Abstract
This research quantitatively studied the effect of temperature on the synthesis of TiB2 particle reinforcement via a casing method in which a salts-metal reaction was involved. Experimental results showed that the yield of TiB2 reached 89.5 pct and most TiB2 particles ranged from 400 to 800 nm at 1173 K (900 °C) with a 10-minute reaction time; when the reaction time was 30 minutes, the TiB2 particles had similar yield and size distribution. At 973 K (700 °C) with a 10-minute reaction time, most TiB2 particles were less than 300 nm, whereas the yield was just 28.1 pct; as the time was prolonged to 30 minutes, some smaller-sized TiB2 particles were synthesized and the yield of TiB2 was 36.4 pct. At a higher temperature [1173 K (900 °C)], the synthesis of TiB2 mainly followed the precipitation-growth process at reaction interface. At a lower temperature [973 K (700 °C)], the precipitation-growth process and dissolution reaction between Al3Ti and AlB2 both contributed to the formation of TiB2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is well known that in situ TiB2 particles can be synthesized via the salts-metal reaction by adding the mixed K2TiF6-KBF4 salts into molten Al at high temperatures.[1,2] Due to its unique characteristics, such as low cost, high efficiency and short synthesizing time, the salts-metal reaction has been widely used in practical productions for producing in situ TiB2/Al composites and the existence of TiB2 particles can improve the mechanical properties of the Al alloys significantly.[3,4] This type of material has been obtained more attentions in the aerospace and automobile industries. In nature, the salts-metal reaction belongs to a chemical reaction. The synthesizing temperature is one of the most important factors, which influences the formation of the products during the reaction. Generally, a high temperature is beneficial for the formation of TiB2 particles, and the temperature used in the salts-metal reaction is usually higher than 1073 K (800 °C). It has been reported that a lower temperature can lead to the formation of Al3Ti and AlB2 phases, which decrease the yield of TiB2 phase in the Al matrix.[5] Thereby, using a higher temperature of the Al melt is always recommended in order to improve the production of TiB2 particles in the reaction.
Although the salts-metal reaction has become the most common method for synthesizing in situ TiB2/Al composites, the relationship between the temperature and the formation of TiB2, however, has not been investigated clearly. A quantitative study of the effect of temperature on the synthesis of TiB2 regarding the yield of TiB2 particles and their size distribution has not been reported by other researchers. In addition, the understanding of the formation mechanism of TiB2 in the salts-metal reaction is still unclear.
So far, two main viewpoints with regard to the formation process of TiB2 have been proposed. One refers that TiB2 particles are formed by a dissolution-precipitation mechanism when the concentrations of Ti and B reach saturation in molten Al (alloys).[6] The detailed mechanism can be summarized as follows.
First, K2TiF6 and KBF4 are melted on the Al surface, both of which are separately decomposed to KF (liquid), TiF4 (gas), and BF3 (gas), which are expressed as follows:
Then, Ti and B atoms are released and diffuse into liquid Al through the aluminothermic reduction of TiF4 and BF3 gases at the salts-melt interface, which are expressed as below:
When the solutes [Ti] and [B] in liquid Al reach saturation, they might be separated out as compounds Al3Ti, AlB2, and TiB2, according to the following reactions:
Among the compounds involved in the salts-metal reaction, TiB2 is the most thermodynamically stable phase due to its lowest free energy of formation.[7] The overall reaction showing the formation of TiB2 can be written as
The other one refers that TiB2 particles are synthesized resulting from the reaction between Al3Ti and AlB2, which are formed due to the reactions of K2TiF6-Al and KBF4-Al.[8] The following reactions describe the formation process of TiB2:
An overall reaction for synthesizing TiB2 can be given as follows:
Actually, the above two mechanisms are based on the equilibrium thermodynamics of the Al-Ti-B system. The practical salts-metal reaction, however, is a typical non-equilibrium thermodynamic reaction. An analysis considering the reaction temperature and reaction time might be more suitable to reflect the actual formation process of TiB2 during the reaction.
In order to investigate the influences of reaction temperature and time on the formation of TiB2 particles in molten Al regarding the yields and size distributions of TiB2 particulates, two reaction temperatures [1173 K and 973 K (900 °C and 700 °C)] with two reaction times (10 and 30 minutes) were studied. A quantitative analysis about the formation of TiB2 under different experimental parameters was firstly investigated in this research. The formation process of TiB2 particles was also explored from the perspective of the temperature.
