Abstract
Summary
We measured serum parathyroid hormone in 8409 Romanian subjects and found a mild seasonal variation with highest levels in March and lowest levels in September. PTH was dependent on serum vitamin D, particularly below 12.82 ng/mL. Together, these suggest that vitamin D supplementation is warranted only in at-risk groups.
Purpose
Seasonal variation of parathyroid hormone (PTH) and its dependency on serum 25-hydroxy vitamin D (25(OH)D) levels are well-described. However, there are few studies from Europe, and genetic, nutritional, and cultural differences are important. We describe the seasonal variation of serum PTH and its relation with serum 25(OH)D levels in Romania.
Methods
We retrieved from our endocrinology center database all PTH measurements between 2011 and 2019 together with age, sex, diagnosis, and date of blood sampling. Simultaneous serum 25(OH)D levels were partially available. Intact PTH was measured using the same electrochemiluminescence assay.
Results
There were 8409 subjects (median age 49 (36, 60) years; 20.5% men) without a diagnosis of hyperparathyroidism (primary or secondary to chronic kidney disease), hypoparathyroidism, or low bone mass. Serum PTH showed a mild seasonal variation with highest levels at the end of March (47.61 pg/mL) and lowest levels at the end of September (43.15 pg/mL). All sex and age subgroups showed highest levels in the spring and lowest levels in the fall. Males had significantly lower PTH levels than females irrespective of season. PTH correlated inversely with serum 25(OH)D with a breakpoint of 12.82 ng/mL in 2800 subjects who had a simultaneous measurement of 25(OH)D. Increasing age was associated with increasing PTH levels independently of serum 25(OH)D.
Conclusions
We showed a mild seasonal variation of serum PTH in Romania, at an inverse sinusoidal pattern than serum 25(OH)D. The breakpoint on the PTH–25(OH)D correlation suggests that vitamin D supplementation is warranted only in at-risk groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serum 25-hydroxy vitamin D (25(OH)D) has a strong seasonal variation in the temperate climate, with the nadir in the early spring and the highest levels in the early autumn [1]. In the Northern Hemisphere, these correspond to the beginning of March and the beginning of September [2]. Romania, located between 44° N and 48° N latitude, has a similar variation of serum 25(OH)D [3, 4]. The prevalence of vitamin D insufficiency and deficiency is high in women and in old age particularly in the winter season [3, 4].
There is a widely demonstrated inverse correlation between serum 25(OH)D and serum parathyroid hormone (PTH) [5] and has also been shown for our country [6]. Most studies showed a threshold of 25(OH)D above which serum PTH reached a plateau [5] although few studies revealed a continuous relation [7]. Moreover, the 25(OH)D–PTH relation has been used to define vitamin D insufficiency [8].
A seasonal variation of serum PTH can be easily presumed, at an inverse sinusoidal pattern than serum 25(OH)D. This seasonal 25(OH)D–PTH relation has been consistently demonstrated for the temperate climate, including in studies with more than one million samples [9]. In these studies, the maximal seasonal variation (min to max) of serum PTH varied between 3 pg/mL [10] and 13 pg/mL [11] with a peak and a nadir in early spring and early autumn respectively. Studies conducted in Europe are less numerous and showed a small (4 pg/mL in Sweden) [12] or no PTH variation (Spain) [13]. Interestingly, a seasonal variation of PTH has been shown even in patients with primary hyperparathyroidism [14] or chronic dialysis [15].
Seasonal variation of PTH and its amplitude could be an indicator of vitamin D status and the need for vitamin D supplementation in the general population. It also might reflect genetic, nutritional, and cultural differences between racial and ethnic groups. Our aim was to describe the seasonal variation of serum PTH and its relation with serum 25(OH)D levels in Romania.
Methods
Subjects
We retrospectively retrieved from the electronic database of our endocrinology center all PTH and 25(OH)D measurements between January 1, 2011 and November 30, 2019. For each measurement, we also retrieved the corresponding sex, age, and diagnosis of the subject, and the date of blood sampling. The study was approved by the Institutional Ethic Committee.
