ratio of CO/CO2 to a certain extent.
Influence of temperature on electronic conductivity
The electronic conductivity as a function of temperature is shown in Figure 11. Just like the total conductivity and ionic conductivity, the relationship between electronic conductivity and temperature also obeys the Arrhenius law, and the corresponding activation energies of the electronic conductivity are shown in Table II.
Conclusions
The electrical conductivity of TiO2-SiO2-CaO slags was measured by a four-terminal technique, results of which show that the temperature dependences of ionic, electronic, and total conductivity obey the Arrhenius law. The SPC method was employed for the measurement of the electronic transference numbers, which exhibits a strong dependence on oxygen potential, while is independent of temperature. The total electrical, electronic, and ionic conductivities present similar increasing trends with increasing the ratio of CO/CO2, which is resulted from increasing of Ti3+.
References
S. C. Britten, and U. B. Pal: Metall. Mater. Trans. B, 2000, vol. 31, pp. 733-52.
S. Sun, and S. Jahnashahi: Metall. Mater. Trans. B, 2000, vol. 31B, pp. 937-43.
Y. Li and I.P. Ratchev: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 624-30.
D. Wang, A. J. Gmitter, and D. R. Sadoway: J. Electrochem. Soc., 2011, vol. 158, pp. E51-4.
D. R. Sadoway: J. Mater. Res., 1995, vol. 10, pp. 487-92.
S. L. Schiefelbein, N. A. Fried, K. G. Rhoads, and D. R. Sadoway: Rev. Sci. Instrum., 1998, vol. 69, pp. 3308-13.
H. J. Kim, J. Paramore, A. Allanore, and D. R. Sadoway: J. Electrochem. Soc., 2011, vol. 158, pp. E101.
N. A. Fried, G. K. Rhoads, and D. R. Sadoway: Electrochim. Acta, 2001, vol. 46, pp. 3351- 8.
A. Adachi, and K. Ogino: Technol. Rep Osaka Univ., 1957, vol. 7(244), pp. 121-6.
K. Narita, T. Onoye, T. Ishll, and K. Uemura: ISIJ Int., 1975, vol. 61(14), pp. 2943-51.
T. Hoster, and J. P. Essen: Arch. Eisenhutten., 1983, vol. 54, pp. 389-94.
M. Barati, and K. S. Coley: Metall. Mater. Trans. B, 2006, vol. 37, pp. 41-9.
L. Bobok,L. Bodnar, and J. Schmiedl: Hutnicke Listy, 1982, vol. 37(6), pp. 419-24.
S. N. Shin, S. A. Lyamkin, R. I. Gulyaeva, and V. M. Chumarev: Rasplavy, 1998, vol. 5, pp. 20-4.
M. Kawahara, K. J. Morinaga, and T. Yanagase: Can. Metall. Quart., 1983, vol. 22(2), pp. 143-7.
P. G. Bruce, and C. A. Vincent: J. Electroanal. Chem. Interfacial Elec-trochem., 1987, vol. 225, pp. 1-17.
T. Tran, S. Sun, and S. Jahanshahi: Iron and Steel Soc. AIME, 1997, 28 pp. 115-23.
J. H. Liu, G. H. Zhang, and K. C. Chou: Can. Metall. Quart., 2015, vol. 54, pp. 170-6.
W. R. Dickson, and E.B. Dismukes: Trans. AIME, 1962, vol. 224, pp. 505-11.
E. A. Dancy, and D. J. Derge: Trans. AIME, 1966, vol. 236, pp. 1642-8.
G, Tranell, O. Ostrovski, and S. Jahanshahi: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 61-7.
W. P. Close, and J. F. Tillman: Glass Technol, 1969, vol. 10, pp. 134-46.
M. Barati and K. S. Coley: Metall. Mater. Trans. B, 2006, vol. 67, pp. 51-60.
Acknowledgments
Thanks are given to the financial supports from the Specialized Research Fund for the Doctoral Program of Higher Education (20130006120007) and the National Natural Science Foundation of China (51174022, 51304018 and 51474141).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 22, 2015.
Rights and permissions
About this article
Cite this article
Liu, JH., Zhang, GH., Wu, YD. et al. Electrical Conductivity and Electronic/Ionic Properties of TiO x -CaO-SiO2 Slags at Various Oxygen Potentials and Temperatures. Metall Mater Trans B 47, 798–803 (2016). https://doi.org/10.1007/s11663-015-0499-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0499-3