Abstract
Melt-SHS (self-propagating high-temperature synthesis), based on the SHS process and oxide reaction method, was used for preparation of TiB2/Al composites. The mass ratio of two reactants, Ti powder/TiO2, in initial powder mixture was varied from 0:1 to 1:0. The results showed that the 5 wt% TiB2/Al composites could be successfully produced by a reaction of aluminum powder, TiO2, and B2O3 in Al melt at 950 °C, while the reaction rate was slow. The addition of titanium powder helps to reduce the content of Al2O3 and destroy the coating structure of Al2O3 covered TiB2 particles, which leads to the acceleration of reaction process and improvement of particle concentration. A significant improvement was that TiB2 particles were dispersively distributed when the mass ratio of Ti powder/TiO2 was 2:3. As a result, the 5 wt% TiB2/Al composites fabricated by melt-SHS process with modified reactants ratio showed excellent tensile properties with the ultimate tensile strength as high as 114.24 MPa. Besides, the composite also showed superior ductility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
I. INTRODUCTION
Insitu particulate reinforced aluminum matrix composites (Al MMCs) are widely used in the aerospace and automotive industries due to their excellent properties, such as high specific strength, low weight, and chemical inertness.1 It consists of matrix and the reinforcement, and the reinforcement is one of the most important parts. TiB2 particles reinforced Al MMCs is one of the most widely used particulate reinforced Al MMCs because of its outstanding properties, such as low density, high melting point, high elastic modulus, and good lattice matching relations with the aluminum matrix.2
It has been reported in many studies that TiB2 particles can be synthesized by in situ synthesis method, and the process offers significant advantages over the conventional processing from both technical and economic standpoints,2,3 such as its excellent interfacial compatibility between matrices and reinforcements, thermodynamically stable, and distribution uniformity of the reinforcements in the matrix.
In situ TiB2 particles reinforced aluminum matrix composites can be synthesized by the salt-metal reaction, self-propagating high-temperature synthesis (SHS), hot pressing, pressureless sintering, and mechanochemical synthesis.3,4 The salt-metal reaction is the most commonly used in situ methods.5 In this processing, the mixed K2TiF6 and KBF4 were used as reactants, which reacted with molten Al at a high temperature to form TiB2 particles in the Al matrix.6 However, the method has fluoride emissions and the dross needs to be disposed. As one of the fabrication techniques to synthesize in situ ceramic particulate reinforced MMCs, SHS technology has received much attention because of its low energy consumption and high product purity. Nevertheless, the price of the reactants is expensive, and procedure is complicated and time consuming because the final products are porous and need to combine with a densification step; the react temperature cannot be controlled easily as well. To decrease the cost of reactants, Al–TiO2–B,7 TiO2–B4C–C,8 Al–Ti–B,9,10 Al–TiO2–B2O3–Na3AlF6,11 and Al–TiO2–B2O312 systems were studied to fabricate TiB2 particles reinforced aluminum matrix composites. Due to the high price of the elemental powder, the oxide and compounds of titanium and boron were popular as reactants in the new system. However, another main product, Al2O3, needs to be cleared, and this undoubtedly leads the amount of reactants and reaction process to be more difficult to control.
In this study, a modified method melt-SHS process which combined the advantages of low price of oxide reactant and SHS process was used to synthesize Al/TiB2 composites. Furthermore, the melt-SHS method is environmentally friendly due to the clean products. The reactants, H3BO3, TiO2, Al powder, and Ti powder, were used in the process since Ti powder could be used as a reductant and a combustion promoter. To confirm the optimum reactant ratio and produce the low-cost and efficient composites, the different ratios of H3BO3, TiO2, and Ti powder were applied.
II. EXPERIMENTAL PROCEDURE
A. Raw materials
High purity aluminum (>99.9 wt%) was used as the base metal. Two kinds of chemical powders, namely TiO2 (200 mesh) and H3BO3 (200 mesh), and two types of metal powders, namely Al powder (300 mesh) and Ti powder (300 mesh), were used to synthesize the TiB2 particles.