2 Experimental
2.1 Synthesis of TiB2 Particles via Salts-Metal Reaction
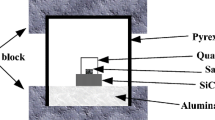
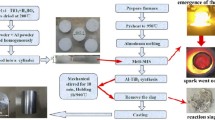
A 500 g pure Al ingot was melted in a graphite crucible in an electrical resistant furnace. After the temperature of the Al melt reached and kept stable at 1173 K (900 °C) [or 973 K (700 °C)], the mixed K2TiF6-KBF4 salt powders were added into the Al melt, and the addition of the mixed salts corresponded to the composition of Al-5 wt pct TiB2. Then the melt was stirred manually for a few seconds by using a niobium bar. After 10 min, the Al melt containing reaction products was stirred again, for the newly formed products might deposit during the reaction due to the higher density than pure Al. After the slag on the melt surface was removed, the melt was poured into a steel mold to form an ingot. For the samples fabricated in 30 min, the melt was stirred in 10-minute intervals. 4 samples were fabricated in total. For simplicity, the 4 samples were referred as S900-10, S900-30, S700-10, and S700-30, respectively (the first and second numbers stands for the reaction temperature (°C) and time (minutes) in each sample).
2.2 Extraction Procedure for Obtaining TiB2 Particles
In this research, some TiB2 particles were extracted from each sample for calculating the yield of TiB2 particles and measuring their size distribution. The detailed extraction procedure for obtaining TiB2 particles is given as (1) A small ingot was cut from the sample, and the surface of which was cleaned by sand paper. (2) The small ingot was dissolved in a 15 vol pct aqueous HCl solution in a beaker at room temperature. After dissolution, a layer of particles were deposited at the bottom of the beaker, and then the HCl solution was decanted. (3) TiB2 particles were washed with water for several times until the supernatant displayed neutral pH. (4) These TiB2 particles were washed with ethanol, and then dried by using an electric hair dryer. According to our proceeding work,[9] pure TiB2 phase can be obtained through the above process and no other phases can influence the calculation of the yield of TiB2 particles and the measurement of their size distribution.
2.3 Examination of Samples
In each group of experiment, the products generated in the reaction were consisted of ingot and slag. A small sample was cut from the ingot, and ground using SiC sand papers successively following the order of 180, 240, 400, and 600 mesh. Then the ground sample was polished using micro-sized diamond compound, following the order of 3 and 1 μm. After that the polished sample was cleaned in the ultrasonic cleaner for a few minutes to clean the polished surface. The by-product slag was milled to powders by using a glass mortar.
The sample and slag powder were both examined by X-ray diffraction (XRD, Bruker D8, AXS, Karlsruhe, Germany) using Cu Kα radiation at 40 kV and 40 mA and a scan rate of 0.10°/s. The microstructures of samples were analyzed by scanning electron microscopy (SEM, S-4800, Hitachi, Ltd., Tokyo, Japan) equipped with an energy dispersive spectroscope (EDS) device for identifying the components in the samples. An optical microscopy (OM) was also applied to observe the microstructures of samples.
2.4 Calculation of the Yield of TiB2 Particles
Three small ingots with a weight of about 8 grams cut from each sample were treated by a completed extraction process, respectively. Three groups of extracted powders were weighted by using an electric balance. Then the actual weight percentage of TiB2 phase in the Al matrix could be calculated. Since the sample was fabricated corresponding to the composition of Al-5 wt pct TiB2, the yield of TiB2 could be further obtained. An average value of the yield was computed in order to decrease the experimental error.
2.5 Measurement of the Size Distributions of TiB2 Particles
Zetasizer Nano ZS (Malvern) was used to measure the size distributions of TiB2 particulates extracted from each sample. This device was able to measure the particles with the size ranging from 3 nm to 5 μm. Before the test, a small amount of TiB2 powders were diluted with DI water. In order to reduce error, 10 measurements were conducted for each sample.