Data from National Meteorological Administration of Romania showed a stable seasonality for the studied period with no significant differences between similar seasons or between the studied period and the Romanian climate. Therefore, we were able to group together serum PTH and 25(OH)D levels from similar days or seasons of all studied years.
There were 28,118 PTH measurements. Of these, 16,859 were made on different subjects and 11,259 were repeated measurements. In those subjects with multiple assessments available, we used only the first measurement. Of the 16,859 different subjects, we excluded 1572 (9.32%) patients with a diagnosis of primary hyperparathyroidism, 536 (3.17%) patients with a diagnosis of chronic kidney disease (including end-stage renal disease and dialysis), 386 (2.28%) patients with a diagnosis of hypoparathyroidism (including pseudohypoparathyroidism), and 5645 (33.48%) patients that had a diagnosis of low bone mass (osteoporosis or osteopenia) with 8720 subjects remaining. Of these, we excluded the subjects with PTH serum values over 200 pg/mL (41 subjects (0.47%)) or below 15 pg/mL (270 subjects (3.09%)) due to a high probability of an underlying disorder that was not mentioned in the diagnosis by the treating physician. A total of 8409 subjects were retained for the primary analysis. There were 1892 (22.5%) subjects sampled in the winter, 2252 (26.8%) in the spring, 1914 (22.8%) in the summer, and 2351 (27.9%) in the autumn. As for genetic background, 3467 (41.2%) subjects were born in Bucharest and Ilfov counties, 2749 (32.7%) subjects were born in Muntenia, 823 (9.8%) were born in Oltenia, 629 (7.5%) in Moldavia, 359 (4.3%) in Dobrogea, 289 (3.4%) in Transylvania, 38 (0.5%) in Banat, 30 (0.4%) in Maramures, 19 (0.2%) in Crisana, and 6 (0.07%) were born outside Romania. Of the 8409 subjects with a PTH measurement, 2800 (33.29%) had a simultaneous measurement of serum 25(OH)D and were included in the secondary analysis group. Data on vitamin D or calcium supplementation was not available for either primary or secondary analysis. Subjects’ characteristics can be found in Table 1.
PTH and 25(OH)D assays
Intact PTH was measured by electrochemiluminescence on a Cobas E601 C analyzer (Roche Diagnostics, Indianapolis, IN; with a measuring range 1.2–5000 pg/mL, reference range 15–65 pg/mL, functional sensitivity 6.0 pg/mL, and a variation coefficient of 20%).
25(OH)D was measured by chemiluminescence on a Liaison XL analyzer (DiaSorin, Saluggia, Italy; with a measuring range 4–150 ng/mL, functional sensitivity 4 ng/mL, and variation coefficient of 20%) or by electrochemiluminescence on a Cobas E601 C analyzer (Roche Diagnostics, Indianapolis, IN; with a measuring range 3–70 ng/mL, functional sensitivity 4.01 ng/mL, and variation coefficient of 18.5%).
Statistical analysis
We tested for normal distribution of PTH and 25(OH)D serum concentrations using D’Agostino-Pearson test. Both values were skewed and normal distribution was rejected. All continuous variables are expressed as median (25th, 75th percentiles). Prevalence data are expressed as number (percentage). For comparisons between two groups (Autumn vs. Spring, men vs. women), we used the Mann-Whitney U test. For comparisons between more than three groups (age groups), we used the Kruskal-Wallis test.
For a better description of the annual variation of serum PTH and 25(OH)D levels, we plotted all individual values against the corresponding day of serum sample (counted from the beginning of the year of the blood drawing). A sine function with a period of 365 was fitted. Minimum and maximum of the curve were found by solving the first derivative of the fitted curve equation. Statistical analysis was carried out using the SigmaPlot 12.5 software (San Jose, CA) and the MedCalc 14.8.1 software (Ostend, Belgium).