B. Processing
With the atomic ratio Ti/4B, TiO2 and H3BO3 were first dried at 200 °C for 2 h, and then a percentage of aluminum and titanium powder were added in the mixed powder and mechanical stirred homogeneously. The final mixed powder was pressed into a cylinder (φ62.5 × 12 mm3) to make a powder block. After that, Al ingot was melted in a graphite crucible resistance furnace as the temperature reached 950 °C, which was measured by a thermocouple, then the prepared powder block was pressed into the melt. Melt-SHS reaction between the powder cylinder and the molten Al to form TiB2 particles. The whole preparation process is shown in Fig. 1, and the synthesis process is in the air. Two times were marked: one is the response time t1 which is the time from the cylinder touched aluminum melt to the emergence of the spark. The other is the reaction time denoted as t2, which indicates the time from the emergence of the spark until the spark went out. After the reaction, the melt was mechanically stirred for 10 min, the reaction holding time was set as 30 min at 800 °C, and finally the melt was poured into a steel mold (φ95 × 150 mm3) at 720 °C. Then the TiB2/Al composite billets were extruded into bar composites with an extrusion ratio of 25:1 at about 400 °C. The four composites correspond to the four quality ratio of Ti powder/TiO2 in the initial powder mixture which varied from 0:1 to 1:0 (showed in Table I).
The mechanical properties were evaluated by the tensile test, which was carried out on a computerized universal testing machine at a velocity of 0.05 mm/s. Three tests were conducted for each composite to get a precise value for each property.
C. Characterization
The metallographic specimens were intercepted from each bottom of the samples. X-ray diffraction (D8 ADVANCE, Bruker, Karlsruhe, Germany) was used to analyse the diffraction phases in the composite. Scanning electron microscopy (QUANTA200, FEI Company, Hillsboro, Oregon) equipped with an energy-dispersive X-ray spectroscopy (EDS) was used to observe the morphology and distribution of particles in the composites. Besides, the software Image-Pro plus 6 (Media Cybernetics, Rockville, Maryland) was used to statistically analyze the particle size distribution.
III. RESULTS AND DISCUSSION
A. Thermodynamic analysis
Before the preparation of powder block, TiO2 and H3BO3 mixture was dried to remove the water in boric acid. It was easier to produce crystalline lumps since boric acid was dried separately. The reaction of the dehydration process can be written as function (1),
The formation of ceramic particles via melt-SHS process is a thermodynamically chemical reaction. The possible reaction basically has the following several parts: (i) at a predetermined temperature, Al powder produces sparks as an igniter composition, and the combustion wave diffuses along the powder cylinder. (ii) The aluminothermy reduction of B2O3 and TiO2 at the molten flux-liquid metal interface forms B, Ti atoms, and Al2O3, which can be written as Eqs. (2) and (3). (iii) The formation of Al3Ti, AlB2, and TiB2 by combination reaction of B, Ti, and Al. The reactions are shown in Eqs. (4), (5), and (7). (iv) The boron atoms react with Al3Ti and generate TiB2. The last part reaction is exhibited as Eq. (6),
The temperature dependence of the reaction enthalpy (ΔHT) and free energy (ΔGT) changes for these six reactions are summarized in Fig. 2. The results indicate that the values of ΔHT and ΔGT in all functions are negative, and further illustrate that the reactions are exothermic reactions and can proceed spontaneously. The lower the ΔGT is, the higher possibility the reaction goes. It is easy to observe that Eq. (7) has the greatest possibility over other functions in the parts (iii) and (iv), and TiB2 particle is the most stable phase. A notable finding is that the TiB2 is the main product, and TiAl3 and AlB2 are much less competitive due to the higher ΔGT.