3 Results and Discussion
3.1 S900-10 Sample
3.1.1 XRD analysis
Figure 1 shows the XRD patterns of the slag and sample produced at 1173 K (900 °C) with a 10-minute reaction time. It is obvious that KAlF4 and K3AlF6 were the two main phases in the slag, as shown in Figure 1(a), indicating that Ti and B elements were both transferred from the molten mixed salts to molten Al. In the S900-10 sample, TiB2 was the only newly formed phase during the salts-metal reaction, the diffraction peaks of which could be observed clearly, as shown in Figure 1(b) (inset graph). The results of the two XRD patterns indicate that the chemical formula shown in reaction [12] is more suitable to describe the process of the reaction of K2TiF6-KBF4-Al system.
3.1.2 SEM analysis
Figure 2 shows the typical microstructure of the S900-10 sample. Only one type of newly formed phase existed in the Al matrix, which was identified as TiB2 by EDS. These small-sized TiB2 particulates aggregated to form clusters at α-Al grain boundaries, as shown in Figure 2(a). Figure 2(b) clearly shows that these TiB2 particles synthesized via the salts-metal reaction had different sizes, and most of them were much smaller than 1 μm. In Figure 2(c), it can be found that some TiB2 particles had a larger size exceeding 1 μm.
3.2 S900-30 Sample
3.2.1 XRD analysis
Figure 3 shows the XRD patterns of the slag and sample (S900-30) produced at 1173 K (900 °C) with a 30-minute reaction time. Similar to the products produced at 1173 K (900 °C) with a 10-minute reaction time, KAlF4 and K3AlF6 were the two main phases in the slag (Figure 3(a)), and TiB2 was the only newly formed phase in the sample (Figure 3(b)).
3.2.2 SEM analysis
The typical microstructure of the S900-30 sample is presented in Figure 4. Overall, it had similar microstructural features with the S900-10 sample. Only TiB2 particulates were in situ formed, which existed in the Al matrix as clusters located at the grain boundaries (Figure 4(a)). Most TiB2 particles were smaller than 1 μm in size (Figure 4(b)), whereas a very small amount of TiB2 particles were greater than 1 μm, as shown in Figure 4(c).
3.3 S700-10 Sample
3.3.1 XRD analysis
Figure 5 shows the XRD patterns of the slag and S700-10 obtained in the salts-metal reaction at 973 K (700 °C) with a 10-minute reaction time. KAlF4 and K3AlF6 detected by XRD were the main phases in the slag, as shown in Figure 5(a). No phase containing obvious Ti or B was found in the slag, suggesting that Ti and B elements were both transferred to molten Al from salts. Some rather weak diffraction peaks of TiB2 phase were found in the S700-10 sample (Figure 5(b)), indicating that the mixed-salts reaction proceeded according to the chemical formula shown in reaction [12] at the low temperature of 973 K (700 °C).
3.3.2 SEM analysis
Figure 6 shows the typical microstructure of the S700-10 sample. Three types of reinforcements existed in the Al matrix. Some blocky particles ranged in size from 1 to 3 μm were detected as Al3Ti by EDS. Some rather small-sized TiB2 particles were also found in the matrix, most of which existed along the chain-like reinforcement. After examined by EDS, the main phase of chain-like reinforcement was identified as Al3Ti, and also some separately tiny TiB2 particles were located in Al3Ti, as shown in Figure 6(b). Since the content of Al3Ti phase in the matrix was rather low, it is hard to detect in the XRD examination (Figure 5(b)). In addition, a little amount of AlB2 phase might exist in the Al matrix, but it is hard to examine them out in the present research due to the limitation of device.
3.4 S700-30 Sample
3.4.1 XRD analysis
Figure 7 shows the XRD patterns of the slag and S700-30 sample obtained in the salts-metal reaction at 973 K (700 °C) with a 30-minute reaction time. As shown in Figure 7(a), the slag was consisted of KAlF4 and K3AlF6 phases. Weak TiB2 peaks were also found in the sample, showing that the content of TiB2 phase was still low (Figure 7(b)) in the Al matrix by a 30-minute reaction.