Results
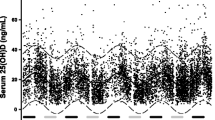
Serum PTH showed a slight seasonal variation with the peak in March and the nadir in September. The sinusoidal-fitted equation with period of 365 between serum PTH level f(x) and the day of the blood sampling x (R = 0.077; p < 0.001) is f(x) = 45.3857 + 2.231 ∙ sin(2πx/365–6.1918) had an amplitude of 4.46 pg/mL. The highest serum PTH concentration (max f(x) = 47.61 pg/mL) corresponds to day 86 (March 27), while the lowest concentration (min f(x) = 43.15 pg/mL) corresponds to day 268 (September 25). Sex and age subgroup analysis by season (Table 2) showed a similar variation with the highest levels during spring and the lowest levels during fall. Also, the proportion of subjects with PTH values above normal was higher in spring compared with that in fall. Males had significantly lower PTH serum levels than females irrespective of the season. Also, increasing age was associated with a significant increase of serum PTH in all seasons.
The secondary analysis was performed in the group of individual subjects with concomitant measurement of serum PTH and 25(OH)D to assess the delay between 25(OH)D nadir/peak and PTH peak/nadir respectively. After fitting a sinusoidal curve with a period of 365 days to each data set, the following equations were obtained: 25(OH)D = 22.366 + 3.9671 ∙ sin(2πx/365–2.5274) and PTH = 45.5894 + 1.9726 ∙ sin(2πx/365–6.1609). The time lag between 25(OH)D nadir/peak and PTH peak/nadir levels was 28 days (Fig. 1).
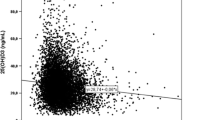
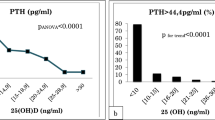
There was a negative association between serum 25(OH)D and serum PTH. Piecewise regression revealed a breakpoint at a serum 25(OH)D level of 12.82 ng/mL. The slope and correlation coefficients were − 2.35 and 0.21 (p < 0.01) below the breakpoint (n = 505) and − 0.31 and 0.15 (p < 0.01) respectively above the breakpoint (n = 2295) (Fig. 2a). Two stepwise multiple regression models (below and above the breakpoint) with serum PTH as the dependent variable and serum 25(OH)D, age, and sex as independent variables showed that sex was not significantly associated with PTH while 25(OH)D and sex were significant predictors. The models explained 6.4% and 10.0% of the PTH variation below and above the breakpoint respectively. As expected, below the breakpoint, PTH was dependent more on serum 25(OH)D (regression coefficient = − 2.13, r partial = − 0.19) than on age (regression coefficient = 0.19, r partial = 0.14), while above the breakpoint, PTH was dependent more on age (regression coefficient = 0.27, r partial = 0.28) than on serum 25(OH)D (regression coefficient = − 0.26, r partial = − 0.13). The median (25, 75 percentiles) of serum PTH for every 5 ng/mL interval of serum 25(OH)D can be found in Fig. 2b. The dependency of serum PTH on age and serum 25(OH)D level can also be seen in Fig. 3. In subjects with serum 25(OH)D levels over 20 ng/mL, high PTH values (over the upper limit of normal) are virtually restricted to those over 41 years of age.
a Correlation (solid line) between serum 25(OH)D and PTH. Horizontal dashed line represents the upper normal limit for serum PTH. Vertical dashed line represents the breakpoint (25(OH)D = 12.82 ng/mL). The horizontal box-plot represents the distribution of serum 25(OH)D in the 2800 subjects. b Box-plots of serum PTH for every 5 ng/mL interval of serum 25(OH)D
Serum PTH levels by intervals of serum 25(OH)D in subjects < 18 years of age (white bars), 18–40 years of age (rightwards dashed bars), 41–65 years of age (crosshatched bars), and > 65 years of age (gray bars). Note the statistically significant increase of serum PTH by age for every serum 25(OH)D category. The dashed horizontal line stands for the serum PH upper limit of normal
Discussion
Serum 25(OH)D has a seasonal variation in the temperate climate with the lowest levels in the early spring and the highest levels in the early autumn [2, 9]. Romania, situated in the Northern Hemisphere, Europe, between 44° N and 48° N latitude, shows a similar variation [3]. Serum PTH follows a temporal variation at an inverse sinusoidal pattern than serum 25(OH)D, and this relation has been consistently demonstrated in studies conducted in various parts of the world [9, 11, 16], but Europe is underrepresented [12, 13]. This study presents the seasonal variation of serum PTH and its relation to vitamin D status in a significant sample of Romanian population. To our knowledge, it is the first study to assess serum PTH across the year in Romania and Eastern Europe.