The SHS reaction maintains by the heat released in the process of the reaction. It is necessary to analyse the thermodynamics which enables a SHS reaction to proceed successfully. For the SHS reaction, an experience criterion was put forward; when Tad ≥ 1527 °C, the SHS reaction sustains itself, otherwise needs supplement system energy.13 The adiabatic temperature can be calculated according to the materials formed in the process of reaction and enthalpy of phase changes. The overall reaction showing the formation of TiB2 can be written as
According to the first law of thermodynamics,
The former equation at the interval of room temperature and temperature T is integrated, and the solid phase transformation and melting phenomenon of the material within the scope of the studied temperature T range are considered. Ignoring the vaporization phenomenon, the equation is as follows:
\({C_{{\rm{p}},{\rm{m}}}},C_{{\rm{p}},{\rm{m}}}^\prime ,\>{\rm{and}}\>C_{{\rm{p}},{\rm{m}}}\) are the molar constant pressure heat capacity of substance, J/(mol K). \(H_T^\Theta - H_{298}^\Theta \) is the relative material mole enthalpy, J/mol. Ttr and Tm are the phase transition temperature and melting point of material, K. ΔHtr and ΔHm are the solid phase transformation and melting heat of material, J/mol.
According to the Kirchhoff equation,
In the equation, ΔCp is the D-value between the sum isobaric heat capacity of products and reactants, namely, the heat capacity difference,
In the equation, ni is the mole of reactants participate in the reaction and Cp is the molar constant pressure heat capacity.
Assuming that the reactants are involved in the reaction completely under the condition of adiabatic temperature, the release of heat will be all applied to the reaction. If the reaction proceeds at room temperature (298 K), the equation is as follows:
\(\Delta H_{298}^\Theta \) is the heating effect of the reaction at room temperature. If the reactants are preheated at T0,
If the adiabatic temperature of reaction is 1527 °C, preheat temperature T0 is easily figured out. After calculation, the T0 is 892 °C. Considering that the experiment is under atmospheric conditions, to ensure the smooth progress of the reaction, the starting reaction temperature of 950 °C was used.
B. X-ray diffractions analysis
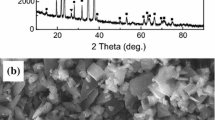
XRD patterns of the four composites and the reaction slag are shown in Fig. 3. The presence of TiB2 peaks and TiAl3 peaks in the 1#, 2#, and 3# composites [Fig. 3(a)] confirms the formation of TiB2 and TiAl3. The TiB2 peaks in 4# composite are not obvious. XRD patterns of the reaction slag [Fig. 3(b)] confirm the formation of TiB2 and Al2O3 in the fabrication of four composites. It can be seen that the 4# slag has more obvious peaks of TiB2 and Al2O3, which means that TiB2 particles which failed to spread to aluminum melt mainly exist in the slag. It is interesting to note that there are no obvious peaks of AlB2 and Al2O3 in the spectra of the TiB2/Al composites, while Al2O3 mainly exists in the reaction slag which had been cleared out of the Al melt.
C. Microstructures of reaction slag
To make sure TiB2 and Al2O3 particles exist in the reaction slag, microstructures and energy spectrum analysis of the slag are shown in Fig. 4. Combining XRD and energy spectrum analysis [Figs. 4(c) and 4(d)], accumulation area of Al2O3 [Fig. 4(a)] and TiB2 [Fig. 4(b)] was found in the slag. It can be concluded that Al2O3 particles were taken away with the reaction slag, and there was still a percentage of TiB2 particles that have not been able to diffuse into the Al melt. This is mainly because the Al2O3 particles gathered in the block after the reaction. Besides, the initial size of TiB2 particles formatted in the block were much less than the holes in the block, which results in the smaller size TiB2 particles diffused to the aluminum melt easily at the end of the reaction. With the increase of the reaction holding time, part of TiB2 particles in the block grew up to a bigger size and stayed in the melt.
D. Microstructure and properties of composites
Microstructures and the particle size distribution of four composites with different mass ratios of Ti powder/TiO2 in raw materials are showed in Fig. 5. And the mechanical properties of 5 wt% TiB2/Al composites were shown in Table II.