3.4.2 SEM analysis
Figure 8 shows the microstructure of the S700-30 sample. Long chain-like reinforcements and cluster-like reinforcements were both found in the Al matrix, as shown in Figure 8(a). It is found that the chain-like reinforcement was composed of Al3Ti and some small-sized TiB2 particles which were located in the Al3Ti phase, as shown in Figure 8(b). The cluster-like reinforcement was consisted of some large-sized blocky Al3Ti particles and some nanometer-sized TiB2 particles, as shown in Figure 8(c). One more thing should be mentioned is that the size of long chain-like reinforcement in S700-30 sampler was smaller than that in the S700-10 sample, which indicated that part of it might dissolve or react as the reaction time was increased. AlB2 phase also might exist in the sample, which was hard to examine out clearly in this research.
3.5 Yields and Size Distributions of TiB2 Particles
The yields of TiB2 particles and their size distributions of each sample are presented in Figure 9. For the S900-10 sample, the yields of TiB2 particles could reach 89.5 pct; most TiB2 particles were less than 1 µm in size, in which about 81 pct of the TiB2 particles ranged in size from 400 to 800 nm, and a very small amount of TiB2 ranged in size from 1 to 2 µm. For the S900-30 sample, the yield of TiB2 particles was about 90.3 pct; overall, the most TiB2 particles were smaller than 1 µm, in which about 83 pct of the TiB2 particles ranged in size from 400 to 800 nm, and a rather small amount of TiB2 particles had the size in the range of 1 to 2 µm. It is clear that the S900-10 and S900-30 samples had similar yield and size distribution of TiB2 particles. For the S700-10 sample, the yield of TiB2 particles was rather low, about 28.1 pct; the size of TiB2 particles was less than 1 µm, in which about 95 pct TiB2 particles were smaller than 300 nm. For the S700-30 sample, the yield of particles could reach 36.4 pct; more than 95 pct of the TiB2 had the size less than 300 nm. Comparing with the S700-10 sample, the content of TiB2 particles with a smaller size was increased in the S700-30 sample, and the ratio of TiB2 smaller than 100 nm was about 30 pct, indicating a certain amount of TiB2 particles with a smaller size were formed as the reaction time was prolonged from 10 to 30 minutes.
The results in this research displayed the differences in the formation of TiB2 at different temperatures [1173 K and 973 K (900 °C and 700 °C)], such as the yields of TiB2 particulates and their size distributions. It is noted that the reaction temperature played a critical role in the salts-metal reaction of K2TiF6-KBF4-Al system in this research. Accordingly, the related formation mechanism of TiB2 might be different at different temperatures. The following discussion about the formation process of TiB2 will be conducted considering the Al melt temperature.
3.6 Formation of TiB2 at a Higher Temperature [1173 K (900 °C)]
In this research, when the reaction temperature was 1173 K (900 °C) and the reaction time was 10 minutes, no Al3Ti phase was found in the Al matrix, the yield of TiB2 reached 89.5 pct, and the size of most of in situ formed TiB2 particulates was in the range of 400 to 800 nm. As the reaction time was increased to 30 minutes, no obvious change occurred in the S900-30 sample, in which the yield of TiB2 was about 90.3 pct and the size of most TiB2 particles was in the range of 400 to 800 nm, as shown in Figure 9(a). It is clear that a higher reaction temperature is beneficial for the formation of TiB2 with a larger size in a short time.
The salts-metal reaction of K2TiF6-KBF4-Al system is an interface reaction. Based on the results of the S900 samples, the possible formation process of TiB2 phase in the salts-metal reaction is discussed below, in which three critical steps in the reaction are listed, respectively.
3.6.1 Formation of the Interface Between Molten Salts and Al Melt
First all of, no obvious KF and AlF3 phases were found in the slags produced in the salts-metal reaction at different temperatures. Thereby, the Ti and B in Al are not from the reactions of TiF4 (gas)-Al and BF3 (gas)-Al, in which the TiF4 and BF3 gases are from the decomposition of K2TiF6 and KBF4. Accordingly, the formation processes described in reactions [1], [2], [3], and [4] are not suitable to explain the formation of TiB2 phase. At least, the formation of most TiB2 phase in the reaction does not follow the chemical reaction shown in reaction [8].
Actually, it has been reported by Birol[10] that even though K2TiF6 has a higher melting point than KBF4, K2TiF6 can be reduced by Al, releasing Ti at approximately 493 K (220 °C); KBF4 starts to be reduced by Al a while later at round 798 K (525 °C), possibly after its polymorphic transformation from orthorhombic to cubic structure is over. In this research, after the mixed salts are added into the molten Al at 1173 K (900 °C), the mixed salts melt quickly on the Al melt surface, and an interface between molten salts and Al melt is formed.