Our study demonstrated a slight seasonal variation of PTH with the peak at the end of March and the nadir at the end of September. Studies on lower number of subjects with aggregate data by season showed a maximum serum PTH during winter and a minimum PTH during summer [10, 12]. Studies including a high number of subjects calculated a peak serum PTH during March (September in the Southern Hemisphere) and a nadir serum PTH during September (March in Southern Hemisphere) [8, 9, 11], consistent with our results. The amplitude between min and max serum PTH was 4.46 pg/mL in our sample, rather low compared with that of other studies on thousands of subjects. Studies from Australia showed an amplitude of 5.6 [8] or 13.2 pg/mL [11], while studies from the USA revealed a min to max difference or 6.4 pg/mL [9]. This difference in amplitude might be due to genetic or dietary factors and not to serum 25(OH)D seasonal variation because similar studies had both lower [8, 9] and higher [11] 25(OH)D amplitudes. Moreover, all these studies [8, 9, 11] reported significantly higher 25(OH)D values (from 20 to 22 ng/mL in the spring to 28–31 ng/mL in the fall) than values reported for Romania (15.7 ng/mL to 24.3 ng/mL) [3] which should convert in a shallower PTH oscillation. Also, some studies included only females [11].
The analysis by season showed that the variation of serum PTH was consistent in all sex and age subgroups with the highest levels during spring and the lowest levels during fall. It is interesting to note that in our sample, men had significantly lower levels of PTH than women in all seasons. This is consistent with our [3] and other [4] previous studies who showed higher levels of serum 25(OH)D in males compared with females in Romania. Studies from Europe showed mixed results with both males or females having higher 25(OH)D levels all year round or just during particular seasons [17, 18]. USA studies revealed higher serum 25(OH)D levels in females and corresponding lower PTH levels [9]. These differences probably incorporate genetic, food, clothing, and cultural variation [18]. In Romania, men tend to wear lighter clothes in the spring-fall interval and are also prone to spend more time outdoors than women.
Age subgroup analysis showed a significant increase in serum PTH with advancing age in all seasons, similar to other studies [19]. Declining renal function is the most probable explanation for the higher PTH levels in subjects over 40 years of age [20], but lower serum 25(OH)D levels [3, 4], decreased calcium intake, malabsorption, or other factors [21] have to be considered with advancing age. The importance of lower calcium intake and malabsorption [22,23,24] is supported by the secondary analysis of our study which showed a significant trend for increasing serum PTH with advancing age for all serum 25(OH)D categories, from deficiency to sufficiency, and for all ages. As renal function is maintained until 40 years of age, the lower PTH levels in children compared with that of young adults might be due to calcium intake and absorption [22,23,24] or other factors [25]. Also, a high calcium intake (as food or supplements) could explain, at least partially, the occurrence of some low PTH serum levels in subjects with vitamin D deficiency.
The proportion of subjects with PTH serum levels above the normal range (over 65 pg/mL) generally follows the sinusoidal variation of PTH with the highest proportions during spring, between 5.18% in 0–17 years of age subgroup and 27.27% in over 65 years of age subgroup. In subjects with serum 25(OH)D levels over 20 ng/mL, high PTH values (over the upper limit of normal) are virtually restricted to those over 41 years of age. This suggests an age and renal effect more than a 25(OH)D-related one. It is interesting to note that the proportion of subjects with significantly increased PTH (over 100 pg/mL) is rather stable across the year suggesting that these values are more probable due to an underlying renal disease, not stated in the diagnosis by the treating physician, and not to the normal seasonal variation of 25(OH)D.