According to XRD [Fig. 3(a)] and EDS analysis [Fig. 5(e)], it can be confirmed that the white particles are TiB2. The morphology of TiB2 particles in composite 1# [Fig. 5(a)] presents the hexagonal shape, and the average size is nearly 0.91 µm [Fig. 5(f)]. Compared to composite 1#, composite 2# [Fig. 5(b)], which has additional Ti powder in raw materials mixture and with a mass ratio of Ti powder/TiO2 of 2:3, exhibits a uniform distribution of TiB2 particles in the Al matrix, the average size is nearly 0.67 µm [Fig. 5(f)], and the contents of TiB2 particles increased. It is seen that when Ti powder was added into the powder block, the assimilation of B increased and showed an increase in the number of the TiB2 particles. Thus the 2# composite showed excellent tensile properties: the ultimate tensile strength (UTS) is up to 114.24 MPa, the hardness is 25.54 HB, and the elongation is 33.15% which is also a superior ductility. After the fast exothermic combustion reaction, the temperature in the combustion zone decreases sharply and the desired phases are vigorously formed in great numbers. With increasing of Ti powder mass ratio further, the contents of TiB2 particles decrease as shown in Figs. 5(c) and 5(d), and the average size increases to 0.72 µm and 0.95 µm, respectively [Fig. 5(f)]. Besides, the distributions of TiB2 particles were uneven when the quality ratio of Ti powder/TiO2 was larger than 2:3 which resulted from the relatively higher adiabatic temperature induced by the addition of Ti powder. And the tensile properties were also not that good, which showed lower UTS, because high temperature led to a large amount of evaporation of aluminum melt and made the TiB2 diffusion become more difficult. According to the data of XRD spectra and the microstructures, it was confirmed that the amount of TiAl3 phase was too few to show in the microstructure. It may be because a long enough reaction holding time results in less available TiAl3 in the melt, as a result of its continuing decomposition to form TiB2.
E. In situ formation of TiB2 particulates
The response time t1 and the reaction time t2 are showed in Table III.
After the powder block was put into an aluminum melt, the composite 1# did not spark immediately. Approximately 60 s later, the spark appeared and extinguished in 2 min. While the composites with the additions of Ti powder (the composite 2#, 3#, 4#) appeared the spark in 10 s and kept for 1 min until the reaction finished. The reactants of composite 1# are H3BO3 and TiO2 which resulted in the products containing a mass of Al2O3. A mount of the reactants was forbidden to touch the Al melt because the powder cylinder was surrounded by the formed Al2O3 which could be deduced from the microstructure of the slag as shown in Figs. 6(a) and 6(b); thus, the reaction speed was reduced greatly. Majority of nanometer TiB2 particles were trapped in the melt slag and could not spread out as shown in Fig. 6(c).
According to the thermodynamic analysis and the microstructures, the reaction process analysis was described in Fig. 7. This reaction process used the heat released by the reaction to synthesise TiB2/Al composites. When the mixed powder cylinder was pressed into the high temperature aluminum liquid, parts of titanium powder first ignition where the powder and liquid aluminum contact because of the flammable properties of titanium powder, and aluminum powder melted due to the low melting point. TiO2 and B2O3 reacted with the molten aluminum to form free B atoms and Ti atoms. Since the Gibbs free energy of aluminum oxide is lower than that for boron oxide, chemical reactions occur at the interface between molten aluminum and boron oxide.12,14 As boron atoms go into solution in the molten aluminum, aluminum oxide covers the top of melt as slag and formed a coating structure. The reaction released heat to melt the rest of the parts of solid titanium powder, but it only occurred in a few atomic layers of the Ti particles surface, and the molten titanium reacted with the free boron to produce TiB2 particles. According to the thermodynamic analysis, it is easy to form AlB2 and TiAl3 phases, respectively, when the free B and Ti atoms met the molten Al. It has been reported15,16 that the dominant bonding in TiB2 is covalent, which leads to its great structural stability at high temperature and the dominant bonding in AlB2 is ionic due to the large charge transfer from aluminum to boron. It means that AlB2 phases were relatively unstable from the view of thermodynamics, and it would decompose back to Al and B atoms. The free B atoms not only combined with Ti atoms to form TiB2 but also took part in the reduction reaction with TiAl3 to form TiB2. The Al2O3 particles were cleaned out of the Al melt with the reaction slag. It has been reported that the removal of Al2O3 promotes the particle wetting.17 With the Al2O3 out of the melt, the wetting angle between the liquid Al and TiB2 decreased, hence also producing a good interfacial integrity. The addition of titanium powder helps to reduce the content of Al2O3 and destroy the coating structure of Al2O3 covered TiB2 particles, which lead to the acceleration of reaction process and improvement of particle concentration. Therefore, the addition of titanium powder could reduce the generation and accumulation of Al2O3 and proceed the reaction.