3.6.2 Precipitation of TiB2
Once the reaction interface is formed, Ti released from K2TiF6 and B released from KBF4 both react with Al at the interface between salts and Al to form Al3Ti and AlB2 by reduction reactions [9] and [10]. According to Al-Ti and Al-B phase diagrams,[11] as shown in Figure 10, Al3Ti and AlB2 are both soluble in molten Al. The newly formed Al3Ti and AlB2 phases can dissolve in molten Al immediately, and then a thin layer of liquid Al containing Ti and B adjacent to the interface can be formed. The processes can be expressed as follows:
The aluminum-rich side of the Al-Ti phase diagram (a), and the aluminum-rich side of the Al-B phase diagram (b). Both of which were reproduced according to Sigworth[9]
Actually, the above processes are significantly influenced by the temperature of molten Al. Higher contents of [Ti] and [B] in the thin layer are obtained easily at a higher reaction temperature [1173 K (900 °C)]. On the one hand, the reduction reactions between salts and Al become more active, and more Al3Ti and AlB2 phases can be obtained at a higher temperature. On the other hand, according to the phase diagrams in Figure 11, the solubilities of Al3Ti and AlB2 phases in molten Al become larger as the temperature is increased. Thereby, increasing the temperature can accelerate the transfer speed of Ti and B to molten Al effectively.
Phase diagram for Al-Ti-B system at 1000 K (727 °C) (Al corner), x represents the molar fraction, which is reproduced according to Jones and Pearson[10]
As mentioned above, Ti and B can be easily obtained in the thin layer of Al at the reaction interface, and the Ti/B ratio in the reaction layer is much closer to ½ at a higher temperature. When the solutes [Ti] and [B] in liquid Al reaches saturation, they might be separated out as the compounds Al3Ti, AlB2, and TiB2 in the ternary system of Al-Ti-B. TiB2 is the most thermodynamically stable phase due to its lowest free energy of formation among the three compounds. In addition, according to the phase diagram of Al-Ti-B shown in Figure 11,[12] rather low contents of Ti and B can lead to the formation of TiB2 phase. In addition, once the concentrations of [Ti] and [B] reach saturation in the Al melt, TiB2 nuclei precipitate from the saturation region in the Al melt.
3.6.3 Growth of TiB2
As the reaction time is increased, more Ti and B are transferred from the molten salts to the reaction layer. TiB2 particles start to grow up due to the deposition of more Ti and B. It is known that the mass transfers of Ti and B from salts to the reaction interface become faster at a higher temperature, and a higher concentration of solutes in liquid Al can be obtained in a shorter time. Thereby, more Ti and B can be provided in the growth process of TiB2, and then the TiB2 particles can grow larger easily. Meanwhile, the salts-melt reaction for synthesizing TiB2 phase becomes faster.
3.7 Formation of TiB2 at a Lower Temperature [973 K (700 °C)]
Figure 9(b) shows the yields and size distributions of TiB2 particulates in the S700 samples. When the salts-metal reaction proceeded at 973 K (700 °C) with a 10-minute reaction time, some Al3Ti (AlB2 also should exist) phase existed in the Al matrix, the yield of TiB2 was just about 28.1 pct, and most of TiB2 particulates were smaller than 300 nm in size. The yield of TiB2 fabricated at 973 K (700 °C) with a 30-minute reaction time was 36.4 pct, which was higher than that of TiB2 in the S700-10 sample by about 29.5 pct. Furthermore, the ratio of TiB2 particles with a smaller size was increased, which indicated that a certain amount of smaller-sized TiB2 particles were synthesized as the reaction time was prolonged from 10 to 30 minutes. In addition, according to the SEM analysis (Figures 6 and 8), the products in the S700 samples were more complicated, in which blocky Al3Ti particulates, tiny TiB2 particulates, and chain-like Al3Ti reinforcement containing TiB2 phase were all formed.