In the secondary analysis on subjects with simultaneous measurement of PTH and 25(OH)D, the inflection points on sinusoidal curve of serum PTH followed the 25(OH)D nadir/peak after 28 days, in good agreement with other studies which calculated a time lag of 2 to 4 weeks [8, 9, 11, 26]. Also, in our previous study on vitamin D seasonal variation in Romania including a much larger sample of subjects, the 25(OH)D serum levels had their nadir/peak about 2–3 weeks before the peak/nadir of PTH in the current study [3]. The reasons for this delay are not fully understood by; it might be related to vitamin D storage and release from the adipose tissue [27] and the mechanism of action at the cellular level [28].
Our study also confirmed the inverse 25(OH)D–PTH relation found in other studies [5, 7, 8, 10, 13], including Romanian subjects [6]. The piecewise regression found a breakpoint of 12.82 ng/mL below which the level of the slope for PTH increased from 0.31 to 2.35 suggesting this as an intervention threshold. This is consistent with studies on other populations confirming the lack of influence of genetic background on the dependency of PTH or other bone markers on vitamin D status [5, 8]. We were not able to find a threshold level of serum 25(OH)D at which serum PTH plateaus.
The biggest limitation of our study is that the included subjects are not a sample of the general population of Romania. Also, as our endocrinology center is located in the southern part of Romania, it receives patients mainly from this part of the country. The place of birth of our subjects confirms this predominance. Another limitation is the retrospective nature of our study. However, this allowed us to enroll an adequate number of subjects. Also, the intake of vitamin D and calcium (dietary or medication) was not available for any subject. This bias was minimized two ways: (1) by selecting only the first value (in case of serial measurements) as subjects with high PTH/low 25(OH)D levels is expected to receive vitamin D supplementation in the future and (2) by excluding subjects with a diagnosis of osteopenia or osteoporosis as they are expected to receive vitamin D and calcium supplementation and antiosteoporotic drugs who may directly or indirectly modify PTH serum levels. According to a 2018 survey of the National Institute of Public Health [29] on a representative Romanian sample, the mean daily calcium intake was 734 mg for men and 709 mg for women. We could not collect data on body mass index, medication or alcohol use, smoking status, place of living, and biochemistry (serum creatinine or creatinine clearance, serum calcium, etc.). However, the large number of clinically based subjects, with uniform test methods, provides important insights and can overcome the absence of the abovementioned parameters.
The main advantage of our study is the very large number of subjects which is of utmost importance when minimal variations (less than 10% of the upper limit of normal in the case of PTH) are expected across the year. Also, the large number of subjects allowed subgroup analysis (by season, age, sex, or 25(OH)D serum levels). As the general results could be replicated in all subgroup analyses, this confirmed that seasonality was not fortuitous.
Taken together, our results showed that the PTH seasonal variation in Romania is mild (6.8% of the upper limit of normal) and probably of no clinical importance. Moreover, it is unlikely that this variation can impact the clinical interpretation of PTH measurements. We also showed that PTH serum levels over 100 pg/mL are very probably the expression of an underlying disease and not the result of seasonal variation or vitamin D deficiency.
We have to notice that the seasonal nadir of serum 25(OH)D, whether from this or previous studies in Romania [3, 4], is higher than the 12.82 ng/mL breakpoint below which the PTH has a much steeper increase. Also, the normal seasonal vitamin D variation does not translate in PTH serum levels above the upper limit of the normal. These suggest that whole population supplementation with vitamin D (by medication or by food fortification) is not recommended for our country. Instead, vitamin D supplementation should be recommended in at-risk groups (e.g., over 65 years of age) or on a case-by-case scenario.