F. Fractograph
Figure 8 shows the fractographs of the composites fabricated by the melt-SHS with the ratio of Ti powder/TiO2 as 2:3 (2# composite) which has the high UTS of 114.24 MPa. As shown in Fig. 8(a), a large number of micron and deep equiaxed dimples dominate in the fracture of the matrix, which indicates that ductile fracture happened during tensile test. TiB2 particles were discovered in the equiaxed dimples as shown in Fig. 8(b). It has been reported that TiB2 particles act as effective barriers to the growth and coalescence of the voids which resulted in the matrix grain refinement.18,19 Due to the great coherent relationship between TiB2 particles and Al matrix, the good wetting, and the strong interface bonding, the composites achieved excellent tensile properties.
IV. CONCLUSIONS
Using the melt-SHS method, 5 wt% TiB2/Al composites were prepared by the reaction of Al powder, Ti powder, TiO2, and H3BO3 in Al melt successfully. The results are showed as follows:
-
(1)
5 wt% TiB2/Al composite could be produced at 950 °C, but the reaction rate is slow when only adding TiO2 as the main source of Ti. The addition of Ti powder into the powder block significantly reduces the response time from more than 40 s to less than 10 s and improves the reaction rate. With the increase of titanium powder in the raw powder materials, the reaction rate increased.
-
(2)
Al2O3 particles mainly exist in the melt slag and can be cleared directly. The addition of titanium powder helps to reduce the content of Al2O3, destroy the coating structure of Al2O3 covered TiB2 particles, speed up the reaction process, and improve particle concentration.
-
(3)
The microstructure of composites mainly presents sub-micron TiB2 particles, and the average size of the particles is 0.67 µm with Ti powder addition to the reactants and the optimized ratio of Ti powder/TiO2 is 2:3. While the distribution of TiB2 particles were heterogeneous and the particle size increased when the quality ratio of Ti powder/TiO2 is larger than 2:3 which resulted from the relatively increased adiabatic temperature induced by the additions of Ti powder.
-
(4)
The 5 wt% TiB2/Al composite fabricated by melt-SHS process with modified reactants ratio showed excellent tensile properties with the UTS as high as 114.24 MPa, and the composite also showed superior ductility.
References
Q. Zhang, W.H. Wu, G.Q. Chen, L.T. Jiang, and B.F. Luan: The thermal expansion and mechanical properties of high reinforcement content SiCp/Al composites fabricated by squeeze casting technology. Composites, Part A 34, 1023–1027 (2003).
C.F. Feng and L. Froyen: Microstructures of in situ Al/TiB2 MMCs prepared by acasting route. J. Mater. Sci. 35, 837–850 (2000).
S. Kumar, V.S. Sarma, and B.S. Murty: A statistical analysis on erosion wear behavior of A356 alloy reinforced with in situ formed TiB2 particles. Mater. Sci. Eng., A 476, 333–340 (2008).