Similarly, a reaction interface between the molten mixed salts and Al is firstly formed in the reaction at a lower temperature. However, more Ti and B might exist as Al3Ti and AlB2, respectively, at the reaction interface, because of the limited solubilities of the two phases at a lower temperature (as shown in Figure 10). It has been proved that the reaction between K2TiF6 and Al occurs at a much higher rate than that between KBF4 and Al.[13] Thereby, the ratio of Ti/B in the thinner layer located at the reaction interface is easy to be greater than ½, and this ratio will become greater as the temperature of molten Al is decreased. Part of Ti reacts with B to form TiB2 phase, following the precipitation-growth process, and the rest of Ti will react with Al to form Al3Ti phase. As reported by Mohanty et al.,[14] the growth of Al3Ti could engulf the entire TiB2 particles. Alternatively, the Al3Ti might also nucleate as individual particulates.[15]
At a lower temperature, since the content of B is rather low in the reaction interface, the growth of TiB2 will be limited. As a result, the size of TiB2 in the S700-10 sample was much smaller than that of TiB2 produced in the S900-10 sample.
In the S700-30 sample, the newly formed TiB2 particulates had a smaller size. It is reasonable that the newly formed TiB2 particulates in the S700-30 sample were obtained according to reaction [11], by which Al3Ti reacted with AlB2 to form TiB2. The detailed formation process has been studied by Emamy et al.[16] and Michael Rajan et al.,[17] respectively. The basic sequence of TiB2 formation can be proposed as follows:
-
1.
The B from AlB2 moves toward Al3Ti phase;
-
2.
Reaction takes place between Ti and B in a gap from Al3Ti surface to formTiB2;
-
3.
Dissolution of Al3Ti phase due to natural cracking and fragmentation of Al3Ti which lead to increased rate of TiB2 formation.
These steps involve the dissolutions of AlB2 and Al3Ti phases and the reaction for synthesizing TiB2. Thereby, the related formation process of TiB2 is a dissolution-reaction process. The detailed process in the present research is give below.
Al3Ti phase in the S700-10 sample mainly existed in the chain-like reinforcements. The area surrounding Al3Ti phase was a [Ti]-rich region. AlB2 phase also existed in molten Al, which could dissolve in Al to generate B atoms. When B atoms contacted with the Al3Ti phase, Ti and B could react to form TiB2. The concentrations of [Ti] and [B] were rather low due to the low temperature of molten Al; thereby, the TiB2 particles synthesized in this period were not able to grow large, and more TiB2 particles with a smaller size were formed. As a result, the percentage of TiB2 particles with smaller size in the S700-30 sample was increased compared with the S700-10 sample, as shown in Figure 9(b).
As the reaction time was increased, the quantities of Al3Ti and AlB2 were both decreased, and more TiB2 could be formed. A comparison about the chain-like reinforcements between the S700-10 sample and the S700-30 sample is given in Figure 12. It is clear that the size of long chain-like reinforcements became small due to the dissolution of Al3Ti phase in the formation of TiB2 particulates. In this research, because the temperature was rather low, a complete formation of TiB2 was hard to achieve in a short holding time. In order to obtain more TiB2 phase, a longer reaction time is needed. Chen et al.[2] reported that a completed reaction for synthesizing TiB2 could not be achieved with a 60-minute holding time after salt addition at 1023 K (750 °C), and large areas of AlB2 and Al3Ti were observed along the grain boundary of the composites. One more thing should be mentioned is about the AlB2 phase. In this research, the phases in the slags produced in the fabrications of the S700-10 and S700-30 samples were similar; on the other hand, as the content of TiB2 was increased in the S700-30 sample, it is reasonable to think some AlB2 should exist in the S700 samples. As reported by Wang et al.,[18] blocky AlB2 could be formed in the Al-B master alloy at 1023 K (750 °C) with a 10-minute reaction time. Wang et al.[19] continued proving that the similar AlB2 particles still existed in the Al-B master alloy at 1023 K (750 °C) with a 30-minute reaction time. Due to the rather small amount of AlB2, it was hard to be detected by XRD in this research.
4 Conclusions
In this research, the influence of reaction temperature on the formation of TiB2 via the salts-metal reaction regarding the yield and size distribution of TiB2 particles was quantitatively analyzed firstly and the formation processes of TiB2 at a higher temperature [1173 K (900 °C)] and a lower temperature [973 K (700 °C)] were both investigated as well. The following conclusions are drawn:
-
1.