In conclusion, our data show a mild seasonal variation of serum PTH in Romania, at an inverse sinusoidal pattern than serum 25(OH)D. Data from serum 25(OH)D seasonal variation and PTH–25(OH)D relation suggest that in Romania, vitamin D supplementation is warranted only in at-risk groups.
References
Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T (2001) A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 86:1212–1221. https://doi.org/10.1210/jcem.86.3.7327
Kasahara AK, Singh RJ, Noymer A et al (2013) Vitamin D (25OHD) serum seasonality in the United States. PLoS One 8:e65785. https://doi.org/10.1371/journal.pone.0065785
Niculescu DA, Capatina CAM, Dusceac R, Caragheorgheopol A, Ghemigian A, Poiana C (2017) Seasonal variation of serum vitamin D levels in Romania. Arch Osteoporos 12:113. https://doi.org/10.1007/s11657-017-0407-3
Chirita-Emandi A, Socolov D, Haivas C, Calapiș A, Gheorghiu C, Puiu M (2015) Vitamin D status: a different story in the very young versus the very old Romanian patients. PLoS One 10:e0128010. https://doi.org/10.1371/journal.pone.0128010
Sai AJ, Walters RW, Fang X, Gallagher JC (2011) Relationship between vitamin D, parathyroid hormone, and bone health. J Clin Endocrinol Metab 96:E436–E446. https://doi.org/10.1210/jc.2010-1886
Grigorie D, Sucaliuc A, Ivan M et al (2008) High prevalence of vitamin D deficiency in 1048 Romanian women with postmenopausal osteoporosis. Acta Endocrinol 4:33–45. https://doi.org/10.4183/aeb.2008.33
Valcour A, Blocki F, Hawkins DM, Rao SD (2012) Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab 97:3989–3995. https://doi.org/10.1210/jc.2012-2276
Shah S, Chiang C, Sikaris K, Lu Z, Bui M, Zebaze R, Seeman E (2017) Serum 25-hydroxyvitamin D insufficiency in search of a bone disease. J Clin Endocrinol Metab 102:2321–2328. https://doi.org/10.1210/jc.2016-3189
Kroll MH, Bi C, Garber CC, Kaufman HW, Liu D, Caston-Balderrama A, Zhang K, Clarke N, Xie M, Reitz RE, Suffin SC, Holick MF (2015) Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS One 10:e0118108. https://doi.org/10.1371/journal.pone.0118108
Hegarty V, Woodhouse P, Khaw K-T (1994) Seasonal variation in 25-hydroxyvitamin D and parathyroid hormone concentrations in healthy elderly people. Age Ageing 23:478–482. https://doi.org/10.1093/ageing/23.6.478
Pasco JA, Henry MJ, Kotowicz MA, Sanders KM, Seeman E, Pasco JR, Schneider HG, Nicholson GC (2004) Seasonal periodicity of serum vitamin D and parathyroid hormone, bone resorption, and fractures: the Geelong Osteoporosis Study. J Bone Miner Res 19:752–758. https://doi.org/10.1359/JBMR.040125
Piirainen R, Englund E, Henriksson AE (2016) The impact of seasonal variation of 25-hydroxyvitamin D and parathyroid hormone on calcium levels. Clin Biochem 49:850–853. https://doi.org/10.1016/j.clinbiochem.2016.06.009
González-Molero I, Morcillo S, Valdés S, Pérez-Valero V, Botas P, Delgado E, Hernández D, Olveira G, Rojo G, Gutierrez-Repiso C, Rubio-Martín E, Menéndez E, Soriguer F (2011) Vitamin D deficiency in Spain: a population-based cohort study. Eur J Clin Nutr 65:321–328. https://doi.org/10.1038/ejcn.2010.265
Nevo-Shor A, Kogan S, Joshua B-Z, Bahat-Dinur A, Novack V, Fraenkel M (2016) Seasonal changes in serum calcium, PTH and vitamin D levels in patients with primary hyperparathyroidism. Bone 89:59–63. https://doi.org/10.1016/j.bone.2016.05.012