Z.W. Liu, M. Rakita, W. Xu, X.M. Wang, and Q.Y. Han: Ultrasound assisted salts-metal reaction for synthesizing TiB2 particles at low temperature. Chem. Eng. J. 263, 317–324 (2015).
Q. Gao, S.S. Wu, S.L. Lü, X.C.H. Duan, and P. An: Preparation of in situ 5 vol% TiB2 particulate reinforced Al–4.5Cu alloy matrix composites assisted by improved mechanical stirring process. Mater. Des. 94, 79–86 (2016).
S.C. Tjong and Z.Y. Ma: Microstructural and mechanical characteristics of in situ metal matrix composites. Mater. Sci. Eng., R 29, 49–113 (2000).
C.F. Feng and L. Froyen: On the reaction kinetics of an Al–TiO2–B system for producing in situ Al/(Al2O3 + TiB2) composites. J. Mater. Sci. Lett. 19, 103–105 (2000).
R. Koc and D.B. Hodge: Production of TiB2 from a precursor containing carbon coated TiO2 and B4C. J. Mater. Sci. Lett. 19, 667–669 (2000).
P.J. Li, E.G. Kandalova, V.I. Nikitin, A.R. Luts, A.G. Makarenko, and Y.F. Zhang: Effect of fluxes on structure formation of SHS Al–Ti–B grain refiner. Mater. Lett. 57, 3694–3698 (2003).
V.I. Nikitin, W.Q. Jie, E.G. Kandalova, A.G. Makurenko, and L. Yong: Preparation of Al–Ti–B grain refiner by SHS technology. Scr. Mater. 42, 561–566 (2000).
Y.F. Yang and Q.C. Jiang: Effect of TiB2/TiC ratio on the microstructure and mechanical properties of high volume fractions of TiB2/TiC reinforced Fe matrix composite. Int. J. Refract. Met. Hard Mater. 38, 137–139 (2003).
E.M. Sharifi, F. Karimzadeh, and M.H. Enayati: Synthesis of titanium diboride reinforced alumina matrix nanocomposite by mechanochemical reaction of Al–TiO2–B2O3. J. Alloys Compd. 502, 508–512 (2010).
C.L. Yeh and S.H. Su: In situ formation of TiAl–TiB2 composite by SHS. J. Alloys Compd. 407, 150–156 (2006).
A. Changizi, A. Kalkanli, and N. Sevinc: Production of in situ aluminum–titanium diboride composite formed by slag–metal reaction. J. Alloys Compd. 509, 237–240 (2011).
H.L. Zhang, Y.F. Han, J. Wang, Y.B. Dai, and B.D. Sun: An ab initio molecular dynamics study on the structural and electronic properties of AlB2, TiB2 and (Alx, Ti(1−x))B2 in Al–Ti–B master alloys. J. Alloys Compd. 585, 529–534 (2014).
X.Z. Li, X.F. Bian, X.J. Li, and X.F. Liu: Ab initio studies of TiB2 and AlB2 in the Al–Ti–B alloy. Acta Metall. Sin. 37, 235–238 (2001).
S. Lakshmi, L. Lu, and M. Gupta: In situ preparation of TiB2 reinforced Al based composites. J. Mater. Process. Technol. 73, 160–166 (1998).
B.S. Murty, S.A. Kori, K. Venkateswarlu, R.R. Bhat, and M. Chakraborty: Manufacture of Al–Ti–B master alloys by the reaction of complex halide salts with molten aluminum. J. Mater. Process. Technol. 80, 152–158 (1999).
F. Chen, Z.N. Chen, F. Mao, T.M. Wang, and Z.Q. Gao: TiB2 reinforced aluminum based in situ composites fabricated by stir casting. Mater. Sci. Eng., A 625, 357–368 (2015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Chai, L., Wang, H. et al. Fabrication of TiB2/Al composite by melt-SHS process with different content of titanium powder. Journal of Materials Research 32, 2352–2360 (2017). https://doi.org/10.1557/jmr.2017.184
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2017.184