Reaction temperature has an important influence on the synthesis of TiB2 in molten Al via the salts-metal reaction. A high temperature [1173 K (900 °C)] is beneficial for the formation of TiB2. A higher temperature can lead to a higher yield of TiB2 particles with a larger size. In contrast, smaller-sized TiB2 particles can be obtained at a lower temperature [973 K (700 °C)], but the formation of TiB2 can be limited significantly, resulting in a rather low yield of TiB2.
-
2.
At a higher reaction temperature [1173 K (900 °C)], the salts-metal reaction for synthesizing TiB2 phase mainly follows the precipitation-growth process, in which the precipitation and growth of TiB2 phase proceed quickly at the reaction interface between salts and liquid Al.
-
3.
At a lower temperature [973 K (700 °C)], the precipitation-growth process and dissolution reaction between AlB2 and Al3Ti both contribute to the formation of TiB2. As the reaction time is prolonged, TiB2 particles with a smaller size can be formed through the process of the dissolution reaction.
References
A. Mandal, R. Maiti, M. Chakraborty, B.S. Murty: Mater. Sci. Eng. A, 2004, vol. 386, pp. 296-300.
Y.F. Han, X.F. Liu, X.F. Bian: Composites Part A, 2002, vol. 33, pp. 133-38.
M.L. Wang, D. Chen, Z. Chen, Y. Wu, F.F. Wang, N.H. Ma, H.W. Wang: Mater. Sci. Eng. A, 2014, vol. 590, pp.246-254.
G. Han, W.Z. Zhang, G.H. Zhang, Z.J. Feng, YJ. Wang: Mater. Sci. Eng. A, 2015, vol. 633, pp. 161-168.
Z.N.Chen, T.M. Wang, Y.P. Zheng, Y.F. Zhao, H.J. kang, L. Gao: Mater. Sci. Eng. A, 2014, vol. 605, pp. 301-309.
S. Lakshmi, L. Lu, M. Gupta: J. Mater. Process. Tech., 1998, vol. 73, pp. 160-66.
T.X. Fan, G. Yang, D. Zhang: Metall. Mater. Trans. A, 2005, vol. 36, pp. 225-33.
C.F. Feng, L. Froyen: J. Mater. Sci., 2000, vol. 35, pp. 837-50.
Z.W. Liu, M. Rakita, W. Xu, X.M. Wang, Q.Y. Han: Chem. Eng. J., 2015, vol. 263, pp. 317-24.
Y. Birol: J. Alloy. Compd., 2009, vol. 480, pp. 311-14.
G.K. Sigworth: Metall. Trans. A, 1984, vol. 15, pp. 277-82.
G.P. Jones, J. Pearson: Metall. Trans. B, 1976, vol. 7, pp. 223-34.
C.D. Mayers, D.G. McCartney, G.J. Tatlock: Mater. Sci. Technol. Lond., 1993, vol. 9, pp. 97-103.
P.S. Mohanty, J.E. Gruzleski: Acta Metallurgica et Materialia., 1995, vol. 43, pp. 2001-12.
M.M. Guzowski, G.K. Sigworth, D.A. Sentner: Metall. Trans. A, 1987, vol. 18, pp. 603-19.
M. Emamy, M. Mahta, J. Rasizadeh: Compos. Sci. Technol., 2006, vol. 66, pp. 1063-66.
H.B. Michael, S. Ramabalan, I. Dinaharan, S.J. Vijay: Mater. Design., 2013, vol. 44, pp. 438-45.
T.M. Wang, Z.N. Chen, H.W. Fu, J. Xu, Y. Fu, T.J. Li: Scripta Materilia., 2011, vol. 64, pp. 1121-24.
T.M. Wang, H.W. Fu, Z.N. Chen, J. Xu, J. Zhu, F. Cao, T.J. Li: J. Alloy. Compd., 2012, vol. 511, pp. 45-49.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Manuscript submitted February 21, 2015.
Rights and permissions
About this article
Cite this article
Liu, Z., Han, Q., Huang, Z. et al. A Quantitative Study of the Synthesis of TiB2 Particles via Salts-Metal Reaction at Different Temperatures. Metall Mater Trans A 47, 916–926 (2016). https://doi.org/10.1007/s11661-015-3268-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-015-3268-7