Kleine C-E, Obi Y, Streja E, Hsiung JT, Park C, Holick MF, Kalantar-Zadeh K (2019) Seasonal variation of serum 25-hydroxyvitamin D and parameters of bone and mineral disorder in dialysis patients. Bone 124:158–165. https://doi.org/10.1016/j.bone.2019.03.003
Itoh H, Mori I, Matsumoto Y et al (2011) Vitamin D deficiency and seasonal and inter-day variation in circulating 25-hydroxyvitamin D and parathyroid hormone levels in indoor daytime workers: a longitudinal study. Ind Health 49:475–481. https://doi.org/10.2486/indhealth.ms1255
Gaksch M, Jorde R, Grimnes G, et al (2017) Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One 12: . doi: https://doi.org/10.1371/journal.pone.0170791
Grant WB, Fakhoury HMA, Karras SN, al Anouti F, Bhattoa HP (2019) Variations in 25-hydroxyvitamin d in countries from the middle east and europe: the roles of UVB exposure and diet. Nutrients 11: . doi: https://doi.org/10.3390/nu11092065
Farrell CJL, Nguyen L, Carter AC (2018) Parathyroid hormone: data mining for age-related reference intervals in adults. Clin Endocrinol 88:311–317. https://doi.org/10.1111/cen.13486
Weinstein JR, Anderson S (2010) The aging kidney: physiological changes. Adv Chronic Kidney Dis 17:302–307
Carrivick SJ, Walsh JP, Brown SJ, Wardrop R, Hadlow NC (2015) Brief report: does PTH increase with age, independent of 25-hydroxyvitamin D, phosphate, renal function, and ionized calcium? J Clin Endocrinol Metab 100:2131–2134. https://doi.org/10.1210/jc.2014-4370
Steingrimsdottir L, Gunnarsson O, Indridason OS et al (2005) Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. J Am Med Assoc 294:2336–2341. https://doi.org/10.1001/jama.294.18.2336
Patel P, Zulf Mughal M, Patel P et al (2016) Dietary calcium intake influences the relationship between serum 25-hydroxyvitamin D3(25OHD) concentration and parathyroid hormone (PTH) concentration. Arch Dis Child 101:316–319. https://doi.org/10.1136/archdischild-2015-308985
Portale AA, Lonergan ET, Tanney DM, Halloran BP (1997) Aging alters calcium regulation of serum concentration of parathyroid hormone in healthy men. Am J Phys 272:E139–E146. https://doi.org/10.1152/ajpendo.1997.272.1.E139
Stagi S, Cavalli L, Ricci S, Mola M, Marchi C, Seminara S, Brandi ML, de Martino M (2015) Parathyroid hormone levels in healthy children and adolescents. Horm Res Paediatr 84:124–129. https://doi.org/10.1159/000432399
Rapuri PB, Karimi Kinyamu H, Christopher Gallagher J, Haynatzka V (2002) Seasonal changes in calciotropic hormones, bone markers, and bone mineral density in elderly women. J Clin Endocrinol Metab 87:2024–2032. https://doi.org/10.1210/jcem.87.5.8475
Martinaityte I, Kamycheva E, Didriksen A, Jakobsen J, Jorde R (2017) Vitamin D stored in fat tissue during a 5-year intervention affects serum 25-hydroxyvitamin d levels the following year. J Clin Endocrinol Metab 102:3731–3738
Bikle DD (2014) Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 21:319–329
National Institute of Public Health (2019) Nutritional Status and Food Consumption in Romania - 2018 (Starea de nutritie si consumul de alimente in Romania - 2018). https://cnmrmc.insp.gov.ro/images/rapoarte/starea-de-nutritie.pdf. Accessed 22 Apr 2020
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Niculescu, D.A., Deacu, L.G., Caragheorgheopol, A. et al. Seasonal periodicity of serum parathyroid hormone and its relation with vitamin D in Romania. Arch Osteoporos 15, 66 (2020). https://doi.org/10.1007/s11657-020-00744-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-020-00744-1